From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus: A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct, and Early microRNA Signatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric and Laboratory Determinations
2.3. Circulating miRNAs Profiling
2.4. Global Ct Mean Normalization and Bioinformatic Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Popupulation
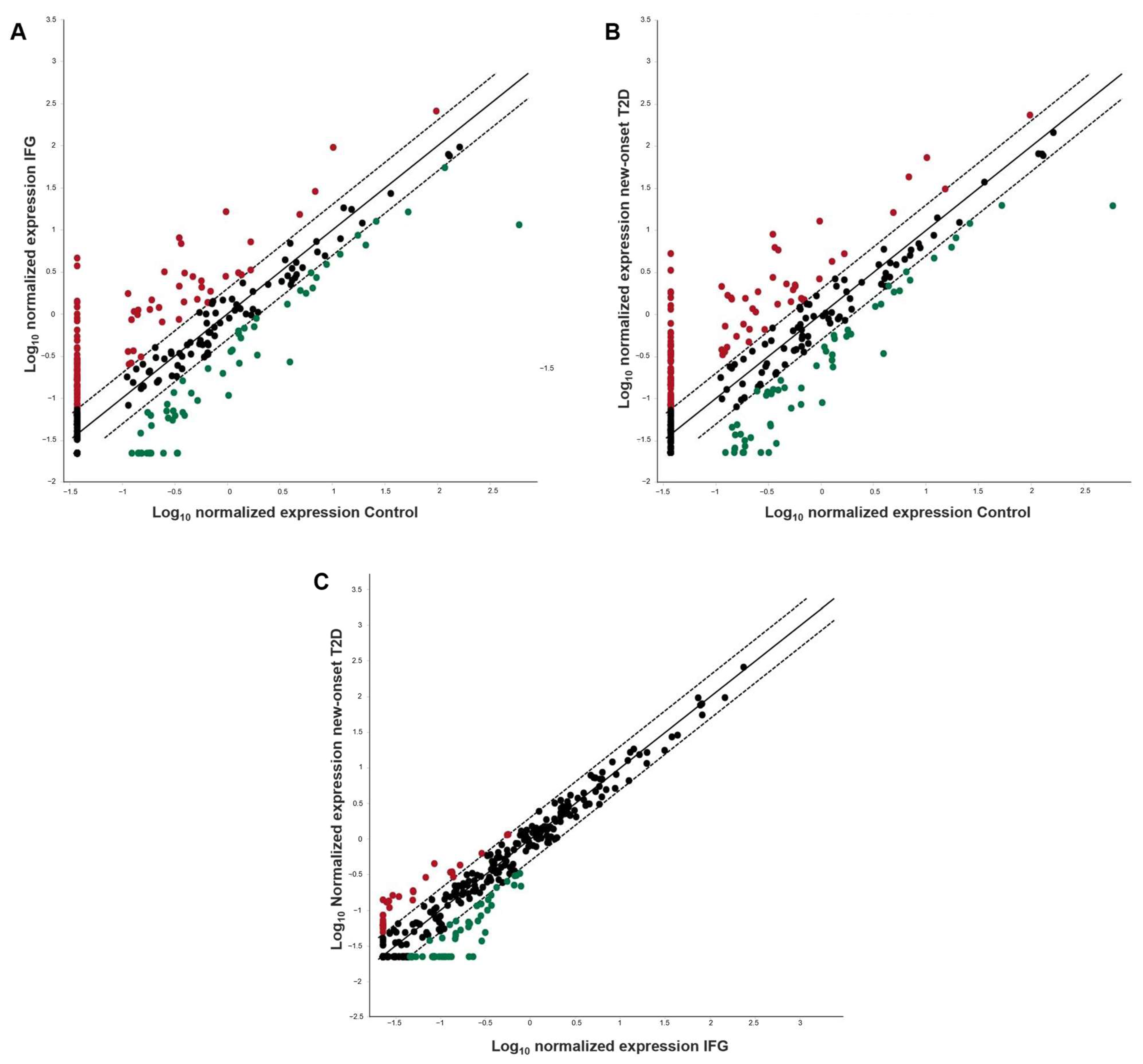
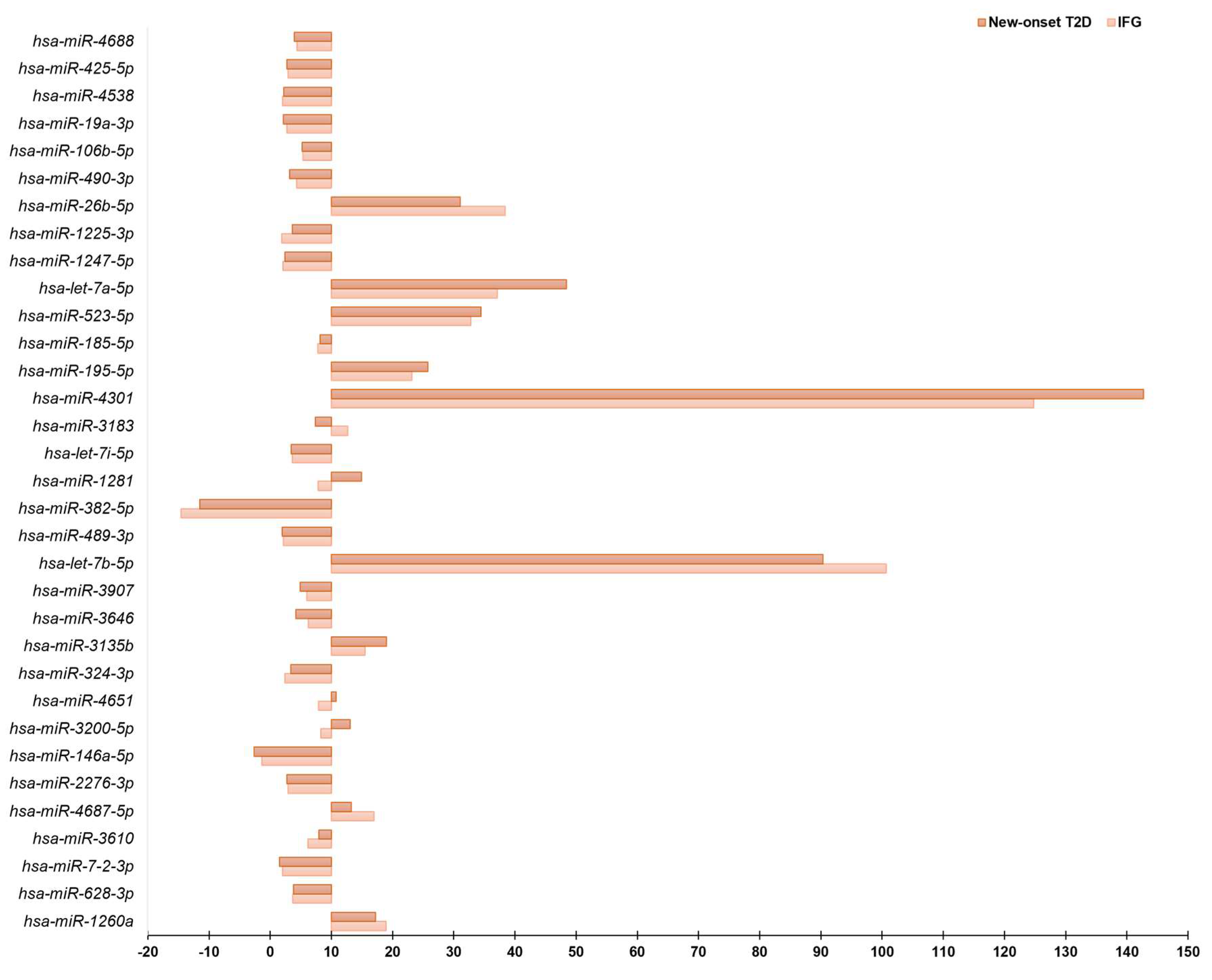
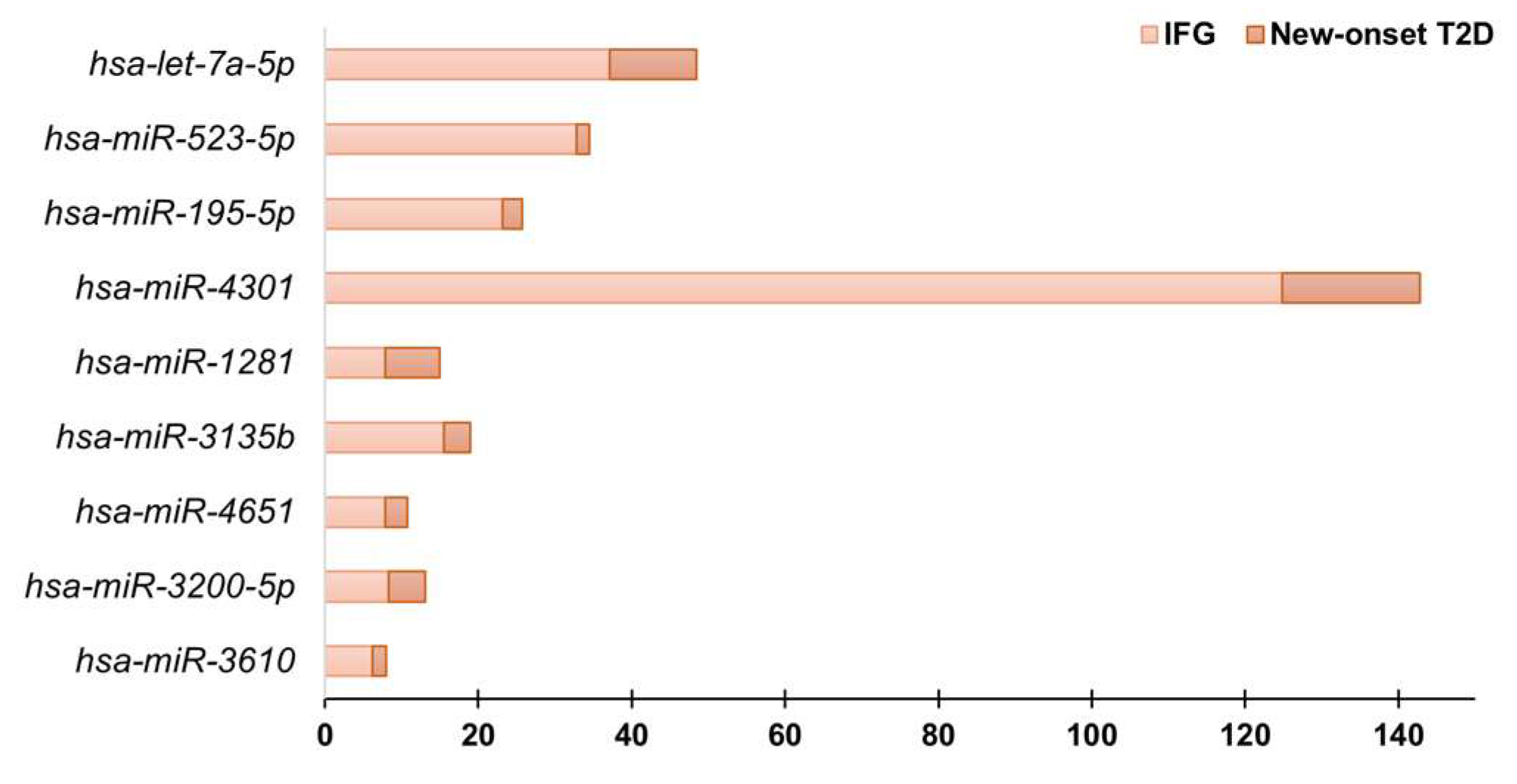
3.2. Circulating miRNAs Profiling by Real-Time PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Inukai, S.; Slack, F. MicroRNAs and the genetic network in aging. J. Mol. Biol. 2013, 425, 3601–3608. [Google Scholar] [CrossRef]
- Garzon, R.; Croce, C.M. MicroRNAs in normal and malignant hematopoiesis. Curr. Opin. Hematol. 2008, 15, 352–358. [Google Scholar] [CrossRef]
- Latronico, M.V.; Condorelli, G. MicroRNAs and cardiac pathology. Nat. Rev. Cardiol. 2009, 6, 419–429. [Google Scholar] [CrossRef]
- Dimmeler, S.; Nicotera, P. MicroRNAs in age-related diseases. EMBO Mol. Med. 2013, 5, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Steitz, J.A. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 2007, 128, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.-H.; Miano, J.M.; Ivey, K.N.; Srivastava, D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef]
- Strachan, T.; Read, A. Human Molecular Genetics; Chapter 9; Organization of the Human Genome: Geneva, Switzerland, 2010. [Google Scholar]
- Friedländer, M.R.; Lizano, E.; Houben, A.J.; Bezdan, D.; Báñez-Coronel, M.; Kudla, G.; Mateu-Huertas, E.; Kagerbauer, B.; González, J.; Chen, K.C.; et al. Evidence for the biogenesis of more than 1000 novel human microRNAs. Genome Biol. 2014, 15, R57. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Mirabelli, M.; Salatino, A.; Pullano, S.A.; Aversa, A.; Foti, D.P.; Brunetti, A. Insights into the World of MicroRNAs. In Biomarkers in Diabetes. Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Greco, M.; Presta, P.; Duni, A.; Vita, C.; Pappas, E.; Mirabelli, M.; Lakkas, L.; Naka, K.K.; Brunetti, A.; et al. A small circulating miRNAs signature predicts mortality and adverse cardiovascular outcomes in chronic hemodialysis patients. Clin. Kidney J. 2023, 16, 868–878. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients with Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 528. [Google Scholar] [CrossRef]
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol. Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 736–743. [Google Scholar] [CrossRef]
- Ortiz-Martínez, M.; González-González, M.; Martagón, A.J.; Hlavinka, V.; Willson, R.C.; Rito-Palomares, M. Recent Developments in Biomarkers for Diagnosis and Screening of Type 2 Diabetes Mellitus. Curr. Diabetes Rep. 2022, 22, 95–115. [Google Scholar] [CrossRef]
- Nigi, L.; Grieco, G.E.; Ventriglia, G.; Brusco, N.; Mancarella, F.; Formichi, C.; Dotta, F.; Sebastiani, G. MicroRNAs as Regulators of Insulin Signaling: Research Updates and Potential Therapeutic Perspectives in Type 2 Diabetes. Int. J. Mol. Sci. 2018, 19, 3705. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S. microRNAs in Pancreatic β-Cell Physiology. Adv. Exp. Med. Biol. 2015, 887, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Mahdy, H.A.; Gomaa Eldeib, M.; Doghish, A.S. miRNAs as cornerstones in diabetic microvascular complications. Mol. Genet. Metab. 2023, 138, 106978. [Google Scholar] [CrossRef]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407-15. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Niemira, M.; Maliszewska, K.; Erol, A.; Bielska, A.; Szalkowska, A.; Adamska-Patruno, E.; Szczerbinski, L.; Gorska, M.; Kretowski, A. Circulating miRNAs as a Predictive Biomarker of the Progression from Prediabetes to Diabetes: Outcomes of a 5-Year Prospective Observational Study. J. Clin. Med. 2020, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Salatino, A.; Mirabelli, M.; Chiefari, E.; Greco, M.; Di Vito, A.; Bonapace, G.; Brunetti, F.S.; Crocerossa, F.; Epstein, A.L.; Foti, D.P.; et al. The anticancer effects of Metformin in the male germ tumor SEM-1 cell line are mediated by HMGA1. Front. Endocrinol. 2022, 13, 1051988. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.J.F.; Floege, J.; Biessen, E.A.L.; Jankowski, J.; van der Vorst, E.P.C. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int. J. Mol. Sci. 2020, 21, 6547. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Mirabelli, M.; Salatino, A.; Tocci, V.; Cianfrone, P.; Foti, D.P.; Brunetti, A. Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines 2022, 3, 175–186. [Google Scholar] [CrossRef]
- Kang, K.; Peng, X.; Luo, J.; Gou, D. Identification of circulating miRNA biomarkers based on global quantitative real-time PCR profiling. J. Anim. Sci. Biotechnol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Chekka, L.M.S.; Langaee, T.; Johnson, J.A. Comparison of Data Normalization Strategies for Array-Based MicroRNA Profiling Experiments and Identification and Validation of Circulating MicroRNAs as Endogenous Controls in Hypertension. Front. Genet. 2022, 13, 836636. [Google Scholar] [CrossRef]
- Marima, R.; Hull, R.; Dlamini, Z.; Penny, C. The profiling, identification, quantification and analysis of differentially expressed genes (DEGs) in response to drug treatment in lung cancer. MethodsX 2021, 8, b101381. [Google Scholar] [CrossRef]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rasoulinejad, S.A.; Akbari, A.; Nasiri, K. Interaction of miR-146a-5p with oxidative stress and inflammation in complications of type 2 diabetes mellitus in male rats: Anti-oxidant and anti-inflammatory protection strategies in type 2 diabetic retinopathy. Iran. J. Basic Med. Sci. 2021, 24, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.Y.; Wang, S.J.; Wang, H.J. Non-canonical Interaction Between O-Linked N-Acetylglucosamine Transferase and miR-146a-5p Aggravates High Glucose-Induced Endothelial Inflammation. Front. Physiol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, B.; Ghaedi, H.; Meshkani, R.; Torkamandi, S.; Saffari, S.; Iranpour, M.; Omrani, M.D. Association of MiR-146a Expression and Type 2 Diabetes Mellitus: A Meta-Analysis. Int. J. Mol. Cell Med. 2017, 6, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef]
- Régnier, C.H.; Song, H.Y.; Gao, X.; Goeddel, D.V.; Cao, Z.; Rothe, M. Identification and characterization of an IkappaB kinase. Cell 1997, 90, 373–383. [Google Scholar] [CrossRef]
- Woronicz, J.D.; Gao, X.; Cao, Z.; Rothe, M.; Goeddel, D.V. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science 1997, 278, 866–869. [Google Scholar] [CrossRef]
- Sgarra, R.; Pegoraro, S.; Ros, G.; Penzo, C.; Chiefari, E.; Foti, D.; Brunetti, A.; Manfioletti, G. High Mobility Group A (HMGA) proteins: Molecular instigators of breast cancer onset and progression. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 216–229. [Google Scholar] [CrossRef]
- Chiefari, E.; Foti, D.P.; Sgarra, R.; Pegoraro, S.; Arcidiacono, B.; Brunetti, F.S.; Greco, M.; Manfioletti, G.; Brunetti, A. Transcriptional Regulation of Glucose Metabolism: The Emerging Role of the HMGA1 Chromatin Factor. Front. Endocrinol. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Brunetti, L.; Foti, D.; Accili, D.; Goldfine, I.D. Human diabetes associated with defects in nuclear regulatory proteins for the insulin receptor gene. J. Clin. Investig. 1996, 97, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Nevolo, M.T.; Arcidiacono, B.; Maurizio, E.; Nocera, A.; Iiritano, S.; Sgarra, R.; Possidente, K.; Palmieri, C.; Paonessa, F.; et al. HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci. Rep. 2012, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Tanyolaç, S.; Paonessa, F.; Pullinger, C.R.; Capula, C.; Iiritano, S.; Mazza, T.; Forlin, M.; Fusco, A.; Durlach, V.; et al. Functional variants of the HMGA1 gene and type 2 diabetes mellitus. JAMA. 2011, 305, 903–912. [Google Scholar] [CrossRef]
- Chanprasertyothin, S.; Jongjaroenprasert, W.; Ongphiphadhanakul, B. The Association of Soluble IGF2R and IGF2R Gene Polymorphism with Type 2 Diabetes. J. Diabetes Res. 2015, 2015, 216383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Pei, D.; Hung, Y.J.; Lee, C.H.; Hsiao, F.C.; Wu, C.Z.; Lin, J.; Hsu, C.; Chang, J.; Hsieh, C. Associations between genetic variants and the severity of metabolic syndrome in subjects with type 2 diabetes. Genet. Mol. Res. 2015, 14, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, J.; Grinstein, S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004, 447, 549–565. [Google Scholar] [CrossRef]
- Bellomo, E.A.; Meur, G.; Rutter, G.A. Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. J. Biol. Chem. 2011, 286, 25778–25789. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol. Asp. Med. 2013, 34, 220–235. [Google Scholar] [CrossRef]
- Gurzov, E.N.; Stanley, W.J.; Brodnicki, T.C.; Thomas, H.E. Protein tyrosine phosphatases: Molecular switches in metabolism and diabetes. Trends Endocrinol. Metab. 2015, 26, 30–39. [Google Scholar] [CrossRef]
- Nasarre, L.; Juan-Babot, O.; Gastelurrutia, P.; Llucia-Valldeperas, A.; Badimon, L.; Bayes-Genis, A.; Llorente-Cortés, V. Low density lipoprotein receptor-related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Acta Diabetol. 2014, 51, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.L.; Vicenta, L.C.; Victor, G.C.; Dolores, L.C.; Fernando, C.; Ricardo, G.H.; Lina, B. Alterations of specific biomarkers of metabolic pathways in vascular tree from patients with Type 2 diabetes. Cardiovasc. Diabetol. 2012, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Mirabelli, M.; La Vignera, S.; Tanyolaç, S.; Foti, D.P.; Aversa, A.; Brunetti, A. Insulin Resistance and Cancer: In Search for a Causal Link. Int. J. Mol. Sci. 2021, 22, 11137. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Meyerovich, K.; Ortis, F.; Cardozo, A.K. The non-canonical NF-κB pathway and its contribution to β-cell failure in diabetes. J. Mol. Endocrinol. 2018, 61, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Chiefari, E.; Foryst-Ludwig, A.; Currò, G.; Navarra, G.; Brunetti, F.S.; Mirabelli, M.; Corigliano, D.M.; Kintscher, U.; Britti, D.; et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. eBioMedicine 2020, 59, 102912. [Google Scholar] [CrossRef]
- Greco, M.; Mirabelli, M.; Tocci, V.; Mamula, Y.; Salatino, A.; Brunetti, F.S.; Dragone, F.; Sicilia, L.; Tripolino, O.; Chiefari, E.; et al. Prothymosin-Alpha, a Novel and Sensitive Biomarker of the Inflammatory and Insulin-Resistant Statuses of Obese Individuals: A Pilot Study Involving Humans. Endocrines 2023, 4, 427–436. [Google Scholar] [CrossRef]
- De Silva, K.; Demmer, R.T.; Jönsson, D.; Mousa, A.; Forbes, A.; Enticott, J. A data-driven biocomputing pipeline with meta-analysis on high throughput transcriptomics to identify genome-wide miRNA markers associated with type 2 diabetes. Heliyon 2022, 8, e08886. [Google Scholar] [CrossRef]
- Zahari Sham, S.Y.; Ng, C.T.; Azwar, S.; Yip, W.K.; Abdullah, M.; Thevandran, K.; Osman, M.; Seow, H.F. Circulating miRNAs in Type 2 Diabetic Patients with and without Albuminuria in Malaysia. Kidney Blood Press. Res. 2022, 47, 81–93. [Google Scholar] [CrossRef]
- Singh, T.; Kaushik, M.; Mishra, L.C.; Behl, C.; Singh, V.; Tuli, H.S. Exosomal miRNAs as novel avenues for breast cancer treatment. Front. Genet. 2023, 14, 1134779. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 10) | IFG (n = 10) | New-Onset T2D (n = 10) | p Value | |
|---|---|---|---|---|
| Female sex (%) | 6 (60%) | 6 (60%) | 6 (60%) | - |
| Age (yrs) | 53.50 (47–58) | 51.50 (47–61) | 58 (57–65) | 0.167 |
| BMI (kg/m2) | 26 (22–28) | 28 (25–30) | 29 (27–30) | 0.060 |
| SBP (mmHg) | 120 (119–120) | 120 (120–130) | 120 (120–125) | 0.170 |
| DBP (mmHg) | 80 (78–80) | 85 (80–85) | 80 (80–85) | 0.048 |
| Fasting glucose (mg/dL) | 88 (83–91) | 113 (105–118) | 135 (118–165) | <0.001 |
| HbA1c (%) | 5.10 (4.98–5.38) | 5.70 (5.43–6.10) | 6.20 (6.0–6.60) | <0.001 |
| Total cholesterol (mg/dL) | 179 (160–200) | 204 (178–223) | 185 (165–210) | 0.337 |
| LDL-cholesterol (mg/dL) | 110 (93–125) | 142 (109–158) | 113 (103–125) | 0.133 |
| HDL-cholesterol (mg/dL) | 57 (44–60) | 42 (37–56) | 55 (40–56) | 0.207 |
| Triglycerides (mg/dL) | 88 (75–127) | 103 (67–158) | 120 (93–144) | 0.480 |
| CRP (mg/L) | 3.16 (3.16–3.36) | 3.45 (3.16–10) | 4.08 (0.64–5.75) | 0.659 |
| ESR (mm/h) | 6.50 (4.75–14.25) | 9.50 (3.75–28.25) | 9.0 (5.0–10) | 0.806 |
| T2D duration (yrs) | - | - | 2 (0–2) | - |
| IFG vs. Control | New-Onset T2D vs. Control | New-Onset T2D vs. IFG | |||
|---|---|---|---|---|---|
| Mature ID | Fold Regulation | Mature ID | Fold Regulation | Mature ID | Fold Regulation |
| hsa-miR-4301 | 124.8 | hsa-miR-4301 | 142.74 | hsa-miR-4732-3p | 10.29 |
| hsa-let-7b-5p | 100.66 | hsa-let-7b-5p | 90.34 | hsa-miR-142-5p | 9.28 |
| hsa-miR-26b-5p | 38.41 | hsa-miR-1287-5p | 50.12 | hsa-miR-877-3p | 7.75 |
| hsa-let-7a-5p | 37.1 | hsa-let-7a-5p | 48.41 | hsa-miR-3141 | 6.43 |
| hsa-miR-523-5p | 32.75 | hsa-miR-1913 | 42.73 | hsa-miR-2467-3p | 5.91 |
| hsa-miR-1287-5p | 26.97 | hsa-miR-523-5p | 34.47 | hsa-miR-7-5p | 5.52 |
| hsa-miR-1913 | 24.14 | hsa-miR-26b-5p | 31.06 | hsa-miR-324-5p | 5.11 |
| hsa-miR-195-5p | 23.16 | hsa-miR-195-5p | 25.76 | hsa-miR-942-5p | 4.87 |
| hsa-miR-100-5p | 22.37 | hsa-miR-1587 | 21.07 | hsa-miR-550a-5p | 4.64 |
| hsa-miR-1260a | 18.94 | hsa-miR-3135b | 18.99 | hsa-miR-10b-5p | 4.33 |
| hsa-miR-365b-3p | 17.43 | hsa-miR-1260a | 17.23 | hsa-miR-361-3p | 3.87 |
| hsa-miR-4687-5p | 16.95 | hsa-miR-1260b | 16.53 | hsa-miR-2110 | 3.85 |
| hsa-miR-3135b | 15.49 | hsa-miR-20b-5p | 15.97 | hsa-miR-548o-5p | 3.79 |
| hsa-let-7f-5p | 14.66 | hsa-miR-1281 | 14.9 | hsa-miR-331-3p | 3.74 |
| hsa-miR-3131 | 13.12 | hsa-miR-3131 | 14.49 | hsa-miR-769-5p | 3.66 |
| hsa-miR-3183 | 12.67 | hsa-miR-4687-5p | 13.24 | hsa-miR-18a-5p | 3.66 |
| hsa-miR-20b-5p | 10.8 | hsa-miR-3200-5p | 13.06 | hsa-miR-3191-3p | 3.64 |
| hsa-miR-1260b | 10.44 | hsa-miR-100-5p | 12.88 | hsa-miR-140-5p | 3.61 |
| hsa-miR-223-3p | 9.4 | hsa-let-7c-5p | 11.94 | hsa-miR-1587 | 3.59 |
| hsa-miR-370-3p | 8.9 | hsa-miR-365b-3p | 11.77 | hsa-miR-130b-3p | 3.49 |
| hsa-miR-3200-5p | 8.3 | hsa-let-7f-5p | 11.37 | hsa-miR-99a-5p | 3.35 |
| hsa-miR-3911 | 8.02 | hsa-miR-671-3p | 11.29 | hsa-miR-3185 | 3.33 |
| hsa-miR-4651 | 7.91 | hsa-miR-625-3p | 11.29 | hsa-miR-596 | 3.3 |
| hsa-miR-1281 | 7.85 | hsa-miR-4651 | 10.76 | hsa-miR-181c-5p | 3.26 |
| hsa-miR-378g | 7.85 | hsa-miR-4505 | 9.9 | hsa-miR-138-1-3p | 3.17 |
| hsa-miR-30e-3p | 7.8 | hsa-miR-144-3p | 9.76 | hsa-miR-4505 | 3.15 |
| hsa-miR-185-5p | 7.75 | hsa-miR-1237-3p | 9.56 | hsa-miR-1539 | 3.1 |
| hsa-miR-1237-3p | 7.59 | hsa-miR-378g | 9.36 | hsa-miR-29b-3p | 3 |
| hsa-let-7c-5p | 7.23 | hsa-miR-1183 | 9.11 | hsa-miR-193a-5p | 2.92 |
| hsa-miR-144-3p | 7.08 | hsa-miR-596 | 8.92 | hsa-miR-376c-3p | 2.82 |
| hsa-miR-671-3p | 7.08 | hsa-miR-1910-5p | 8.86 | hsa-miR-3176 | 2.56 |
| hsa-miR-101-3p | 7.03 | hsa-miR-3141 | 8.44 | hsa-miR-1183 | 2.4 |
| hsa-miR-1301-3p | 6.88 | hsa-miR-185-5p | 8.15 | hsa-miR-501-5p | 2.38 |
| hsa-miR-1910-5p | 6.7 | hsa-miR-3610 | 7.93 | hsa-miR-574-3p | 2.32 |
| hsa-miR-1290 | 6.38 | hsa-miR-877-3p | 7.71 | hsa-miR-1207-5p | 2.3 |
| hsa-miR-3646 | 6.2 | hsa-miR-1207-5p | 7.61 | hsa-miR-28-3p | 2.27 |
| hsa-miR-3610 | 6.16 | hsa-miR-3911 | 7.55 | hsa-miR-874-3p | 2.24 |
| hsa-miR-4291 | 5.95 | hsa-miR-99a-5p | 7.55 | hsa-miR-92b-3p | 2.19 |
| hsa-miR-3907 | 5.95 | hsa-miR-3183 | 7.4 | hsa-miR-361-5p | 2.19 |
| hsa-miR-1203 | 5.95 | hsa-let-7e-5p | 7.35 | hsa-miR-3622a-5p | 2.18 |
| hsa-miR-1587 | 5.87 | hsa-miR-223-3p | 7.2 | hsa-miR-339-3p | 2.16 |
| hsa-miR-4732-5p | 5.79 | hsa-miR-373-5p | 6.31 | hsa-miR-375 | 2.16 |
| hsa-miR-625-3p | 5.63 | hsa-miR-4274 | 6.18 | hsa-miR-4306 | 2.13 |
| hsa-miR-106b-5p | 5.36 | hsa-miR-370-3p | 6.05 | hsa-miR-154-5p | 2.12 |
| hsa-miR-181c-3p | 5.25 | hsa-miR-101-3p | 5.93 | hsa-miR-199b-5p | 2.1 |
| hsa-miR-18a-3p | 5.08 | hsa-miR-181c-5p | 5.69 | hsa-miR-454-3p | 2.08 |
| hsa-miR-4274 | 5.01 | hsa-miR-4689 | 5.65 | hsa-miR-1976 | 2.02 |
| hsa-miR-219a-1-3p | 4.8 | hsa-miR-142-5p | 5.57 | hsa-miR-378b | 2.01 |
| hsa-let-7e-5p | 4.74 | hsa-miR-1301-3p | 5.53 | hsa-miR-625-3p | 2.01 |
| hsa-miR-34c-3p | 4.54 | hsa-miR-106b-5p | 5.23 | ||
| hsa-miR-877-5p | 4.51 | hsa-miR-3907 | 4.92 | ||
| hsa-miR-1909-5p | 4.48 | hsa-miR-1290 | 4.81 | ||
| hsa-miR-146b-5p | 4.45 | hsa-miR-877-5p | 4.71 | ||
| hsa-miR-4688 | 4.39 | hsa-miR-1909-5p | 4.68 | ||
| hsa-miR-363-3p | 4.39 | hsa-miR-4732-5p | 4.68 | ||
| hsa-miR-490-3p | 4.33 | hsa-miR-320e | 4.52 | ||
| hsa-miR-373-5p | 4.18 | hsa-miR-138-1-3p | 4.49 | ||
| hsa-miR-4689 | 3.93 | hsa-let-7d-5p | 4.46 | ||
| hsa-miR-4267 | 3.85 | hsa-miR-10a-5p | 4.25 | ||
| hsa-miR-1183 | 3.79 | hsa-miR-1203 | 4.25 | ||
| hsa-miR-629-5p | 3.77 | hsa-miR-3646 | 4.19 | ||
| hsa-miR-10a-5p | 3.69 | hsa-miR-34c-3p | 4.05 | ||
| hsa-miR-628-3p | 3.66 | hsa-miR-181c-3p | 4.02 | ||
| hsa-miR-345-5p | 3.64 | hsa-miR-1539 | 3.96 | ||
| hsa-let-7i-5p | 3.61 | hsa-miR-3185 | 3.96 | ||
| hsa-miR-320e | 3.4 | hsa-miR-4688 | 3.96 | ||
| hsa-let-7d-5p | 3.37 | hsa-miR-3191-3p | 3.91 | ||
| hsa-miR-1207-5p | 3.3 | hsa-miR-4291 | 3.88 | ||
| hsa-miR-27b-3p | 3.17 | hsa-miR-363-3p | 3.88 | ||
| hsa-miR-4505 | 3.15 | hsa-miR-219a-1-3p | 3.83 | ||
| hsa-miR-4516 | 3.12 | hsa-miR-628-3p | 3.8 | ||
| hsa-miR-330-3p | 3.06 | hsa-miR-1225-3p | 3.62 | ||
| hsa-miR-487b-3p | 2.94 | hsa-miR-2467-3p | 3.55 | ||
| hsa-miR-425-5p | 2.91 | hsa-miR-375 | 3.5 | ||
| hsa-miR-2276-3p | 2.89 | hsa-let-7i-5p | 3.43 | ||
| hsa-miR-675-3p | 2.87 | hsa-miR-324-3p | 3.36 | ||
| hsa-miR-605-5p | 2.78 | hsa-miR-27b-3p | 3.36 | ||
| hsa-miR-596 | 2.7 | hsa-miR-4516 | 3.33 | ||
| hsa-miR-19a-3p | 2.7 | hsa-miR-92b-3p | 3.2 | ||
| hsa-miR-451a | 2.65 | hsa-miR-675-3p | 3.2 | ||
| hsa-miR-7-1-3p | 2.54 | hsa-miR-490-3p | 3.15 | ||
| hsa-miR-127-3p | 2.5 | hsa-miR-146b-5p | 3.09 | ||
| hsa-miR-324-3p | 2.43 | hsa-miR-4267 | 2.98 | ||
| hsa-miR-193b-3p | 2.33 | hsa-miR-425-3p | 2.96 | ||
| hsa-miR-378a-5p | 2.3 | hsa-miR-942-5p | 2.92 | ||
| hsa-miR-99a-5p | 2.26 | hsa-miR-374c-5p | 2.86 | ||
| hsa-miR-374c-5p | 2.19 | hsa-miR-3176 | 2.8 | ||
| hsa-miR-425-3p | 2.16 | hsa-miR-550a-5p | 2.78 | ||
| hsa-miR-489-3p | 2.15 | hsa-miR-2276-3p | 2.75 | ||
| hsa-miR-1247-5p | 2.12 | hsa-miR-425-5p | 2.75 | ||
| hsa-miR-188-5p | 2.1 | hsa-miR-190a-5p | 2.67 | ||
| hsa-miR-4422 | 2.06 | hsa-miR-10b-5p | 2.6 | ||
| hsa-miR-664a-3p | 2.06 | hsa-miR-139-3p | 2.58 | ||
| hsa-miR-7-2-3p | 2 | hsa-miR-188-5p | 2.46 | ||
| hsa-miR-4538 | 2 | hsa-miR-451a | 2.41 | ||
| hsa-miR-1247-5p | 2.39 | ||||
| hsa-miR-664a-3p | 2.37 | ||||
| hsa-miR-361-3p | 2.32 | ||||
| hsa-miR-2110 | 2.31 | ||||
| hsa-miR-605-5p | 2.29 | ||||
| hsa-miR-4302 | 2.29 | ||||
| hsa-miR-1180-3p | 2.28 | ||||
| hsa-miR-548o-5p | 2.28 | ||||
| hsa-miR-193b-3p | 2.28 | ||||
| hsa-miR-331-3p | 2.25 | ||||
| hsa-miR-7-1-3p | 2.25 | ||||
| hsa-miR-769-5p | 2.2 | ||||
| hsa-miR-4538 | 2.18 | ||||
| hsa-miR-140-5p | 2.17 | ||||
| hsa-miR-19a-3p | 2.17 | ||||
| hsa-miR-378b | 2.04 | ||||
| hsa-miR-4454 | 2.04 | ||||
| IFG vs. Control | New-Onset T2D vs. Control | New-Onset T2D vs. IFG | |||
|---|---|---|---|---|---|
| Mature ID | Fold Regulation | Mature ID | Fold Regulation | Mature ID | Fold Regulation |
| hsa-miR-328-3p | −52.22 | hsa-miR-328-3p | −30.54 | hsa-miR-629-5p | −6.13 |
| hsa-miR-324-5p | −15.1 | hsa-miR-598-3p | −13.96 | hsa-miR-152-3p | −5.53 |
| hsa-miR-154-5p | −14.89 | hsa-miR-152-3p | −12.84 | hsa-miR-1307-3p | −5.3 |
| hsa-miR-382-5p | −14.59 | hsa-miR-502-3p | −11.74 | hsa-miR-3651 | −5.23 |
| hsa-miR-7-5p | −11.05 | hsa-miR-382-5p | −11.49 | hsa-miR-345-5p | −5.08 |
| hsa-miR-409-3p | −9.49 | hsa-miR-409-3p | −11.49 | hsa-miR-4323 | −4.52 |
| hsa-miR-210-3p | −8.44 | hsa-miR-181d-5p | −8.07 | hsa-miR-30e-3p | −4.25 |
| hsa-miR-181d-5p | −8.26 | hsa-miR-28-5p | −7.91 | hsa-miR-487b-3p | −4.05 |
| hsa-miR-28-5p | −8.09 | hsa-miR-1307-3p | −7.58 | hsa-miR-18a-3p | −3.83 |
| hsa-miR-4258 | −7.71 | hsa-miR-154−5p | -7.03 | hsa-miR-378a-5p | −3.75 |
| hsa-miR-340-5p | −6.85 | hsa-miR-424-3p | −6.98 | hsa-miR-502-3p | −3.72 |
| hsa-miR-627-5p | −6.8 | hsa-miR-132-3p | −6.79 | hsa-miR-181a-5p | −3.67 |
| hsa-miR-4732-3p | −6.35 | hsa-miR-181a-5p | −6.65 | hsa-miR-378e | −3.2 |
| hsa-miR-1976 | −6.26 | hsa-miR-30a-3p | −6.56 | hsa-miR-590-5p | −3 |
| hsa-miR-151a-5p | −5.92 | hsa-miR-4323 | −6.25 | hsa-miR-421 | −2.84 |
| hsa-miR-34a-5p | −5.53 | hsa-miR-3651 | −6.03 | hsa-miR-301a-3p | −2.84 |
| hsa-miR-29b-3p | −5.53 | hsa-miR-210-3p | −5.91 | hsa-miR-206 | −2.74 |
| hsa-miR-132-3p | −5.53 | hsa-miR-627-5p | −5.87 | hsa-let-7a-3p | −2.72 |
| hsa-miR-224-5p | −5.49 | hsa-miR-339-5p | −5.47 | hsa-miR-598-3p | −2.72 |
| hsa-miR-598-3p | −5.12 | hsa-miR-34a-5p | −5.4 | hsa-miR-374a-5p | −2.72 |
| hsa-miR-339-5p | −4.98 | hsa-miR-199a-5p | −4.87 | hsa-miR-26b-3p | −2.67 |
| hsa-miR-130b-3p | −4.68 | hsa-miR-4258 | −4.57 | hsa-miR-192-5p | −2.67 |
| hsa-miR-130a-3p | −4.58 | hsa-miR-378a-3p | −4.45 | hsa-miR-505-3p | −2.61 |
| hsa-miR-18a-5p | −4.31 | hsa-miR-340-5p | −4.12 | hsa-miR-199a-5p | −2.6 |
| hsa-miR-424-3p | −3.99 | hsa-miR-93-3p | −3.87 | hsa-miR-3923 | −2.58 |
| hsa-miR-361-5p | −3.94 | hsa-miR-130a-3p | −3.69 | hsa-miR-3653-3p | −2.51 |
| hsa-miR-30a-3p | −3.88 | hsa-miR-15a-5p | −3.35 | hsa-miR-15a-5p | −2.49 |
| hsa-miR-199b-5p | −3.75 | hsa-miR-151a-5p | −3.3 | hsa-miR-1-3p | −2.42 |
| hsa-miR-16-5p | −3.22 | hsa-miR-421 | −3.26 | hsa-miR-378a-3p | −2.2 |
| hsa-miR-342-3p | −3.17 | hsa-miR-199b-3p | −3.19 | hsa-miR-485-5p | −2.15 |
| hsa-miR-93-5p | −3.17 | hsa-miR-1976 | −3.1 | hsa-miR-338-3p | −2.12 |
| hsa-miR-150-5p | −3.15 | hsa-miR-194-5p | −3.06 | hsa-miR-199b-3p | −2.08 |
| hsa-miR-502-3p | −3.15 | hsa-miR-324-5p | −2.95 | hsa-miR-151b | −2.05 |
| hsa-miR-194-5p | −2.98 | hsa-miR-93-5p | −2.93 | ||
| hsa-miR-5095 | −2.98 | hsa-miR-125a-5p | −2.81 | ||
| hsa-miR-193a-5p | −2.9 | hsa-miR-191-5p | −2.78 | ||
| hsa-miR-125a-5p | −2.84 | hsa-miR-224-5p | −2.76 | ||
| hsa-miR-433-3p | −2.65 | hsa-miR-151a-3p | −2.76 | ||
| hsa-miR-24-3p | −2.6 | hsa-miR-24-3p | −2.76 | ||
| hsa-miR-19b-3p | −2.6 | hsa-miR-192-5p | −2.74 | ||
| hsa-miR-186-5p | −2.58 | hsa-miR-19b-3p | −2.68 | ||
| hsa-miR-130b-5p | −2.56 | hsa-miR-16-5p | −2.66 | ||
| hsa-miR-151a-3p | −2.54 | hsa-miR-146a-5p | −2.64 | ||
| hsa-miR-152-3p | −2.32 | hsa-miR-186-5p | −2.61 | ||
| hsa-miR-484 | −2.31 | hsa-miR−433-3p | −2.59 | ||
| hsa-miR-320a | −2.26 | hsa-miR-126-3p | −2.55 | ||
| hsa-miR-93-3p | −2.2 | hsa-miR-505-3p | −2.52 | ||
| hsa-let-7g-5p | −2.14 | hsa-miR-21-5p | −2.37 | ||
| hsa-miR-92a-3p | −2.14 | hsa-miR-151b | −2.35 | ||
| hsa-let-7d-3p | −2.12 | hsa-miR-25-3p | −2.16 | ||
| hsa-miR-25-3p | −2.07 | hsa-miR-342-3p | −2.02 | ||
| hsa-miR-423-5p | −2.02 | hsa-miR-15b-5p | −2.02 | ||
| hsa-miR-191-5p | −2.02 | hsa-miR-7-5p | −2 | ||
| hsa-miR-378a-3p | −2.02 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, M.; Mirabelli, M.; Salatino, A.; Accattato, F.; Aiello, V.; Brunetti, F.S.; Chiefari, E.; Pullano, S.A.; Fiorillo, A.S.; Foti, D.P.; et al. From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus: A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct, and Early microRNA Signatures. Diagnostics 2023, 13, 2443. https://doi.org/10.3390/diagnostics13142443
Greco M, Mirabelli M, Salatino A, Accattato F, Aiello V, Brunetti FS, Chiefari E, Pullano SA, Fiorillo AS, Foti DP, et al. From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus: A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct, and Early microRNA Signatures. Diagnostics. 2023; 13(14):2443. https://doi.org/10.3390/diagnostics13142443
Chicago/Turabian StyleGreco, Marta, Maria Mirabelli, Alessandro Salatino, Francesca Accattato, Vincenzo Aiello, Francesco S. Brunetti, Eusebio Chiefari, Salvatore A. Pullano, Antonino S. Fiorillo, Daniela P. Foti, and et al. 2023. "From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus: A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct, and Early microRNA Signatures" Diagnostics 13, no. 14: 2443. https://doi.org/10.3390/diagnostics13142443
APA StyleGreco, M., Mirabelli, M., Salatino, A., Accattato, F., Aiello, V., Brunetti, F. S., Chiefari, E., Pullano, S. A., Fiorillo, A. S., Foti, D. P., & Brunetti, A. (2023). From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus: A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct, and Early microRNA Signatures. Diagnostics, 13(14), 2443. https://doi.org/10.3390/diagnostics13142443














