Myocardial Infarction in Young Athletes
Abstract
1. Introduction
2. Case Reports
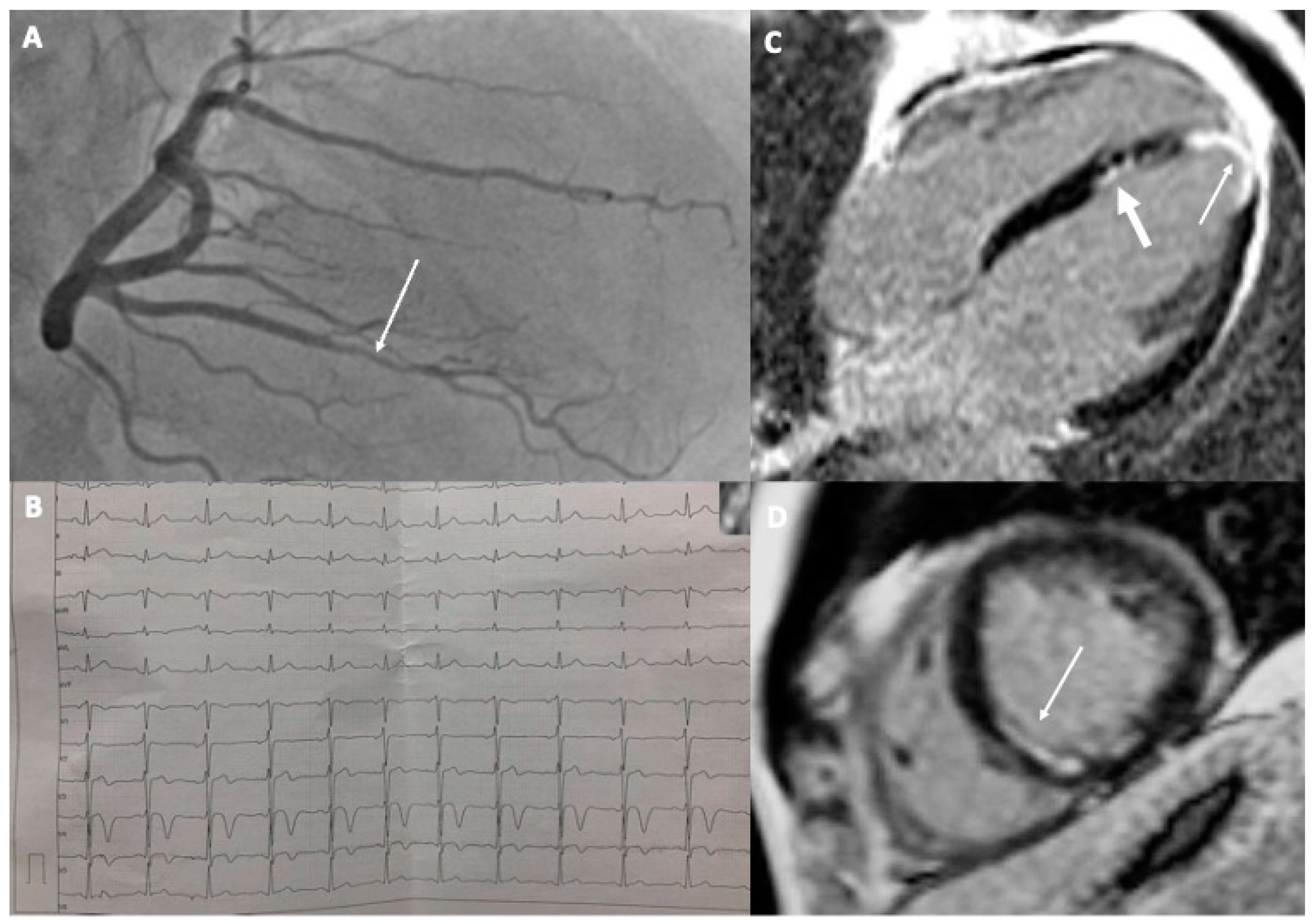
2.1. Case 1
2.2. Case 2
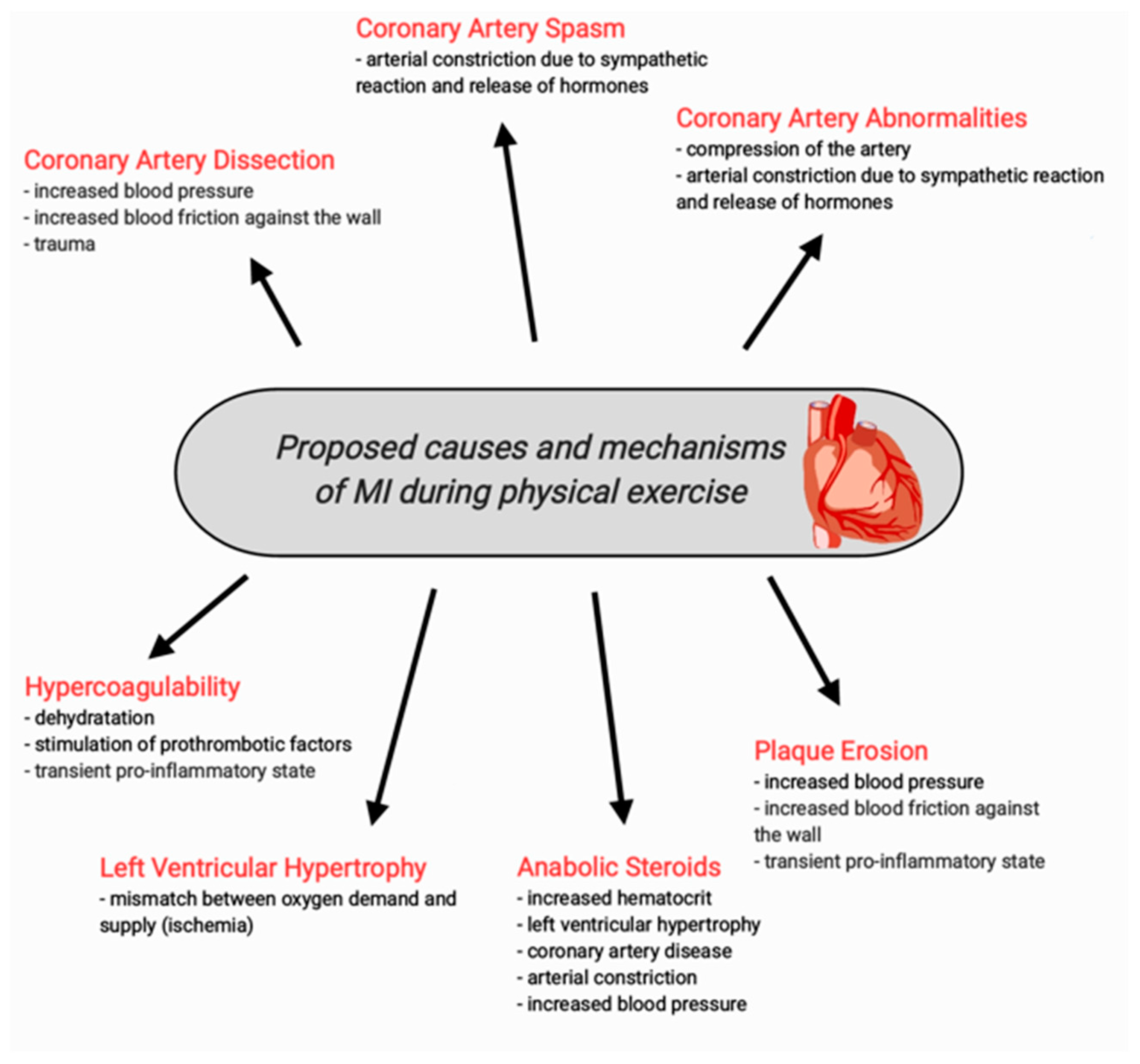
3. Causes of MI in Young Athletes—The Literature Review
3.1. Spontaneous Coronary Artery Dissection (SCAD)
3.2. MI after Chest Trauma
3.3. Abnormalities of the Coronary Arteries
3.4. Coronary Artery Spasm
3.5. Plaque Erosion
3.6. Hypercoagulability
3.7. Left Ventricular Hypertrophy
3.8. MI Induced by Anabolic Steroids
4. Considerations for Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute myocardial infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Ojha, N.; Dhamoon, A.S. Myocardial Infarction; StatPearls Publishing: St. Petersburg, FL, USA, 2021. [Google Scholar]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.-L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Landry, C.H.; Allan, K.S.; Connelly, K.A.; Cunningham, K.; Morrison, L.J.; Dorian, P. Sudden Cardiac Arrest during Participation in Competitive Sports. N. Engl. J. Med. 2017, 377, 1943–1953. [Google Scholar] [CrossRef]
- Gebel, K.; Ding, D.; Chey, T.; Stamatakis, E.; Brown, W.J.; Bauman, A.E. Effect of Moderate to Vigorous Physical Activity on All-Cause Mortality in Middle-aged and Older Australians. JAMA Intern. Med. 2015, 175, 970–977. [Google Scholar] [CrossRef]
- Asatryan, B.; Vital, C.; Kellerhals, C.; Medeiros-Domingo, A.; Gräni, C.; Trachsel, L.D.; Schmied, C.M.; Saguner, A.M.; Eser, P.; Herzig, D.; et al. Sports-related sudden cardiac deaths in the young population of Switzerland. PLoS ONE 2017, 12, e0174434. [Google Scholar] [CrossRef] [PubMed]
- Vicent, L.; Ariza-Solé, A.; Juanatey, J.R.G.; Uribarri, A.; Ortiz, J.; Lopez-De-Sa, E.; Sans-Roselló, J.; Querol, C.T.; Codina, P.; Sousa-Casasnovas, I.; et al. Exercise-related severe cardiac events. Scand. J. Med. Sci. Sports 2018, 28, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Vacek, T.; Yu, S.; Rahman, S.; Grubb, B.; Kosinski, D.; Verghese, C.; Eltahawy, E.; Qaiser, S. Recurrent myocardial infarctions in a young football player secondary to thrombophilia, associated with elevated factor VIII activity. Int. Med. Case Rep. J. 2014, 7, 147–154. [Google Scholar] [CrossRef]
- Thompson, C.S.; Pass, M.; Timothy, T.; Hung, J.; Egred, M. Acute myocardial infarction in a young elite cyclist: A missed opportunity. BMJ Case Rep. 2019, 12, e228560. [Google Scholar] [CrossRef]
- Stuart, S.D.F.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell. Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2016, 389, 197–210. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Saw, J. Spontaneous Coronary Artery Dissection. Can. J. Cardiol. 2013, 29, 1027–1033. [Google Scholar] [CrossRef]
- Klemenc, M.; Budihna, G.; Bedencic, M.; Bartolic, A.; Kranjec, I. Acute myocardial infarction in a young athlete: Optical coherence tomographic features of the culprit lesion. SAGE Open Med. Case Rep. 2016, 4, 2050313X16642333. [Google Scholar] [CrossRef]
- Wagers, T.P.; Stevens, C.J.; Ross, K.V.; Leon, K.K.; Masters, K.S. Spontaneous Coronary Artery Dissection (SCAD): FEMALE SURVIVORS’EXPERIENCES OF STRESS AND SUPPORT. J. Cardiopulm. Rehabil. Prev. 2018, 38, 374. [Google Scholar] [CrossRef]
- Aslam, A.; Stojanovska, J.; Khokhar, U.S.; Weinberg, R.L.; Ganesh, S.K.; Labounty, T.; Sutton, N.R.; Patel, S. Spontaneous Coronary Artery Dissection: An Underdiagnosed Clinical Entity—A Primer for Cardiac Imagers: A review publication of the Radiological Society of North America, Inc. Radiographics 2021, 41, 1897–1915. [Google Scholar] [CrossRef]
- Pergola, V.; Continisio, S.; Mantovani, F.; Motta, R.; Mattesi, G.; Marrazzo, G.; Dellino, C.M.; Montonati, C.; De Conti, G.; Galzerano, D.; et al. Spontaneous coronary artery dissection: The emerging role of coronary computed tomography. Eur. Heart J.-Cardiovasc. Imaging 2023, 24, 839–850. [Google Scholar] [CrossRef]
- Lolay, G.A.; Abdel-Latif, A.K. Trauma induced myocardial infarction. Int. J. Cardiol. 2015, 203, 19–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Mieghem, N.M.; van Weenen, S.; Nollen, G.; Ligthart, J.; Regar, E.; van Geuns, R.-J. Traumatic Coronary Artery Dissection: Potential cause of sudden death in soccer. Circulation 2013, 127, e280–e282. [Google Scholar] [CrossRef] [PubMed]
- Fanari, Z.; Hadid, M.; Hammami, S.; Qureshi, W.A. Traumatic myocardial infarction in a young athletic patient after a football game. Del. Med. J. 2014, 86, 213–215. [Google Scholar] [PubMed]
- Riede, F.-N.; Bulla, S.; Grundmann, S.; Werner, M.; Riede, U.-N.; Otto, C. Isolated hypoplastic circumflex coronary artery: A rare cause of haemorrhagic myocardial infarction in a young athlete. Diagn. Pathol. 2013, 8, 91. [Google Scholar] [CrossRef]
- Harmon, K.G.; Asif, I.M.; Maleszewski, J.J.; Owens, D.S.; Prutkin, J.M.; Salerno, J.C.; Zigman, M.L.; Ellenbogen, R.; Rao, A.L.; Ackerman, M.J.; et al. Incidence, Cause, and Comparative Frequency of Sudden Cardiac Death in National Collegiate Athletic Association Athletes: A Decade in Review. Circulation 2015, 132, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.F.; Sheppard, M.N. A silent cause of sudden cardiac death especially in sport: Congenital coronary artery anomalies. Br. J. Sports Med. 2013, 48, 1151–1156. [Google Scholar] [CrossRef]
- Saitto, G.; Lio, A.; Polizzi, V.; Russo, M.; Nicolò, F.; Ranocchi, F.; Musumeci, F. Surgical Management of Acute Myocardial Infarction Caused by Intramural Anomalous Left Coronary Artery in a Young Female Athlete. Tex. Heart Inst. J. 2022, 49, e207425. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, K.; Sharma, M.; Toor, A.; Toor, R.; Costacurta, G. Anomalous Left Main Coronary Artery Arising from the Right Sinus of Valsalva in a Young Man Presenting with Recurrent Syncope and Myocardial Infarction. Case Rep. Cardiol. 2018, 2018, 9805061. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chen, C.-H. Myocardial Bridging: An Up-to-Date Review. J. Am. Coll. Cardiol. 2015, 27, 521–528. [Google Scholar]
- Jamshidi, P.; Studer, M.; Erne, P. Myocardial infarction after an ice-hockey match: Coincidence of myocardial bridging and coronary spasm. Int. J. Cardiol. 2006, 113, E70–E72. [Google Scholar] [CrossRef] [PubMed]
- Agirbasli, M.; Martin, G.S.; Stout, J.B.; Jennings, H.S.; Lea, J.W.; Dixon, J.H. Myocardial bridge as a cause of thrombus formation and myocardial infarction in a young athlete. Clin. Cardiol. 1997, 20, 1032–1036. [Google Scholar] [CrossRef]
- Zeina, A.R.; Shefer, A.; Sharif, D.; Rosenschein, U.; Barmeir, E. Acute myocardial infarction in a young woman with normal coronary arteries and myocardial bridging. Br. J. Radiol. 2008, 81, e141–e144. [Google Scholar] [CrossRef]
- Zhu, C.-G.; Liu, J.; Liu, W.-D.; Xu, Y.-L.; Wu, N.-Q.; Guo, Y.-L.; Tang, Y.-D.; Jiang, L.-X.; Li, J.-J. Myocardial infarction caused by myocardial bridging in a male adolescent athlete. J. Cardiovasc. Med. 2012, 13, 138–140. [Google Scholar] [CrossRef]
- Guerra, E.; Bergamaschi, L.; Tuttolomondo, D.; Pizzi, C.; Sartorio, D.; Gaibazzi, N. Contrast Stress Echocardiography Findings in Myocardial Bridging Compared to Normal Coronary Course, with and without Coronary Artery Disease. J. Am. Soc. Echocardiogr. 2023. ahead of print. [Google Scholar] [CrossRef]
- El-Maraghi, N.R.; Sealey, B.J. Recurrent myocardial infarction in a young man due to coronary arterial spasm demonstrated at autopsy. Circulation 1980, 61, 199–197. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Myerburg, R.J.; Levine, B.D.; Udelson, J.E.; Kovacs, R.J. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 8: Coronary Artery Disease A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e310–e314. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Munir, J.A.; McIntyre, P.Z.; Ferguson, M.A. STEMI in a 24-year-old man after use of a synephrine-containing dietary supplement: A case report and review of the literature. Tex. Heart Inst. J. 2009, 36, 586–590. [Google Scholar]
- Unnikrishnan, D.; Annam, R.; Jacob, A.; Thyagarajan, B.; Farrugia, P. STEMI in a Young Male after Use of Synephrine-Containing Dietary Supplement. Case Rep. Cardiol. 2018, 2018, 7074104. [Google Scholar] [CrossRef] [PubMed]
- Smedema, J.P.; Muller, G.J. Coronary spasm and thrombosis in a bodybuilder using a nutritional supplement containing synephrine, octopamine, tyramine and caffeine. S. Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 2008, 98, 372–373. [Google Scholar]
- Colleran, R.; Joner, M.; Foin, N.; Byrne, R. Acute myocardial infarction in a young endurance athlete caused by probable plaque erosion. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, e246–e247. [Google Scholar] [CrossRef]
- Kato, A.; Minami, Y.; Katsura, A.; Muramatsu, Y.; Sato, T.; Kakizaki, R.; Nemoto, T.; Hashimoto, T.; Fujiyoshi, K.; Meguro, K.; et al. Physical exertion as a trigger of acute coronary syndrome caused by plaque erosion. J. Thromb. Thrombolysis 2020, 49, 377–385. [Google Scholar] [CrossRef]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes with a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef]
- Virmani, R.; Robinowitz, M.; McAllister, H.A. Nontraumatic death in joggers. A series of 30 patients at autopsy. Am. J. Med. 1982, 72, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, C.; Santarella, L.; Costa, M.G.; Manfrini, O.; Flacco, M.E.; Capasso, L.; Chiarini, S.; Di Baldassarre, A.; Manzoli, L. Pathophysiological mechanisms linking depression and atherosclerosis: An overview. J. Biol. Regul. Homeost. Agents 2012, 26, 775–782. [Google Scholar] [PubMed]
- Cwikiel, J.; Seljeflot, I.; Berge, E.; Arnesen, H.; Wachtell, K.; Ulsaker, H.; Flaa, A. Pro-coagulant activity during exercise testing in patients with coronary artery disease. Thromb. J. 2017, 15, 3. [Google Scholar] [CrossRef]
- Paczuski, R.; Cieślicka, M. The moderate physical exercise significantly increases von Willebrand’s factor’s activity and concentration in the blood. Pol. Ann. Med. 2013, 20, 100–105. [Google Scholar] [CrossRef]
- Christou, G.A.; Christou, K.A.; Nikas, D.N.; Goudevenos, J.A. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur. J. Prev. Cardiol. 2016, 23, 1785–1796. [Google Scholar] [CrossRef]
- Melhem, A.J.M., Jr.; Araújo, A.C.; Figueiredo, F.N.S.; Figueiredo, D.L.A. Acute Myocardial Infarction in a Young Bodybuilder: A Case Report and Review of the Literature. Am. J. Case Rep. 2020, 21, e924796. [Google Scholar] [CrossRef] [PubMed]
- Ehses, W.; Niklaus, K.; Brockmann, M.; Angenendt, W.; Saborowski, F. Fatal Arrhythmia in a Juvenile Athlete due to Myocardial Hypertrophy and Infarction. Int. J. Sports Med. 2000, 21, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Day, S.; Olivotto, I. Leisure-time and competitive sport participation: A changing paradigm for HCM patients. Eur. J. Prev. Cardiol. 2023, 30, 488–495. [Google Scholar] [CrossRef]
- Samreen, F.; Popal, U.; Baloch, Z.A.Q. Anabolic Steroid-Induced Myocardial Infarction in a Young Male. Cureus 2021, 13, e13054. [Google Scholar] [CrossRef]
- Cohen, J.; Collins, R.; Darkes, J.; Gwartney, D. A league of their own: Demographics, motivations and patterns of use of 1955 male adult non-medical anabolic steroid users in the United States. J. Int. Soc. Sports Nutr. 2007, 4, 12. [Google Scholar] [CrossRef]
- Jain, V.; Goel, G. Acute myocardial infarction in young newbie bodybuilder using multiple steroid and protein supplements. J. Cardiol. Cases 2019, 21, 134–136. [Google Scholar] [CrossRef]
- Tan, B.E.-X.; Chowdhury, M.; Hall, C.; Baibhav, B. Exogenous Testosterone Abuse and Myocardial Infarction in a Young Bodybuilder. Am. J. Med. 2020, 133, e665–e666. [Google Scholar] [CrossRef]
- Shahsavari Nia, K.; Rahmani, F.; Ebrahimi Bakhtavar, H.; Hashemi Aghdam, Y.; Balafar, M. A Young Man with Myocardial Infarction due to Trenbolone Acetate; a Case Report. Emergency 2014, 2, 43–45. [Google Scholar] [PubMed]
- Finkle, W.D.; Greenland, S.; Ridgeway, G.K.; Adams, J.L.; Frasco, M.A.; Cook, M.B.; Fraumeni, J.F., Jr.; Hoover, R.N. Increased Risk of Non-Fatal Myocardial Infarction Following Testosterone Therapy Prescription in Men. PLoS ONE 2014, 9, e85805. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.P.; Pereira, A.; Guedes, H.; Lourenço, C.; Azevedo, J.; Pinto, P. Anabolic Drugs and Myocardial Infarction—A Clinical Case Report. Arq. Bras. Cardiol. 2015, 105, 316–319. [Google Scholar] [CrossRef]
- Ciliberti, G.; Westaby, J.; Papadakis, M.; Behr, E.R.; Sharma, S.; Finocchiaro, G.; Sheppard, M.N. Coronary Artery Dissection and Myocardial Infarction with Nonobstructed Coronary Arteries: Insights From a UK Nationwide Autopsy-Based Registry—Brief Report. Arter. Thromb. Vasc. Biol. 2023, 43, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2021, 118, 2281–2292. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotka, M.; Małek, Ł.A. Myocardial Infarction in Young Athletes. Diagnostics 2023, 13, 2473. https://doi.org/10.3390/diagnostics13152473
Dotka M, Małek ŁA. Myocardial Infarction in Young Athletes. Diagnostics. 2023; 13(15):2473. https://doi.org/10.3390/diagnostics13152473
Chicago/Turabian StyleDotka, Mariusz, and Łukasz A. Małek. 2023. "Myocardial Infarction in Young Athletes" Diagnostics 13, no. 15: 2473. https://doi.org/10.3390/diagnostics13152473
APA StyleDotka, M., & Małek, Ł. A. (2023). Myocardial Infarction in Young Athletes. Diagnostics, 13(15), 2473. https://doi.org/10.3390/diagnostics13152473





