Variations of Serum CRP Levels in Periodontal Health and Diseases: A Clinico-Biochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methodology and Sample Collection
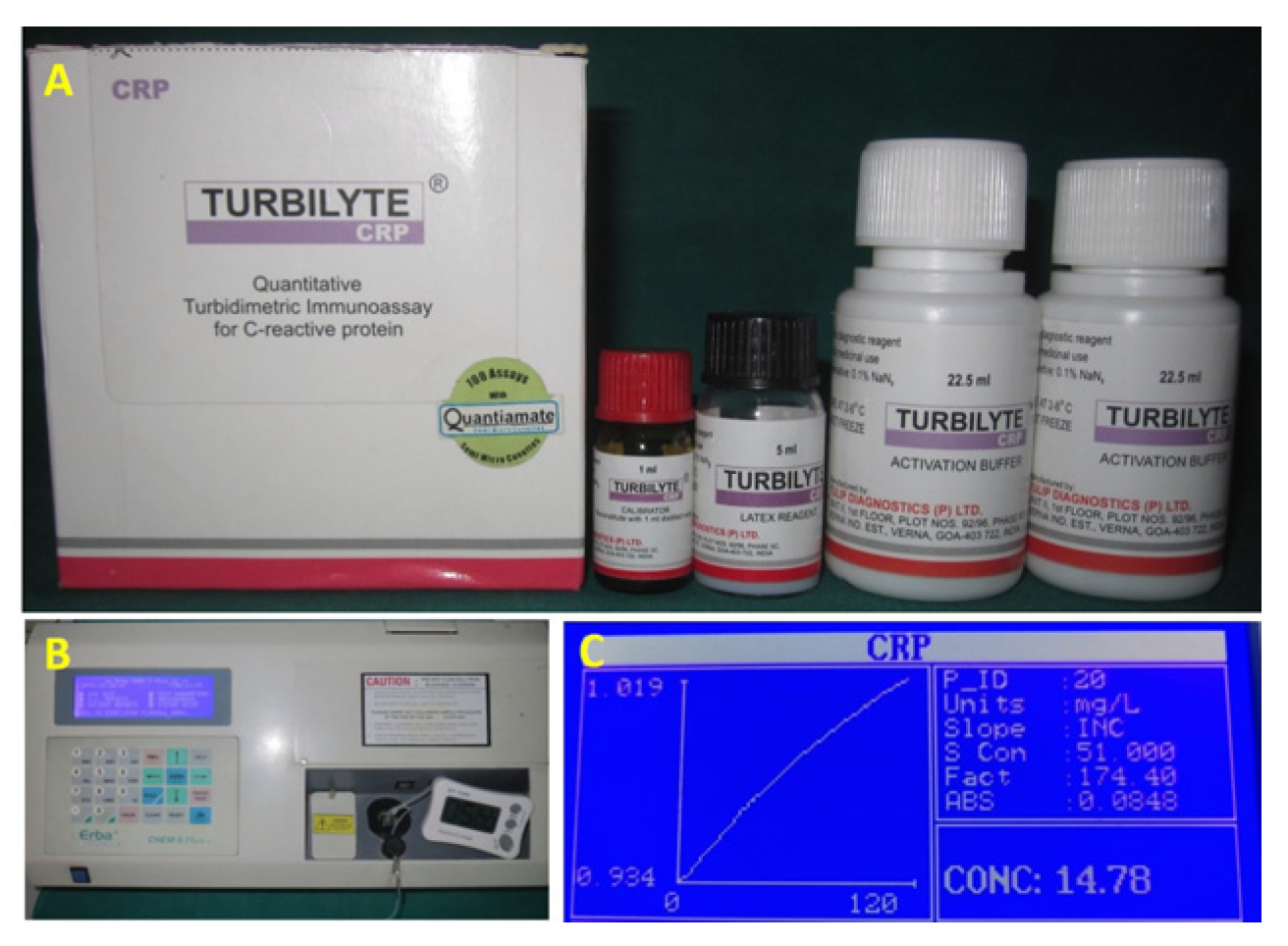
2.3. Laboratory Procedure for Assessment of C-Reactive Protein Levels in Serum
2.4. Test Procedure
2.5. Calculations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Dawson, D., III; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K. The periodontal war: Microbes and immunity. Periodontology 2000 2017, 75, 52–115. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.; Gao, X.; Jagannathan, N. A qualitative analysis of students’ perceptions of videos to support learning in a psychomotor skills course. Eur. J. Dent. Educ. 2019, 23, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.S.; Ricardo, L.B.; Oliveira, A.M.; Horta, D.V.; Papoila, A.L.; Deus, J.R.; Canena, J. C-reactive protein at 24 hours after hospital admission may have relevant prognostic accuracy in acute pancreatitis: A retrospective cohort study. GE Port. J. Gastroenterol. 2015, 22, 198–203. [Google Scholar] [CrossRef]
- Hu, L.; Shi, Q.; Shi, M.; Liu, R.; Wang, C. Diagnostic value of PCT and CRP for detecting serious bacterial infections in patients with fever of unknown origin: A systematic review and meta-analysis. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e61–e69. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Alizadeh, N.; Varshochi, M.; Kafil, H.S. Immunologic biomarkers for diagnostic of early-onset neonatal sepsis. J. Matern.-Fetal Neonatal Med. 2019, 32, 143–153. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Lagrand, W.K.; Visser, C.A.; Hermens, W.T.; Niessen, H.W.; Verheugt, F.W.; Wolbink, G.-J.; Hack, C.E. C-reactive protein as a cardiovascular risk factor: More than an epiphenomenon? Circulation 1999, 100, 96–102. [Google Scholar] [CrossRef]
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef]
- Aoyama, N.; Suzuki, J.-i.; Kobayashi, N.; Hanatani, T.; Ashigaki, N.; Yoshida, A.; Shiheido, Y.; Sato, H.; Izumi, Y.; Isobe, M. Increased oral porphyromonas gingivalis prevalence in cardiovascular patients with uncontrolled diabetes mellitus. Int. Heart J. 2018, 59, 802–807. [Google Scholar] [CrossRef]
- Sesso HBuring, J.E.; Rifai, N.; Blake, G.J.; Gaziano, J.M.; Ridker, P.M. C-reactive protein and the risk of developing hypertension. JAMA 2003, 290, 2945–2951. [Google Scholar] [CrossRef]
- Bolla, V.; Kumari, P.S.; Munnangi, S.R.; Kumar, D.S.; Durgabai, Y.; Koppolu, P. Evaluation of serum c-reactive protein levels in subjects with aggressive and chronic periodontitis in comparison with healthy controls: A clinico-biochemical study. Int. J. Appl. Basic Med. Res. 2017, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Han, C.Y.; Oram, J.F.; Heinecke, J.W. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: Markers or mediators of cardiovascular disease? J. Lipid Res. 2005, 46, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-reactive protein and periodontitis: A systematic review and meta-analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Wu, T.; Trevisan, M.; Genco, R.J.; Falkner, K.L.; Dorn, J.P.; Sempos, C.T. Examination of the relation between periodontal health status and cardiovascular risk factors: Serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am. J. Epidemiol. 2000, 151, 273–282. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cha, G.S.; Kim, H.-J.; Kwon, E.-Y.; Lee, J.-Y.; Choi, J.; Joo, J.-Y. Porphyromonas gingivalis accelerates atherosclerosis through oxidation of high-density lipoprotein. J. Periodontal Implant. Sci. 2018, 48, 60. [Google Scholar] [CrossRef]
- Slade, G.; Offenbacher, S.; Beck, J.; Heiss, G.; Pankow, J. Acute-phase inflammatory response to periodontal disease in the US population. J. Dent. Res. 2000, 79, 49–57. [Google Scholar] [CrossRef]
- Gupta, S.; Suri, P.; Patil, P.B.; Rajguru, J.P.; Gupta, P.; Patel, N. Comparative evaluation of role of hs C-reactive protein as a diagnostic marker in chronic periodontitis patients. J. Fam. Med. Prim. Care 2020, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Swaroop Chandy, K.J.; Sankaranarayanan, A.; Issac, A.; Babu, G.; Wilson, B.; Joseph, J. Evaluation of C-reactive protein and fibrinogen in patients with chronic and aggressive periodontitis: A clinico-biochemical study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC41. [Google Scholar]
- Berben, L.; Sereika, S.M.; Engberg, S. Effect size estimation: Methods and examples. Int. J. Nurs. Stud. 2012, 49, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Loe, H.; Silness, J. Periodontal disease in pregnancy (I). Prevalence and severity. Acta Odontol. Scand. 1963, 21, 532–551. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Loe, H. Periodontal disease in pregnancy (II). Correlation between oral hygiene and periodontal condition. Acta Odontl. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Chopra, R.; Patil, S.R.; Kalburgi, N.B.; Mathur, S. Association between alveolar bone loss and serum C-reactive protein levels in aggressive and chronic periodontitis patients. J. Indian Soc. Periodontol. 2012, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Sibraa, P.D.; Reinhardt, R.A.; Dyer, J.; DuBois, L. Acute-phase protein detection and quantification in gingival crevicular fluid by direct and indirect immunodot. J. Clin. Periodontol. 1991, 18, 101–106. [Google Scholar] [CrossRef]
- Ito, H.; Numabe, Y.; Hashimoto, S.; Sekino, S.; Murakashi, E.; Ishiguro, H.; Sasaki, D.; Yaegashi, T.; Takai, H.; Mezawa, M. Correlation between gingival crevicular fluid hemoglobin content and periodontal clinical parameters. J. Periodontol. 2016, 87, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Sahana Selvaganesh, D.; Thiyaneswaran, N. Evaluation and Comparison of Wound Healing Efficiency in Obese Patients with Elevated C-Reactive Protein Level: An In-Vivo Study. J. Pharm. Negat. Results 2023, 13, 6738–6744. [Google Scholar]
- Bansal, T.; Pandey, A.; Deepa, D.; Asthana, A.K. C-reactive protein (CRP) and its association with periodontal disease: A brief review. J. Clin. Diagn. Res. JCDR 2014, 8, ZE21. [Google Scholar] [PubMed]
- George, A.K.; Janam, P. The short-term effects of non-surgical periodontal therapy on the circulating levels of interleukin-6 and C-reactive protein in patients with chronic periodontitis. J. Indian Soc. Periodontol. 2013, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Kamil, W.; Al Habashneh, R.; Khader, Y.; Al Bayati, L.; Taani, D. Effects of nonsurgical periodontal therapy on C-reactive protein and serum lipids in Jordanian adults with advanced periodontitis. J. Periodontal. Res. 2011, 46, 616–621. [Google Scholar] [CrossRef]

| Clinical Parameter | Group A | Group B1 | Group B2 | F | p Value |
|---|---|---|---|---|---|
| Gingival Index (GI) | 0.14 ± 0.03 | 2.43 ± 0.33 | 1.05 ± 0.34 | 853.27 | 0.00 * |
| Plaque index (PI) | 0.41 ± 0.07 | 2.49 ± 0.27 | 0.95 ± 0.37 | 749.49 | 0.00 * |
| Probing pocket depth (PPD) | 0.00 ± 0.00 | 2.19 ± 0.49 | 1.20 ± 0.40 | 443.92 | 0.00 * |
| Clinical attachment Loss (CAL) | 0.00 ± 0.00 | 1.49 ± 0.23 | 0.77 ± 0.21 | 840.56 | 0.00 * |
| C–Reactive Protein (CRP) | 0.04 ± 0.02 | 1.67 ± 0.58 | 0.88 ± 0.04 | 216.73 | 0.00 * |
| Parameter | Group | Mean Difference | Std. Error | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | p Value | |||||
| GI | A | B1 | −2.29 | 0.06 | −2.43 | −2.16 | 0.00 * |
| B2 | −0.91 | 0.06 | −1.04 | −0.77 | 0.00 * | ||
| B1 | A | 2.29 | 0.06 | 2.16 | 2.43 | 0.00 * | |
| B2 | 1.39 | 0.06 | 1.25 | 1.52 | 0.00 * | ||
| B2 | A | 0.91 | 0.06 | 0.77 | 1.04 | 0.00 * | |
| B1 | −1.39 | 0.06 | −1.52 | −1.25 | 0.00 * | ||
| PI | A | B1 | −2.08 | 0.06 | −2.22 | −1.95 | 0.00 * |
| B2 | −0.56 | 0.06 | −0.69 | −0.43 | 0.00 * | ||
| B1 | A | 2.08 | 0.06 | 1.95 | 2.22 | 0.00 * | |
| B2 | 1.52 | 0.06 | 1.39 | 1.66 | 0.00 * | ||
| B2 | A | 0.56 | 0.06 | 0.43 | 0.69 | 0.00 * | |
| B1 | −1.52 | 0.06 | −1.66 | −1.39 | 0.00 * | ||
| PPD | A | B1 | −2.20 | 0.07 | −2.37 | −2.02 | 0.00 * |
| B2 | −1.21 | 0.07 | −1.39 | −1.03 | 0.00 * | ||
| B1 | A | 2.20 | 0.07 | 2.02 | 2.37 | 0.00 * | |
| 3 | 0.99 | 0.07 | 0.81 | 1.17 | 0.00 * | ||
| B2 | A | 1.21 | 0.07 | 1.03 | 1.39 | 0.00 * | |
| B1 | −0.99 | 0.07 | −1.17 | −0.81 | 0.00 * | ||
| CAL | A | B1 | −1.49 | 0.04 | −1.58 | −1.40 | 0.00 * |
| B2 | −0.78 | 0.04 | −0.87 | −0.69 | 0.00 * | ||
| B1 | A | 1.49 | 0.04 | 1.40 | 1.58 | 0.00 * | |
| B2 | 0.71 | 0.04 | 0.62 | 0.80 | 0.00 * | ||
| B2 | A | 0.78 | 0.04 | 0.69 | 0.87 | 0.00 * | |
| B1 | −0.71 | 0.04 | −0.80 | −0.62 | 0.00 * | ||
| CRP | A | B1 | −1.63 | 0.08 | −1.82 | −1.44 | 0.00 * |
| B2 | −0.84 | 0.08 | −1.03 | −0.65 | 0.00 * | ||
| B1 | A | 1.63 | 0.08 | 1.44 | 1.82 | 0.00 * | |
| B2 | 0.79 | 0.08 | 0.60 | 0.98 | 0.00 * | ||
| B2 | A | 0.84058 * | 0.08 | 0.65 | 1.03 | 0.00 * | |
| B1 | −0.78942 * | 0.08 | −0.98 | −0.60 | 0.00 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shankar, S.; Manjunath, S.; Alqahtani, S.M.; Ganji, K.K.; Nagate, R.R.; Ghokale, S.T.; Nagarajappa, A.K.; Javali, M.A.; Tikare, S.; Khader, M.A. Variations of Serum CRP Levels in Periodontal Health and Diseases: A Clinico-Biochemical Study. Diagnostics 2023, 13, 2483. https://doi.org/10.3390/diagnostics13152483
Shankar S, Manjunath S, Alqahtani SM, Ganji KK, Nagate RR, Ghokale ST, Nagarajappa AK, Javali MA, Tikare S, Khader MA. Variations of Serum CRP Levels in Periodontal Health and Diseases: A Clinico-Biochemical Study. Diagnostics. 2023; 13(15):2483. https://doi.org/10.3390/diagnostics13152483
Chicago/Turabian StyleShankar, Sidharth, Shiva Manjunath, Saad Mohammad Alqahtani, Kiran Kumar Ganji, Raghavendra Reddy Nagate, Shankar T. Ghokale, Anil Kumar Nagarajappa, Mukhatar Ahmed Javali, Shreyas Tikare, and Mohasin Abdul Khader. 2023. "Variations of Serum CRP Levels in Periodontal Health and Diseases: A Clinico-Biochemical Study" Diagnostics 13, no. 15: 2483. https://doi.org/10.3390/diagnostics13152483
APA StyleShankar, S., Manjunath, S., Alqahtani, S. M., Ganji, K. K., Nagate, R. R., Ghokale, S. T., Nagarajappa, A. K., Javali, M. A., Tikare, S., & Khader, M. A. (2023). Variations of Serum CRP Levels in Periodontal Health and Diseases: A Clinico-Biochemical Study. Diagnostics, 13(15), 2483. https://doi.org/10.3390/diagnostics13152483






