Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools
Abstract
1. Introduction
2. Objectives of the Study
3. Materials and Methods
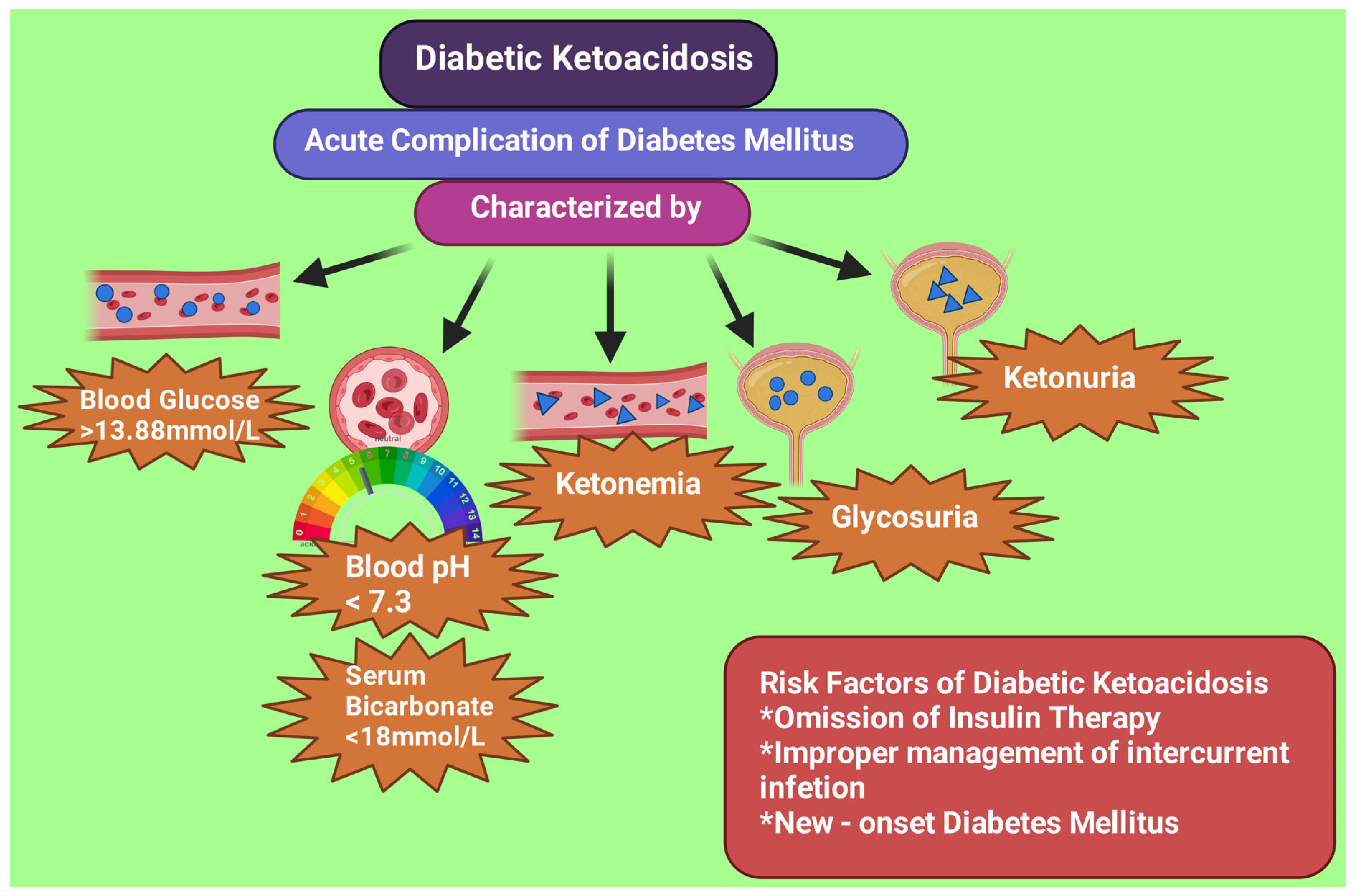
4. Biochemical Criteria for DKA
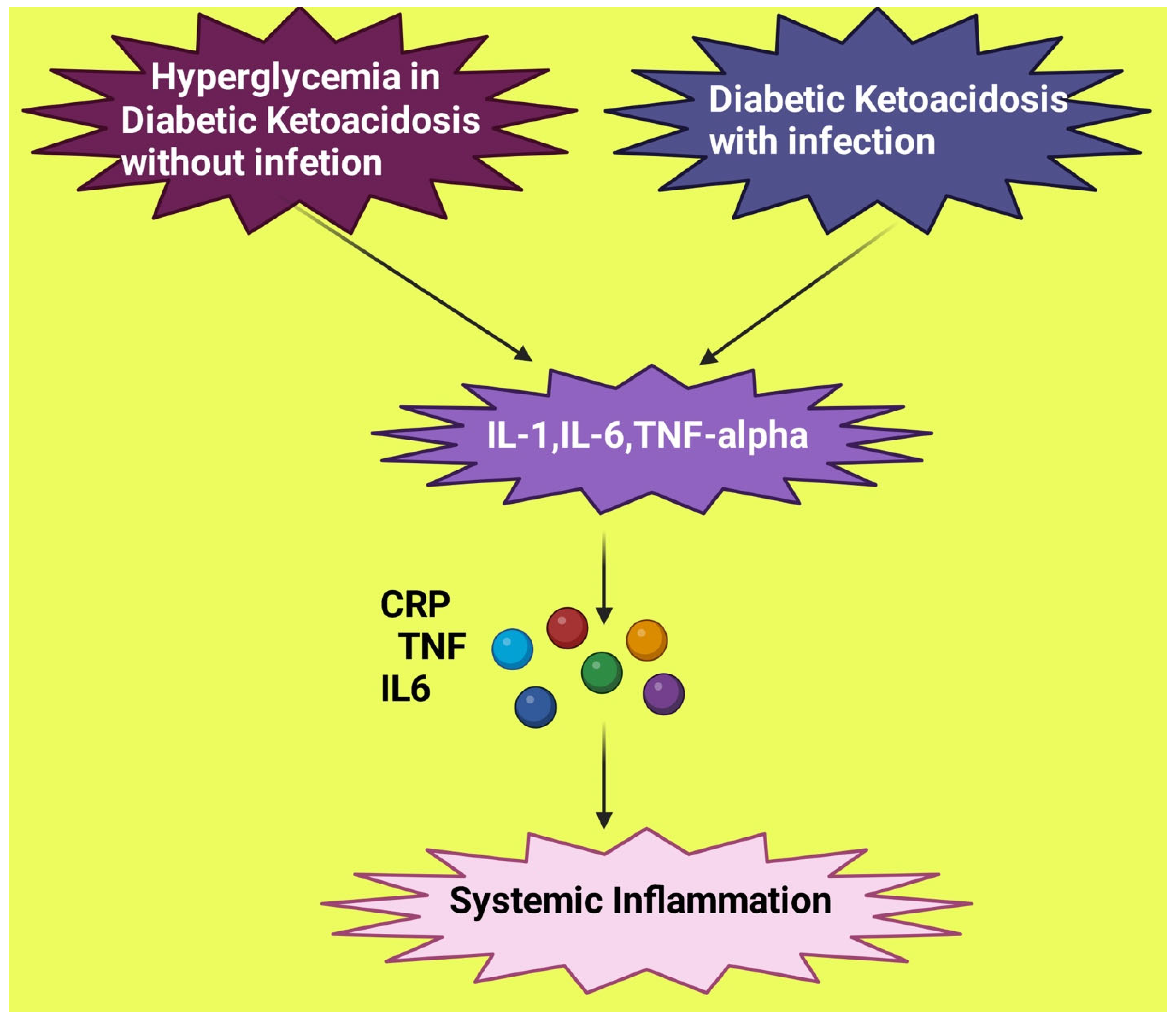
5. DKA Mimicking Bacterial Infection
6. Diagnostic Tools for DKA
6.1. The Link between Serum Lactate and Severity of DKA
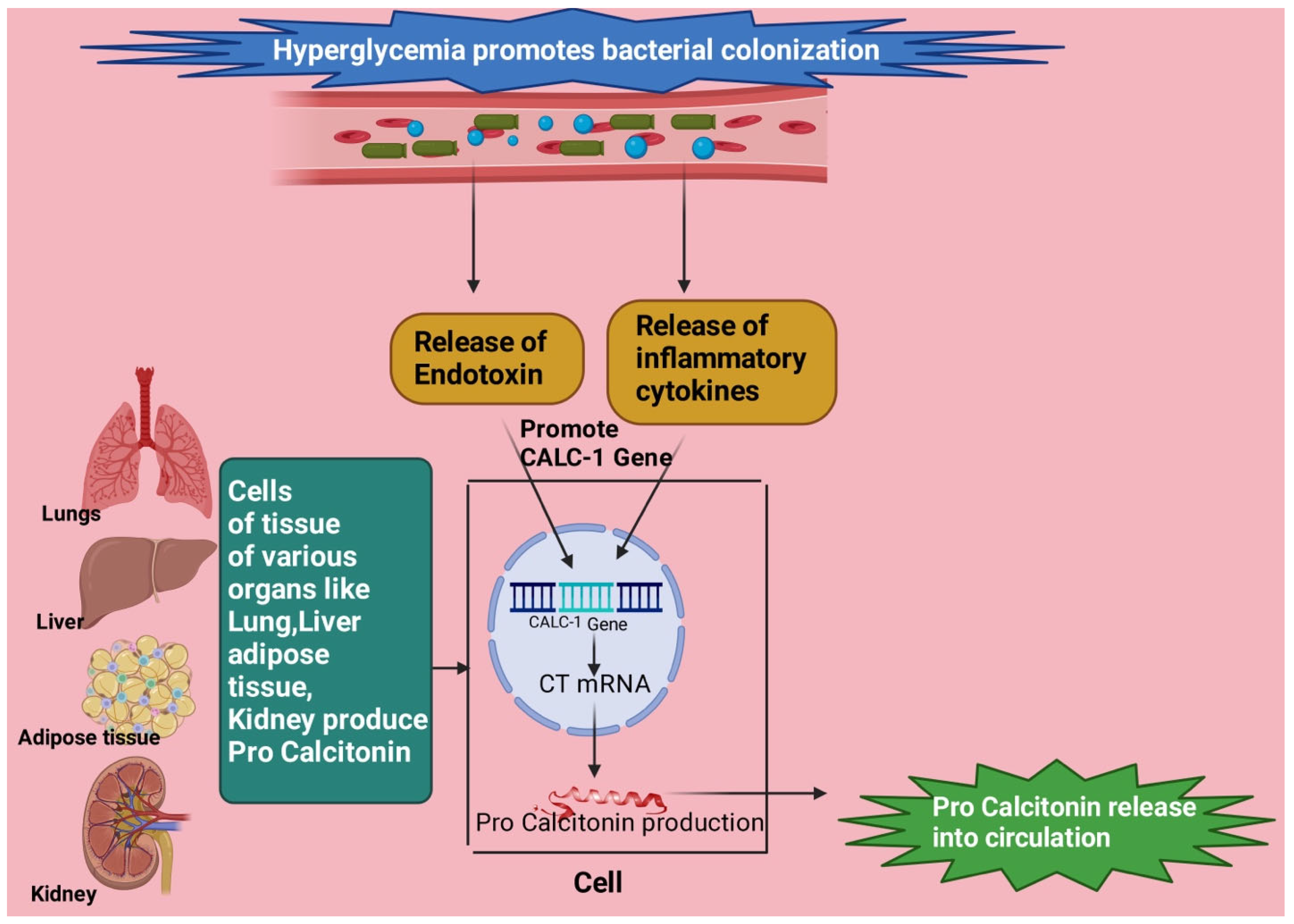
6.2. Procalcitonin (PCT) in DKA
6.3. PCT Levels in Infection
6.4. PCT in DKA Patients with Infection
6.5. Procalcitonin-to-Lactate Ratio (PLR) for Diagnosing DKA with Infection
6.6. Leucocyte Count for DKA with Infection
6.7. Neutrophil-to-Lymphocyte Ratio (NLR) in DKA Patients
6.8. C Reactive Protein (CRP) and Interleukin-6 in DKA with Infection
6.9. Body Temperature in DKA
6.10. Combining Several Biomarkers to Make a Probable Diagnosis of DKA with Infection
6.11. Limitations of this Paper
7. Conclusions
8. Recommendation
9. Article Highlights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Savage, M.W.; Dhatariya, K.K.; Kilvert, A.; Rayman, G.; Rees, J.A.; Courtney, C.H.; Hilton, L.; Dyer, P.H.; Hamersley, M.S.; Joint British Diabetes Societies. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet. Med. 2011, 28, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Huang, C.H.; Lin, W.R.; Lu, P.L.; Chang, K.; Tsai, J.J.; Bojang, K.S.; Lin, C.Y.; Chen, Y.H. Clinical outcomes of septic patients with diabetic ketoacidosis between 2004 and 2013 in a tertiary hospital in Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Alourfi, Z.; Homsi, H. Precipitating factors, outcomes, and recurrence of diabetic ketoacidosis at a university hospital in Damascus. Avicenna J. Med. 2015, 5, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, S.; Ye, S. Systemic Infection Predictive Value of Procalcitonin to Lactic Acid Ratio in Diabetes Ketoacidosis Patients. Diabetes Metab. Syndr. Obes. 2022, 15, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, J.; Wang, G.; Zhong, S.; Su, X.; Huang, Z.; Chen, G.; Zhang, J.; Hou, X.; Yu, X.; et al. Clinical profile of diabetic ketoacidosis in tertiary hospitals in China: A multicentre, clinic-based study. Diabet. Med. 2016, 33, 261–268. [Google Scholar] [CrossRef]
- Mahesh, M.G.; Shivaswamy, R.P.; Chandra, B.S.; Syed, S. The Study of Different Clinical Pattern of Diabetic Ketoacidosis and Common Precipitating Events and Independent Mortality Factors. J. Clin. Diagn. Res. 2017, 11, OC42–OC46. [Google Scholar] [CrossRef]
- Liu, W.Y.; Lin, S.G.; Wang, L.R.; Fang, C.C.; Lin, Y.Q.; Braddock, M.; Zhu, G.Q.; Zhang, Z.; Zheng, M.H.; Shen, F.X. Platelet-to-Lymphocyte Ratio: A Novel Prognostic Factor for Prediction of 90-day Outcomes in Critically Ill Patients With Diabetic Ketoacidosis. Medicine 2016, 95, e2596. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.J. Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz. J. Med. Biol. Res. 2017, 50, e6021. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Lizzo, J.M.; Goyal, A.; Gupta, V. Adult Diabetic Ketoacidosis. [Updated 2 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560723/ (accessed on 28 June 2023).
- Charoenpiriya, A.; Chailurkit, L.; Ongphiphadhanakul, B. Comparisons of biochemical parameters and diabetic ketoacidosis severity in adult patients with type 1 and type 2 diabetes. BMC Endocr. Disord. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. [Updated 9 May 2021]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279052/ (accessed on 28 June 2023).
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, C.A.; Nakhla, M.; Derraik, J.G.; Gunn, A.J.; Daneman, D.; Cutfield, W.S. Preventing Diabetic Ketoacidosis. Pediatr. Clin. N. Am. 2015, 62, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Ata, F.; Iqbal, P.; Bashir, M.; Kartha, A. Clinical and biochemical predictors of intensive care unit admission among patients with diabetic ketoacidosis. World J. Diabetes 2023, 14, 271–278. [Google Scholar] [CrossRef]
- Wolfsdorf, J.; Craig, M.E.; Daneman, D.; Dunger, D.; Edge, J.; Lee, W.R.; Rosenbloom, A.; Sperling, M.A.; Hanas, R.; International Society for Pediatric and Adolescent Diabetes. Diabetic ketoacidosis. Pediatr. Diabetes 2007, 8, 28–43. [Google Scholar] [CrossRef]
- Karavanaki, K.; Karanika, E.; Georga, S.; Bartzeliotou, A.; Tsouvalas, M.; Konstantopoulos, I.; Fotinou, A.; Papassotiriou, I.; Karayianni, C. Cytokine response to diabetic ketoacidosis (DKA) in children with type 1 diabetes (T1DM). Endocr. J. 2011, 58, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, W.H.; Burek, C.L.; Waller, J.L.; Fisher, L.E.; Khichi, M.; Mellick, L.B. Cytokine response to diabetic ketoacidosis and its treatment. Clin. Immunol. 2003, 108, 175–181. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Murphy, M.B.; Kreisberg, R.A. Hyperglycemic crises in adult patients with diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 2739–2748. [Google Scholar] [CrossRef]
- Van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010, 199, 499–508. [Google Scholar] [CrossRef]
- Krzymień, J.; Karnafel, W. Lactic acidosis in patients with diabetes. Pol. Arch. Med. Wewn. 2013, 123, 91–97. [Google Scholar] [CrossRef]
- Gur, A.; Ulutas, Z.; Turgut, K.; Guven, T.; Yucel, N.; Ermis, N. The effect of lactate levels on prognosis in patients with ST-segment elevation myocardial infarction. Ann. Med. Res. 2020, 27, 1924–1929. [Google Scholar] [CrossRef]
- Unal, E.; Pirinccioglu, A.G.; Yanmaz, S.Y.; Yılmaz, K.; Taşkesen, M.; Haspolat, Y.K. A Different Perspective of Elevated Lactate in Pediatric Patients with Diabetic Ketoacidosis. Acta Endocrinol. 2020, 16, 114–117. [Google Scholar] [CrossRef]
- Cully, M.; Thompson, A.D.; DePiero, A.D. Is lactic acidosis predictive of outcomes in pediatric diabetic ketoacidosis? Am. J. Emerg. Med. 2020, 38, 329–332. [Google Scholar] [CrossRef] [PubMed]
- English, P.; Williams, G. Hyperglycaemic crises and lactic acidosis in diabetes mellitus. Postgrad. Med. J. 2004, 80, 253–261. [Google Scholar] [CrossRef]
- Masharani, U.; Strycker, L.A.; Lazar, A.A.; Wu, K.; Brooks, G.A. Hyperlactatemia in diabetic ketoacidosis. Diabet. Med. 2022, 39, e14723. [Google Scholar] [CrossRef]
- Cox, K.; Cocchi, M.N.; Salciccioli, J.D.; Carney, E.; Howell, M.; Donnino, M.W. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J. Crit. Care 2012, 27, 132–137. [Google Scholar] [CrossRef]
- Morgan, T.J.; Scott, P.H.; Anstey, C.M.; Bowling, F.G. Hyperlactatemia in diabetic ketoacidosis is common and can be prolonged: Lactate time-series from 25 intensive care admissions. J. Clin. Monit. Comput. 2021, 35, 757–764. [Google Scholar] [CrossRef]
- Cetin, M.; Kilic, T.Y.; Yesilaras, M.; Uz, I. Clinical utiliy of serum lactate levels in diabetic ketoacidosis in adult patients admitted to emergency department. Ann. Med. Res. 2022, 29, 827–830. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef]
- Howell, M.D.; Donnino, M.; Clardy, P.; Talmor, D.; Shapiro, N.I. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007, 33, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Howell, M.D.; Talmor, D.; Nathanson, L.A.; Lisbon, A.; Wolfe, R.E.; Weiss, J.W. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 2005, 45, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, H.; Li, Y. Hyperlactatemia associated with diabetic ketoacidosis in pediatric intensive care unit. BMC Endocr. Disord. 2021, 21, 110. [Google Scholar] [CrossRef]
- Saad, S.; Mohamed, N.; Moghazy, A.; Ellabban, G.; El-Kamash, S. Venous glucose, serum lactate and base deficit as biochemical predictors of mortality in patients with polytrauma. Ulus. Travma Acil Cerrahi Derg. 2016, 22, 29–33. [Google Scholar] [CrossRef]
- Mistry, N.; Sobolewski, K.; Brophy, A.; Patrick, H.; Patel, A.; Vikraman, P.K. Accuracy of procalcitonin in identifying patients presenting with diabetic ketoacidosis as a result of an infectious etiology. J. Lab. Precis. Med. 2022, 7, 2. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Fischer-Schulz, C.; Seidel-Mueller, E.; Beier, W.; Bergmann, A. Detection of procalcitonin (PCT) in healthy controls and patients with local infection by a sensitive ILMA. Clin. Lab. 2002, 48, 263–270. [Google Scholar] [PubMed]
- Christ-Crain, M.; Müller, B. Biomarkers in respiratory tract infections: Diagnostic guides to antibiotic prescription, prognostic markers, and mediators. Eur. Respir. J. 2007, 30, 556–573. [Google Scholar] [CrossRef]
- Gilbert, D.N. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J. Clin. Microbiol. 2010, 48, 2325–2329. [Google Scholar] [CrossRef]
- Pfäfflin, A.; Schleicher, E. Inflammation markers in point-of-care testing (POCT). Anal. Bioanal. Chem. 2009, 393, 1473–1480. [Google Scholar] [CrossRef]
- Brunkhorst, F.M.; Heinz, U.; Forycki, Z.F. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998, 24, 888–889. [Google Scholar] [CrossRef]
- Limper, M.; de Kruif, M.D.; Duits, A.J.; Brandjes, D.P.; van Gorp, E.C. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J. Infect. 2010, 60, 409–416. [Google Scholar] [CrossRef]
- Lee, H. Procalcitonin as a biomarker of infectious diseases. Korean J. Intern. Med. 2013, 28, 285–291. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin assay in systemic inflammation, infection, and sepsis: Clinical utility and limitations. Crit. Care Med. 2008, 36, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.E.; Fiechtl, J.F.; Brown, M.D.; Ballew, J.J.; Kline, J.A. Procalcitonin test in the diagnosis of bacteremia: A meta-analysis. Ann. Emerg. Med. 2007, 50, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Mega, A.; Grecka, P.; Scarpa, N.; Koratzanis, G.; Thomopoulos, G.; Giamarellou, H. Procalcitonin: A marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med. 2002, 28, 1351–1356. [Google Scholar] [CrossRef]
- Dalton, R.R.; Hoffman, W.H.; Passmore, G.G.; Martin, S.L. Plasma C-reactive protein levels in severe diabetic ketoacidosis. Ann. Clin. Lab. Sci. 2003, 33, 435–442. [Google Scholar]
- Jafar, N.; Edriss, H.; Nugent, K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am. J. Med. Sci. 2016, 351, 201–211. [Google Scholar] [CrossRef]
- Fukunaga, S.; Hoshino, Y.; Sonoda, H.; Kawanishi, M.; Yamauchi, A.; Kato, S.; Yoshikane, K.; Shiina, H.; Tanabe, K.; Ito, T. A Remarkable Elevation in the Procalcitonin Levels Due to Diabetic Ketoacidosis in a Hemodialysis Patient. Intern. Med. 2021, 60, 1231–1235. [Google Scholar] [CrossRef]
- Ivaska, L.; Elenius, V.; Mononen, I.; Ruuskanen, O.; Peltola, V. Discrepancies between plasma procalcitonin and C-reactive protein levels are common in acute illness. Acta Paediatr. 2016, 105, 508–513. [Google Scholar] [CrossRef]
- Anno, T.; Shigemoto, R.; Kawasaki, F.; Irie, S.; Miyashita, N.; Kaku, K.; Kaneto, H. Marked elevation of plasma procalcitonin levels in patients with diabetic ketoacidosis: A possible useful diagnostic biomarker. Diabetes Metab. 2020, 46, 504–505. [Google Scholar] [CrossRef]
- Aksu, N.M.; Aksoy, D.Y.; Akkaş, M.; Yilmaz, H.; Akman, C.; Yildiz, B.O.; Ozmen, M.M.; Usman, A. 25-OH-Vitamin D and procalcitonin levels after correction of acute hyperglycemia. Med. Sci. Monit. 2013, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Charbit, J.; Van der Meersch, G.; Popoff, B.; Picod, A.; Cohen, R.; Chemouni, F.; Gaudry, S.; Bihan, H.; Cohen, Y. Early sepsis markers in patients admitted to intensive care unit with moderate-to-severe diabetic ketoacidosis. Ann. Intensive Care 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Rebelos, E.; Park, N.; Santini, M. Moderate increase of serum levels of procalcitonin in diabetic ketoacidosis. Neth. J. Med. 2018, 76, 454. [Google Scholar]
- Mehanic, S.; Baljic, R. The importance of serum procalcitonin in diagnosis and treatment of serious bacterial infections and sepsis. Mater. Sociomed. 2013, 25, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Y.; Shao, X. Predictive value of procalcitonin for infection of patients with type-2 diabetes mellitus. Exp. Ther. Med. 2019, 18, 722–728. [Google Scholar] [CrossRef]
- Nakamura, M.; Kono, R.; Nomura, S.; Utsunomiya, H. Procalcitonin: Mysterious protein in sepsis. J. Basic Clin. Med. 2013, 2, 7–11. [Google Scholar]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: Systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit. Care Med. 2006, 34, 1996–2003. [Google Scholar] [CrossRef]
- Meisner, M.; Tschaikowsky, K.; Palmaers, T.; Schmidt, J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit. Care 1999, 3, 45–50. [Google Scholar] [CrossRef]
- Reinhart, K.; Meisner, M. Biomarkers in the critically ill patient: Procalcitonin. Crit. Care Clin. 2011, 27, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; Geneva Sepsis Network. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Hausfater, P.; Juillien, G.; Madonna-Py, B.; Haroche, J.; Bernard, M.; Riou, B. Serum procalcitonin measurement as diagnostic and prognostic marker in febrile adult patients presenting to the emergency department. Crit. Care 2007, 11, R60. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017, 15, 15. [Google Scholar] [CrossRef]
- Al-Shammaree, S.A.W.; Abu-ALkaseem, B.A.; Salman, I.N. Procalcitonin levels and other biochemical parameters in patients with or without diabetic foot complications. J. Res. Med. Sci. 2017, 22, 95. [Google Scholar] [CrossRef]
- Rochmah, N.; Faizi, M.; Kurniawan, Y.; Choirunisa, L.; Endaryanto, A. Procalcitonin and White Blood Cells as an Infection Marker in Children with Diabetic Ketoacidosis. J. Indon. Med. Assoc. 2020, 70, 261–264. [Google Scholar] [CrossRef]
- Wolfsdorf, J.; Craig, M.E.; Daneman, D.; Dunger, D.; Edge, J.; Lee, W.; Rosenbloom, A.; Sperling, M.; Hanas, R. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr. Diabetes 2009, 10 (Suppl. S12), 118–133. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wang, X.F.; Li, Z.G.; Liu, Y.F.; Jin, W.M.; Bian, A.L.; Hu, H.Y.; Zhao, H.J. Effect of ratio of serum procalcitonin to lactic acid on diagnosis of infection in patients with diabetic ketoacidosis. Chin. J. Nosocromiology 2019, 29, 1347–1350. [Google Scholar]
- Kitabchi, A.E.; Umpierrez, G.E.; Murphy, M.B.; Kreisberg, R.A. Position statement: Hyperglycemic crises in patients with diabetes mellitus. Diabetes Care 2001, 24, S83–S90. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Umpierrez, G.E. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: Implications for the acute and chronic complications of hyperglycemia. J. Diabetes Complicat. 2012, 26, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Burge, M.R.; Hardy, K.J.; Schade, D.S. Short-term fasting is a mechanism for the development of euglycemic ketoacidosis during periods of insulin deficiency. J. Clin. Endocrinol. Metab. 1993, 76, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, A.L. The management of diabetic ketoacidosis in children. Diabetes Ther. 2010, 1, 103–120. [Google Scholar] [CrossRef]
- Fayfman, M.; Pasquel, F.J.; Umpierrez, G.E. Management of Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Med. Clin. N. Am. 2017, 101, 587–606. [Google Scholar] [CrossRef]
- Jain, S.K.; Kannan, K.; McVie, R. Effect of hyperketonemia on blood monocytes in type-I diabetic patients and apoptosis in cultured U937 monocytes. Antioxid. Redox Signal. 1999, 1, 211–220. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E298–E306. [Google Scholar] [CrossRef]
- Etulain, J.; Negrotto, S.; Carestia, A.; Pozner, R.G.; Romaniuk, M.A.; D’Atri, L.P.; Klement, G.L.; Schattner, M. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb. Haemost. 2012, 107, 99–110. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Stentz, F.B.; Umpierrez, G.E. Diabetic ketoacidosis induces in vivo activation of human T-lymphocytes. Biochem. Biophys. Res. Commun. 2004, 315, 404–407. [Google Scholar] [CrossRef]
- Xu, W.; Wu, H.F.; Ma, S.G.; Bai, F.; Hu, W.; Jin, Y.; Liu, H. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int. J. Med. Sci. 2013, 10, 758–765. [Google Scholar] [CrossRef]
- Karavanaki, K.; Kakleas, K.; Georga, S.; Bartzeliotou, A.; Mavropoulos, G.; Tsouvalas, M.; Vogiatzi, A.; Papassotiriou, I.; Karayianni, C. Plasma high sensitivity C-reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clin. Biochem. 2012, 45, 1383–1388. [Google Scholar] [CrossRef]
- Mertoglu, C.; Gunay, M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S127–S131. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, K.M.; Saulnier, P.J.; Tanamas, S.K.; Vijayakumar, P.; Weil, E.J.; Looker, H.C.; Hanson, R.L.; Lemley, K.V.; Yee, B.; Knowler, W.C.; et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in Type 2 diabetes. Nephrol. Dial. Transplant. 2018, 33, 1001–1009. [Google Scholar] [CrossRef]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Ortega, R.M.; Delgado-Losada, M.L.; López-Parra, A.M.; Aparicio, A. Association between Neutrophil-to-Lymphocyte Ratio with Abdominal Obesity and Healthy Eating Index in a Representative Older Spanish Population. Nutrients 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, Y.H.; Kao, T.W.; Peng, T.C.; Chen, W.L.; Li, Y.W.; Chang, P.K.; Wu, L.W. Neutrophil to lymphocyte ratio as predictor for incident hypertension: A 9-year cohort study in Taiwan. Hypertens. Res. 2019, 42, 1209–1214. [Google Scholar] [CrossRef]
- Nam, K.W.; Kwon, H.M.; Jeong, H.Y.; Park, J.H.; Kim, S.H.; Jeong, S.M. High neutrophil to lymphocyte ratios predict intracranial atherosclerosis in a healthy population. Atherosclerosis 2018, 269, 117–121. [Google Scholar] [CrossRef]
- Wang, J.R.; Chen, Z.; Yang, K.; Yang, H.J.; Tao, W.Y.; Li, Y.P.; Jiang, Z.J.; Bai, C.F.; Yin, Y.C.; Duan, J.M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef]
- Russell, C.D.; Parajuli, A.; Gale, H.J.; Bulteel, N.S.; Schuetz, P.; de Jager, C.P.C.; Loonen, A.J.M.; Merekoulias, G.I.; Baillie, J.K. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 2019, 78, 339–348. [Google Scholar] [CrossRef]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.S.; Mills, P.K.; Srivatsa, S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST-elevation myocardial infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Scutca, A.C.; Nicoară, D.M.; Mărăzan, M.; Brad, G.F.; Mărginean, O. Neutrophil-to-Lymphocyte Ratio Adds Valuable Information Regarding the Presence of DKA in Children with New-Onset T1DM. J. Clin. Med. 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Abeer, M.; El Baky, N.E.D.A.; Kandil, M.E.; Rasshed, I.A.; Ahmed, A.N.; Ibrahim, M.H. Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio in Well Controlled and Uncontrolled Children and Adolescents with Type 1 Diabetes Mellitus. Res. J. Pharm. Biol. Chem. 2021, 12, 13. [Google Scholar]
- Alamri, B.N.; Ferris, J.; Matheson, K.; De Tugwell, B. MON-638 The WBC Differential in Relation to DKA Severity. J. Endocr. Soc. 2020, 4 (Suppl. S1), 638. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, W.; Zhou, Y.; Zhang, T.; Chi, H.; Xu, C. Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells. Open Life Sci. 2021, 16, 1365–1376. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.; Yang, W.; Ren, J.; Ge, W.; Yang, P. Apoptosis as an underlying mechanism in lymphocytes induced by riboflavin and ultraviolet light. Transfus. Apher. Sci. 2020, 59, 102899. [Google Scholar] [CrossRef]
- Mirzaei, S.; Hadadi, Z.; Attar, F.; Mousavi, S.E.; Zargar, S.S.; Tajik, A.; Saboury, A.A.; Rezayat, S.M.; Falahati, M. ROS-mediated heme degradation and cytotoxicity induced by iron nanoparticles: Hemoglobin and lymphocyte cells as targets. J. Biomol. Struct. Dyn. 2018, 36, 4235–4245. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Lou, J.; Zhou, Y.; Bo, L.; Zhu, J.; Zhu, K.; Wan, X.; Cai, Z.; Deng, X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care 2011, 15, R70. [Google Scholar] [CrossRef]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12 (Suppl. S1), S16–S21. [Google Scholar] [CrossRef]
- Gogos, C.A.; Giali, S.; Paliogianni, F.; Dimitracopoulos, G.; Bassaris, H.P.; Vagenakis, A.G. Interleukin-6 and C-reactive protein as early markers of sepsis in patients with diabetic ketoacidosis or hyperosmosis. Diabetologia 2001, 44, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Sigal, R.J.; McGinn, R. Body temperature regulation in diabetes. Temperature 2016, 3, 119–145. [Google Scholar] [CrossRef] [PubMed]
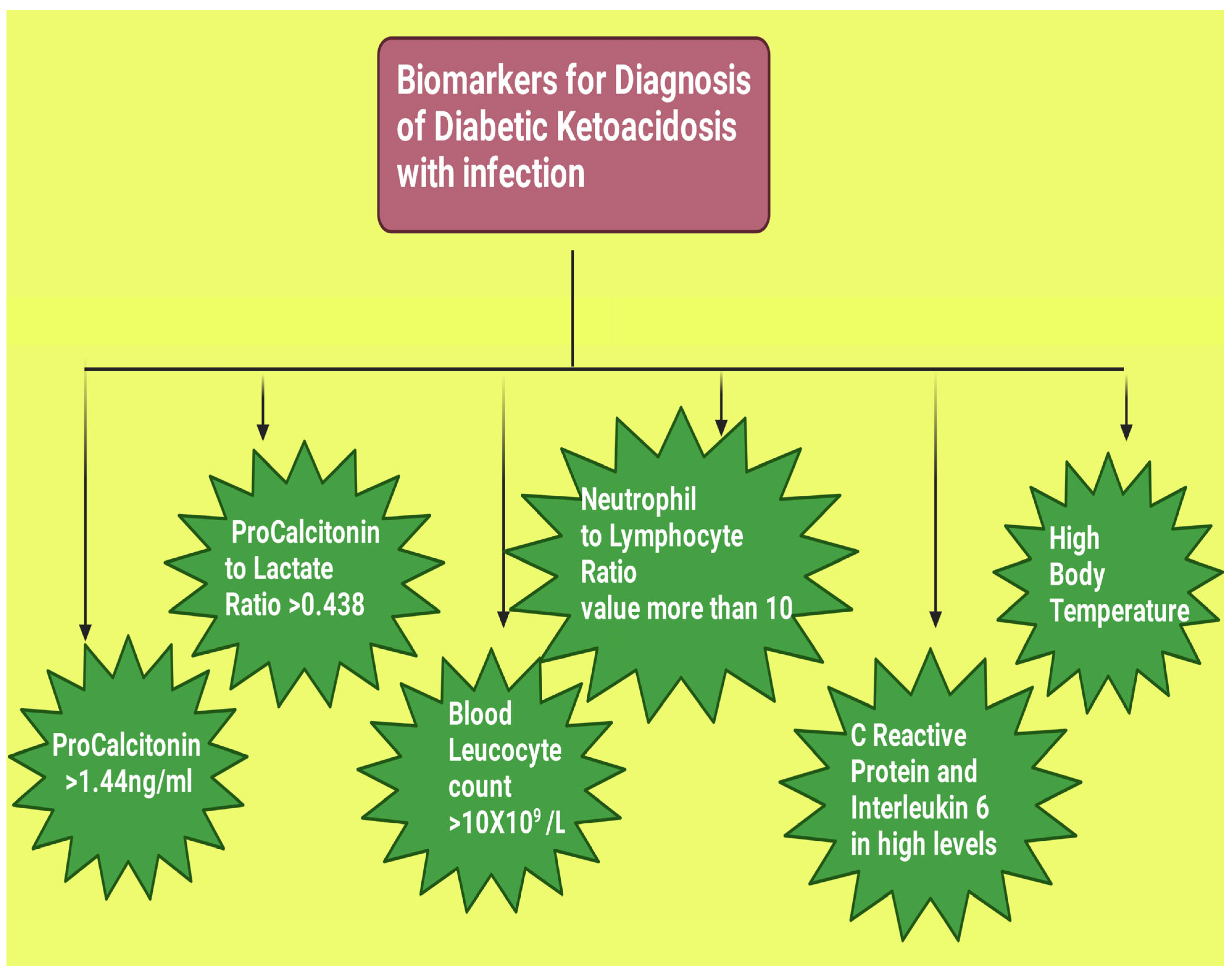
| Biomarkers | Values that May Indicate DKA with Infection |
|---|---|
| PCT level above 1.44 ng/mL on the day of hospital admission is of good predictive value for infection in patients with DKA [55]. |
| PLR threshold level of >0.438 may be considered for identification of infection in its early stages in individuals who develop DKA [4]. |
| The threshold value for the leukocyte count of >10 × 109/L may be considered for DKA with infection [4]. |
| A cut-off value of 10 is usually considered to weakly link to the presence of sepsis, and the higher the value above 10, the more the possibility of a presence of infection [102]. |
| Study conducted by Gogos et al. found a significantly higher CRP level (p < 0.001) and interleukin-6 (p < 0.0001) among patients suffering from DKA with infection when compared to those without infection [103]. |
| Gogos et al. found a body temperature of >38.5 °C in DKA patients with infection [103]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Narwaria, M.; Singh, A.; Kumar, S.; Haque, M. Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnostics 2023, 13, 2441. https://doi.org/10.3390/diagnostics13142441
Ahmad R, Narwaria M, Singh A, Kumar S, Haque M. Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnostics. 2023; 13(14):2441. https://doi.org/10.3390/diagnostics13142441
Chicago/Turabian StyleAhmad, Rahnuma, Mahendra Narwaria, Arya Singh, Santosh Kumar, and Mainul Haque. 2023. "Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools" Diagnostics 13, no. 14: 2441. https://doi.org/10.3390/diagnostics13142441
APA StyleAhmad, R., Narwaria, M., Singh, A., Kumar, S., & Haque, M. (2023). Detecting Diabetic Ketoacidosis with Infection: Combating a Life-Threatening Emergency with Practical Diagnostic Tools. Diagnostics, 13(14), 2441. https://doi.org/10.3390/diagnostics13142441






