Diagnostic Blood Biomarkers for Acute Pulmonary Embolism: A Systematic Review
Abstract
:1. Introduction
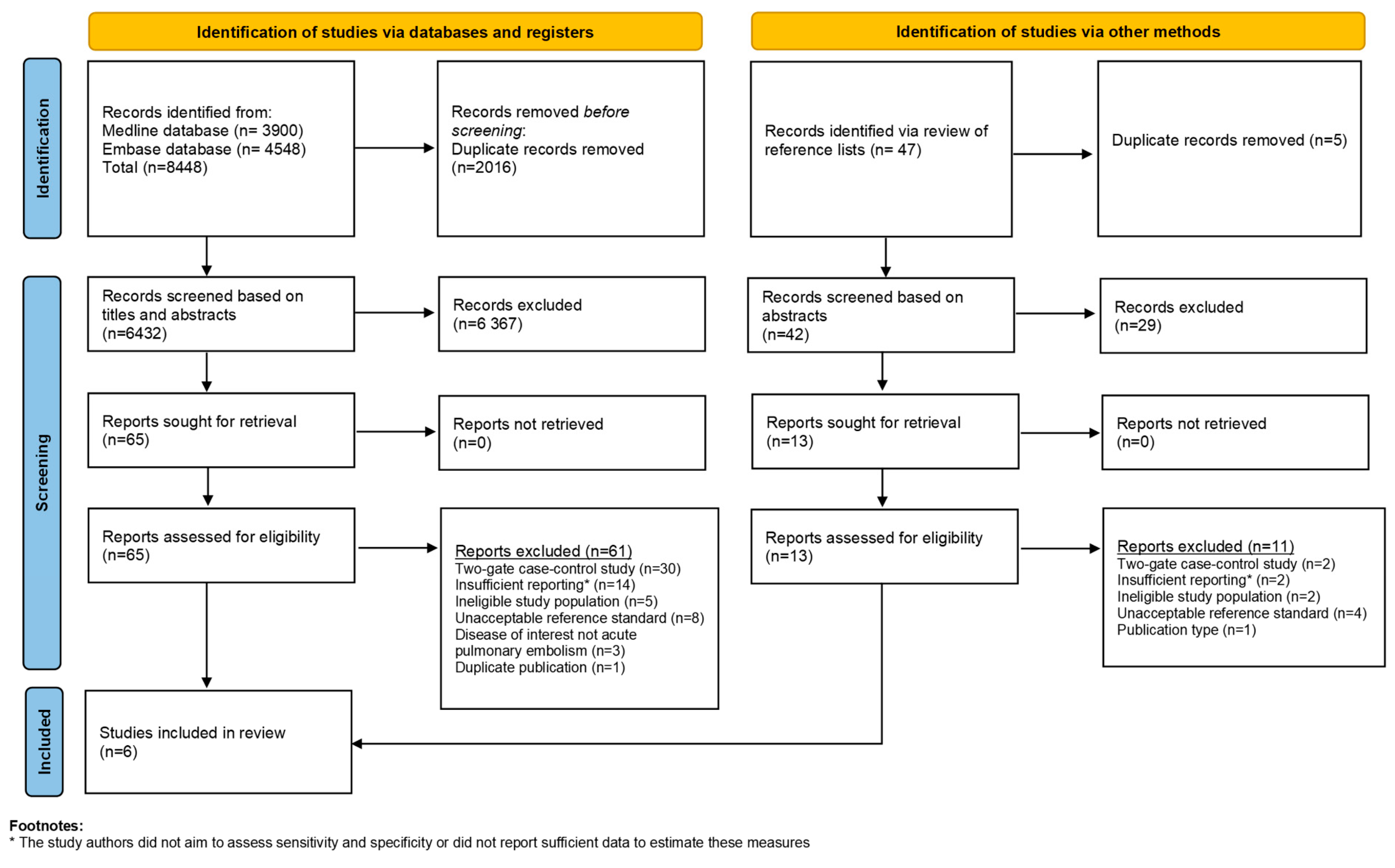
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
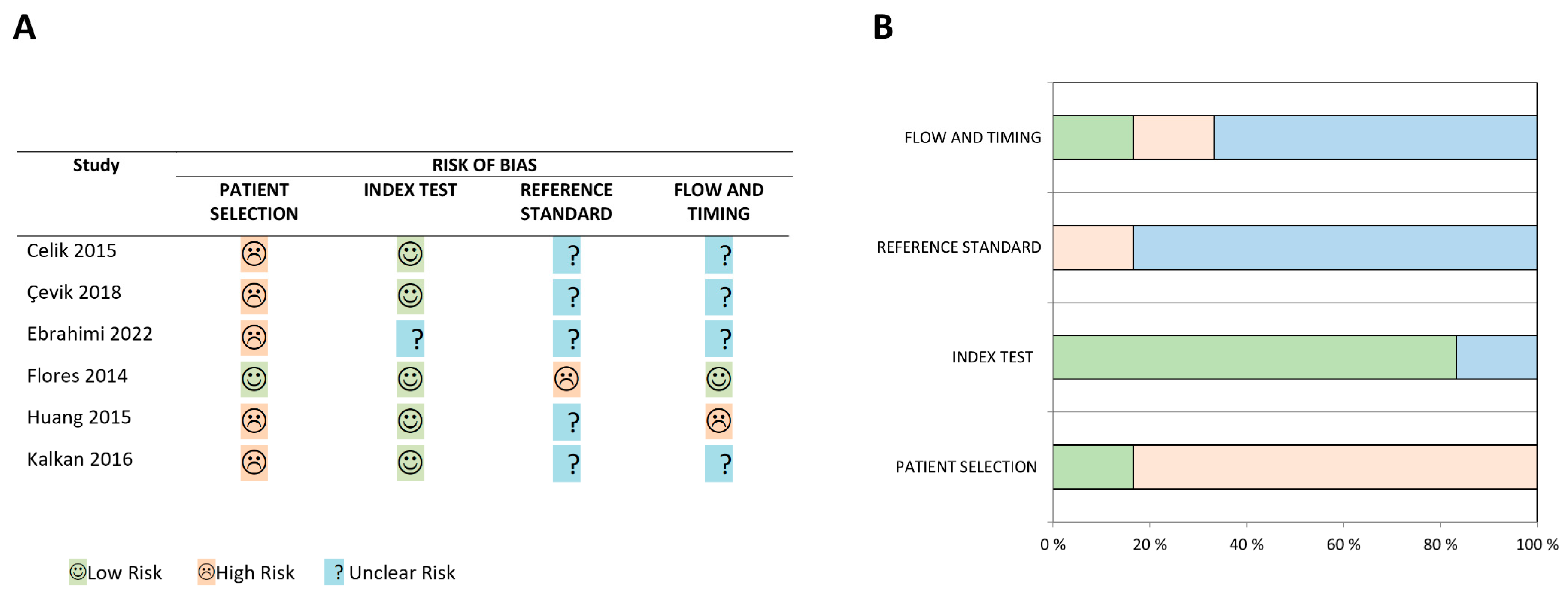
2.4. Risk of Bias Assessments of Included Studies
2.5. Data Synthesis
3. Results
3.1. Risk of Bias Assessment
3.2. Study Characteristics and Findings
4. Discussion
4.1. Summary of Main Findings
4.2. Strengths and Limitations
4.3. Implications for Practice
4.4. Implications for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bikdeli, B.; Wang, Y.; Jimenez, D.; Parikh, S.A.; Monreal, M.; Goldhaber, S.Z.; Krumholz, H.M. Pulmonary Embolism Hospitalization, Readmission, and Mortality Rates in US Older Adults, 1999–2015. JAMA 2019, 322, 574–576. [Google Scholar] [CrossRef]
- Arshad, N.; Isaksen, T.; Hansen, J.-B.; Brækkan, S.K. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur. J. Epidemiol. 2017, 32, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heit, J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 2015, 12, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Tagalakis, V.; Patenaude, V.; Kahn, S.R.; Suissa, S. Incidence of and mortality from venous thromboembolism in a real-world population: The Q-VTE Study Cohort. Am. J. Med. 2013, 126, 832.e13–832.e21. [Google Scholar] [CrossRef] [PubMed]
- Walen, S.; Damoiseaux, R.A.; Uil, S.M.; van den Berg, J.W. Diagnostic delay of pulmonary embolism in primary and secondary care: A retrospective cohort study. Br. J. Gen. Pract. 2016, 66, e444–e450. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.D.; Hasan, O.; Kim, S.; Abrams, R.; Cosby, K.; Lambert, B.L.; Elstein, A.S.; Hasler, S.; Kabongo, M.L.; Krosnjar, N.; et al. Diagnostic error in medicine: Analysis of 583 physician-reported errors. Arch. Intern. Med. 2009, 169, 1881–1887. [Google Scholar] [CrossRef]
- Miniati, M.; Prediletto, R.; Formichi, B.; Marini, C.; Di Ricco, G.; Tonelli, L.; Allescia, G.; Pistolesi, M. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am. J. Respir. Crit. Care Med. 1999, 159, 864–871. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Le Gal, G.; Bates, S.M.; Righini, M.; Haramati, L.B.; Lang, E.; Kline, J.A.; Chasteen, S.; Snyder, M.; Patel, P.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018, 2, 3226–3256. [Google Scholar] [CrossRef] [Green Version]
- Stojanovska, J.; Carlos, R.C.; Kocher, K.E.; Nagaraju, A.; Guy, K.; Kelly, A.M.; Chughtai, A.R.; Kazerooni, E.A. CT Pulmonary Angiography: Using Decision Rules in the Emergency Department. J. Am. Coll. Radiol. 2015, 12, 1023–1029. [Google Scholar] [CrossRef]
- Costantino, M.M.; Randall, G.; Gosselin, M.; Brandt, M.; Spinning, K.; Vegas, C.D. CT angiography in the evaluation of acute pulmonary embolus. AJR Am. J. Roentgenol. 2008, 191, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Germini, F.; Zarabi, S.; Eventov, M.; Turcotte, M.; Li, M.; de Wit, K. Pulmonary embolism prevalence among emergency department cohorts: A systematic review and meta-analysis by country of study. J. Thromb. Haemost. 2021, 19, 173–185. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C. Acute pulmonary embolism. N. Engl. J. Med. 2010, 363, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, M.; Aggarwal, L.; Scott, I.A.; Cocks, N. Underuse of risk assessment and overuse of computed tomography pulmonary angiography in patients with suspected pulmonary thromboembolism. Intern. Med. J. 2017, 47, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.K.; Kline, J.A.; Courtney, D.M.; Camargo, C.A.; Plewa, M.C.; Nordenholz, K.E.; Moore, C.L.; Richman, P.B.; Smithline, H.A.; Beam, D.M. Evaluation of pulmonary embolism in the emergency department and consistency with a national quality measure: Quantifying the opportunity for improvement. Arch. Intern. Med. 2012, 172, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Perelas, A.; Dimou, A.; Saenz, A.; Rhee, J.H.; Teerapuncharoen, K.; Rowden, A.; Eiger, G. CT pulmonary angiography utilization in the emergency department: Diagnostic yield and adherence to current guidelines. Am. J. Med. Qual. 2015, 30, 571–577. [Google Scholar] [CrossRef]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; Berrington de Gonzalez, A. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef] [Green Version]
- Stobbe, C.; Park, S.; Chapman, J. The radiation hypersensitivity of cells at mitosis. Int. J. Radiat. Biol. 2002, 78, 1149–1157. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Salameh, J.P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Reitsma, J.B.; Vandenbroucke, J.P.; Glas, A.S.; Bossuyt, P.M. Case-control and two-gate designs in diagnostic accuracy studies. Clin. Chem. 2005, 51, 1335–1341. [Google Scholar] [CrossRef] [Green Version]
- Wiener, R.S.; Schwartz, L.M.; Woloshin, S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch. Intern. Med. 2011, 171, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Schoepf, U.J.; Costello, P. CT angiography for diagnosis of pulmonary embolism: State of the art. Radiology 2004, 230, 329–337. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Macaskill, P.; Gatsonis, C.; Deeks, J.J.; Harbord, R.M.; Takwoingi, Y. Chapter 10: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; Deeks, J.J., Bossuyt, P., Gatsonis, C., Eds.; The Cochrane Collaboration: London, UK, 2010; p. 61. [Google Scholar]
- Celik, A.; Ozcan, I.T.; Gündes, A.; Topuz, M.; Pektas, I.; Yesil, E.; Ayhan, S.; Kose, A.; Camsari, A.; Cin, V.G. Usefulness of admission hematologic parameters as diagnostic tools in acute pulmonary embolism. Kaohsiung J. Med. Sci. 2015, 31, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Çevik, İ.; Narcı, H.; Dündar, G.A.; Ayrık, C.; Babuş, S.B. Is there a diagnostic value for the platelet indices patients in pulmonary embolism? Hong Kong J. Emerg. Med. 2018, 25, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Arab, M.M.; Zamani Moghadam, H.; Jalal Yazdi, M.; Rayat Doost, E.; Foroughian, M. Risk Stratification of Pulmonary Thromboembolism using Brain Natriuretic Peptide and Troponin I; a Brief Report. Arch. Acad. Emerg. Med. 2022, 10, e8. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.; García-Avello, Á.; Alonso, E.; Ruíz, A.; Navarrete, O.; Álvarez, C.; Lozano, C.; Arribas, I. Tissue plasminogen activator as a novel diagnostic aid in acute pulmonary embolism. Vasa 2014, 43, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Cai, Z.; Chen, P. Diagnostic value of platelet indexes for pulmonary embolism. Am. J. Emerg. Med. 2015, 33, 760–763. [Google Scholar] [CrossRef]
- Kalkan, A.K.; Ozturk, D.; Erturk, M.; Kalkan, M.E.; Cakmak, H.A.; Oner, E.; Uzun, F.; Tasbulak, O.; Yakisan, T.; Celik, A. The diagnostic value of serum copeptin levels in an acute pulmonary embolism. Cardiol. J. 2016, 23, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Leeflang, M.M. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin. Microbiol. Infect. 2014, 20, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfanti, L.; Cervellin, G.; Calamai, S.; Lunian, M.; Aloe, R.; Lippi, G. Diagnostic significance of combining D-dimer with high-sensitivity cardiac troponin I for improving the diagnosis of venous thromboembolism in the emergency department. Acta Biomed. 2021, 92, e2021287. [Google Scholar] [CrossRef] [PubMed]
- Dirican, N.; Duman, A.; Sağlam, G.; Arslan, A.; Ozturk, O.; Atalay, S.; Bircan, A.; Akkaya, A.; Cakir, M. The diagnostic significance of signal peptide-complement C1r/C1s, Uegf, and Bmp1-epidermal growth factor domain-containing protein-1 levels in pulmonary embolism. Ann. Thorac. Med. 2016, 11, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Farm, M.; Antovic, A.; Schmidt, D.E.; Bark, N.; Soutari, N.; Siddiqui, A.J.; Holmström, M.; Pruner, I.; Antovic, J.P. Diagnostic Accuracy in Acute Venous Thromboembolism: Comparing D-Dimer, Thrombin Generation, Overall Hemostatic Potential, and Fibrin Monomers. TH Open 2020, 4, e178–e188. [Google Scholar] [CrossRef]
- Ghahnavieh, A.A.; Golshani, K.; Jafarpisheh, M.; Moaiednia, M.; Memarzade, M.A.; Maghami-Mehr, A. Diagnostic Value of Copeptin in Patients with Suspected Pulmonary Embolism in Emergency Departments. Tanaffos 2019, 18, 262. [Google Scholar]
- Hogg, K.; Hinchliffe, E.; Halsam, S.; Valkov, A.; Lecky, F. Is ischaemia-modified albumin a test for venous thromboembolism? Emerg. Med. J. 2012, 29, 455–459. [Google Scholar] [CrossRef]
- Melanson, S.E.; Laposata, M.; Camargo, C.A., Jr.; Chen, A.A.; Tung, R.; Krauser, D.; Anwaruddin, S.; Baggish, A.; Cameron, R.; Sluss, P.; et al. Combination of D-dimer and amino-terminal pro-B-type natriuretic Peptide testing for the evaluation of dyspneic patients with and without acute pulmonary embolism. Arch. Pathol. Lab. Med. 2006, 130, 1326–1329. [Google Scholar] [CrossRef]
- Wexels, F.; Seljeflot, I.; Pripp, A.H.; Dahl, O.E. D-Dimer and prothrombin fragment 1 + 2 in urine and plasma in patients with clinically suspected venous thromboembolism. Blood Coagul. Fibrinolysis 2016, 27, 396–400. [Google Scholar] [CrossRef]
- Farah, R.; Nseir, W.; Kagansky, D.; Khamisy-Farah, R. The role of neutrophil-lymphocyte ratio, and mean platelet volume in detecting patients with acute venous thromboembolism. J. Clin. Lab. Anal. 2020, 34, e23010. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.A.; Carpenter, C.R.; Newman, T.B. Understanding the direction of bias in studies of diagnostic test accuracy. Acad. Emerg. Med. 2013, 20, 1194–1206. [Google Scholar] [CrossRef]
- Schmidt, R.L.; Factor, R.E. Understanding sources of bias in diagnostic accuracy studies. Arch. Pathol. Lab. Med. 2013, 137, 558–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, B. Post hoc choice of cut points introduced bias to diagnostic research. J. Clin. Epidemiol. 2006, 59, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.; Moons, K.G.; Reitsma, J.B.; Zwinderman, A.H. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: Mechanisms, magnitude, and solutions. Clin. Chem. 2008, 54, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lijmer, J.G.; Mol, B.W.; Heisterkamp, S.; Bonsel, G.J.; Prins, M.H.; van der Meulen, J.H.; Bossuyt, P.M. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999, 282, 1061–1066. [Google Scholar] [CrossRef]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Glas, A.S.; Bossuyt, P.M.; Kleijnen, J. Sources of variation and bias in studies of diagnostic accuracy: A systematic review. Ann. Intern. Med. 2004, 140, 189–202. [Google Scholar] [CrossRef]

| First Author, Publication Year, and Country | Timeframe | Population and Clinical Setting | Total Study Size (n), Prevalence of Acute PE (%) | Mean Age, Proportion of Males (%) | Index Test(s) (Threshold) | Reference Standard |
|---|---|---|---|---|---|---|
| Celik et al. 2015, Turkey [26] | January 2011 to May 2013 | Patients with suspected acute PE and admitted to hospital (department not specified) | 248, 45% | PE+: 59 ± 16 years, 45% PE−: 62 ± 15 years, 46% | Red cell distribution width (<18.9%) | CTPA |
| Çevik et al. 2018, Turkey [27] | March 2013 to October 2015 | Patients with suspected acute PE and admitted to emergency department | 128, 48% | PE+: 64 ± 17 years, not reported, PE−: 65 ± 17 years, not reported | Mean platelet volume (≤9 fL) Platelet distribution width (>12.8 fL) Platelet count (≤254 × 103 cells per mL) | CTPA |
| Ebrahimi et al. 2022, Iran [28] | January 2017 to January 2018 | Patients with suspected acute PE admitted to emergency department. PE was suspected when patients had sudden shortness of breath and chest pain and positive D-dimer test result | 267, 45% | Only reported for the total study sample, 68 ± 12 years, 60% | NT-proBNP (>100 pg/mL) Troponin I (>0.005 ng/mL) | CTPA |
| Flores et al. 2014, Spain [29] | September 2008 to October 2009 | Patients with suspected acute PE admitted to emergency department. Pretest clinical probability was assessed with Wells score. All patients regardless of Wells score were included | 127, 32% | PE+: 62 ± 19 years, 51%, PE−: 50 ± 18 years, 38% | Tissue plasminogen activator (>8.5 ng/mL) | CTPA * V/Q-scan † Necroscopy ‡ |
| Huang et al. 2015, China [30] | September 2009 to January 2014 | Patients with suspected acute PE admitted to hospital (department not specified) | 145, 48% | PE+: 60 ± 14 years, 46%, PE−: 57 ± 16 years, 57% | Mean platelet volume (>8.45 fL) | CTPA |
| Kalkan et al. 2016, Turkey [31] | January 2014 to February 2015 | Patients with suspected acute PE admitted to emergency department. Pretest clinical probability assessed with Wells score and D-dimer measures. Patients with Wells score ≥ 7 or < 7 and positive D-dimer were included | 90, 52% | PE+: 57 ± 16 years, 53%, PE−: 58 ± 16 years, 46% | Copeptin (>4.84 ng/mL) NT-proBNP (>247.4 ng/L) Troponin I (>0.065 ng/mL) | CTPA |
| Index Test (Biomarker) | TP | FP | FN | TN | Total Study Sample | Sensitivity (95% CIs) | Specificity (95% CIs) | Prevalence of Acute PE | Positive Predictive Value | Negative Predictive Value | Overall Percent Agreement * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Copeptin | 32 | 7 | 15 | 36 | 90 | 68% (53–81%) | 84% (69–93%) | 52% | 82% | 71% | 76% |
| MPV † | 21 | 7 | 40 | 60 | 128 | 34% (23–48%) | 90% (80–96%) | 48% | 75% | 60% | 63% |
| MPV ‡ | 62 | 37 | 8 | 38 | 145 | 89% (79–95%) | 51% (39–62%) | 48% | 63% | 83% | 69% |
| NT-proBNP § | 103 | 29 | 18 | 117 | 267 | 85% (78–91%) | 80% (73–86%) | 45% | 78% | 87% | 82% |
| NT-proBNP ¶ | 35 | 8 | 12 | 35 | 90 | 74% (60–86%) | 81% (67–92%) | 52% | 81% | 74% | 78% |
| PDW | 38 | 19 | 23 | 48 | 128 | 62% (49–74%) | 72% (59–82%) | 48% | 67% | 68% | 67% |
| Platelet count | 38 | 29 | 23 | 38 | 128 | 62% (49–74%) | 57% (44–69%) | 48% | 57% | 62% | 59% |
| RDW | 23 | 9 | 88 | 128 | 248 | 21% (14–29%) | 93% (88–97%) | 45% | 72% | 59% | 61% |
| tPA | 39 | 55 | 2 | 31 | 127 | 95% (83–99%) | 36% (26–47%) | 32% | 41% | 94% | 55% |
| Troponin I § | 79 | 85 | 42 | 61 | 267 | 65% (56–74%) | 42% (34–50%) | 45% | 48% | 59% | 52% |
| Troponin I ¶ | 30 | 10 | 17 | 33 | 90 | 64% (49–77%) | 77% (61–88%) | 52% | 75% | 66% | 70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikan, V.E.; Tøndel, B.G.; Morelli, V.M.; Brodin, E.E.; Brækkan, S.K.; Hansen, J.-B. Diagnostic Blood Biomarkers for Acute Pulmonary Embolism: A Systematic Review. Diagnostics 2023, 13, 2301. https://doi.org/10.3390/diagnostics13132301
Wikan VE, Tøndel BG, Morelli VM, Brodin EE, Brækkan SK, Hansen J-B. Diagnostic Blood Biomarkers for Acute Pulmonary Embolism: A Systematic Review. Diagnostics. 2023; 13(13):2301. https://doi.org/10.3390/diagnostics13132301
Chicago/Turabian StyleWikan, Vårin Eiriksdatter, Birgitte Gladsø Tøndel, Vânia Maris Morelli, Ellen Elisabeth Brodin, Sigrid Kufaas Brækkan, and John-Bjarne Hansen. 2023. "Diagnostic Blood Biomarkers for Acute Pulmonary Embolism: A Systematic Review" Diagnostics 13, no. 13: 2301. https://doi.org/10.3390/diagnostics13132301
APA StyleWikan, V. E., Tøndel, B. G., Morelli, V. M., Brodin, E. E., Brækkan, S. K., & Hansen, J.-B. (2023). Diagnostic Blood Biomarkers for Acute Pulmonary Embolism: A Systematic Review. Diagnostics, 13(13), 2301. https://doi.org/10.3390/diagnostics13132301






