Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging
Abstract
:1. Introduction
2. Basics of Dual-Energy CT
2.1. Basic Principles of Dual-Energy CT
2.2. Dual-Energy CT Scanners
2.2.1. Dual-Source CT
2.2.2. Rapid kVp Switching CT
2.2.3. Dual-Layer Detector CT
2.2.4. Split-Filter CT
3. Clinical Application of Dual-Energy CT for Pulmonary Functional Imaging
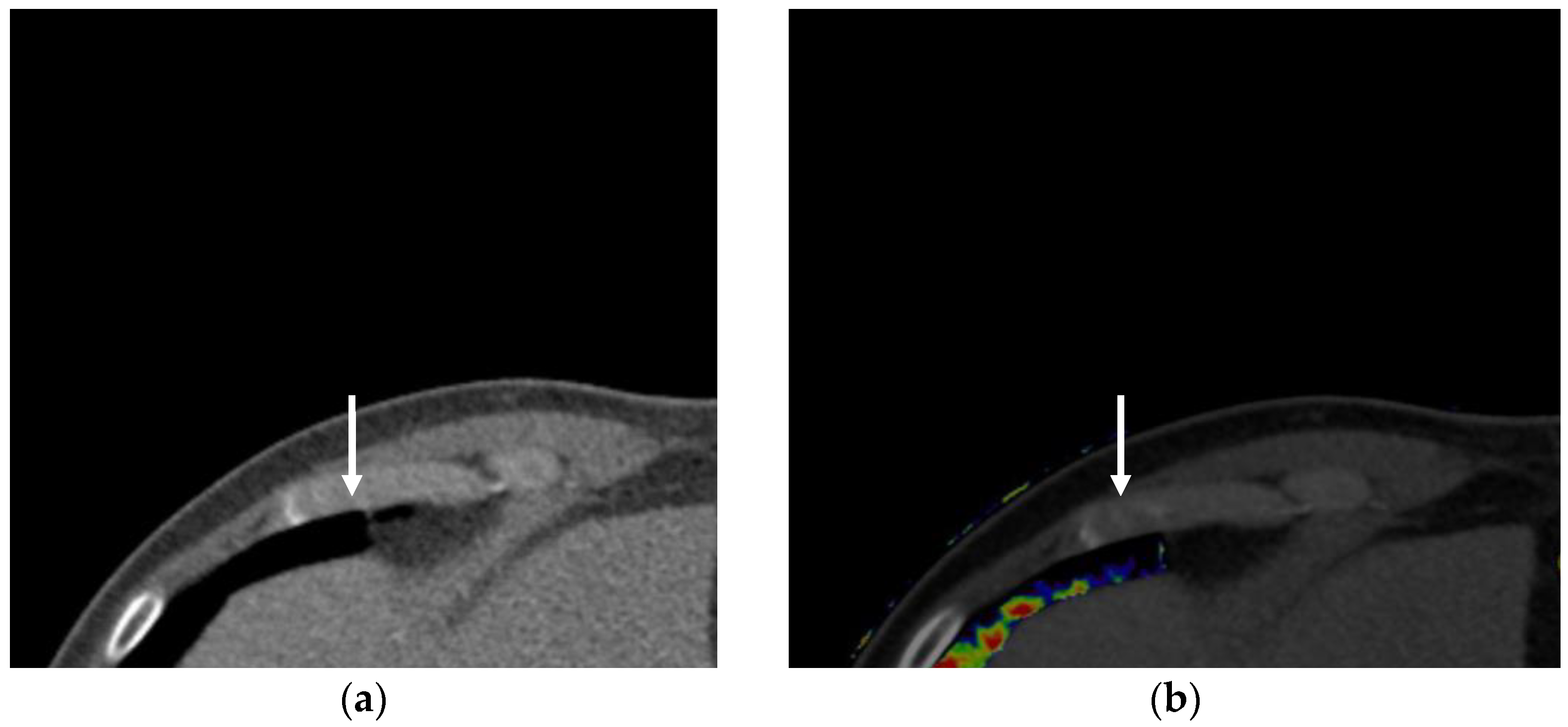
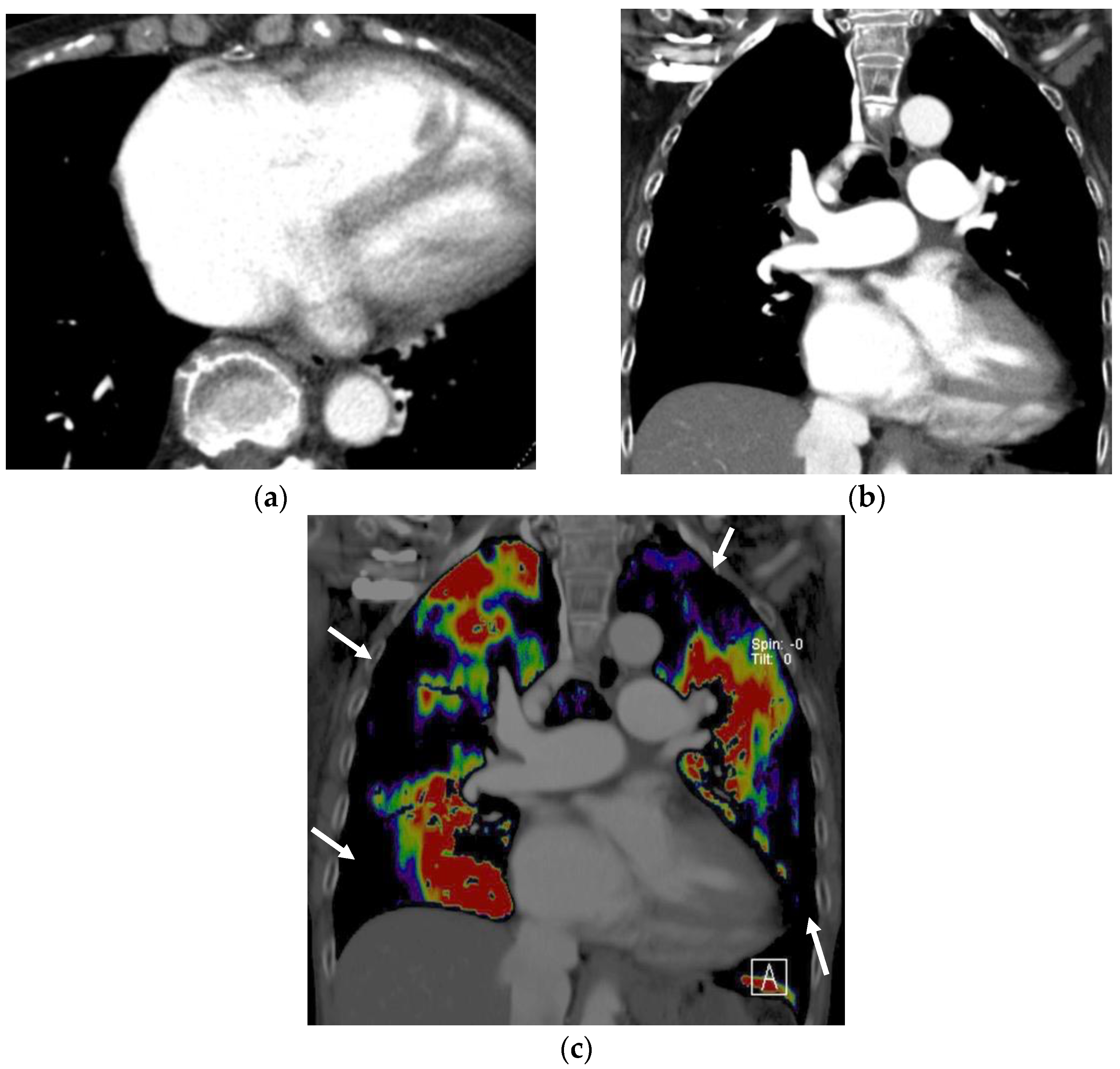
3.1. Iodine Imaging
3.1.1. Perfused Blood Volume in the Lung
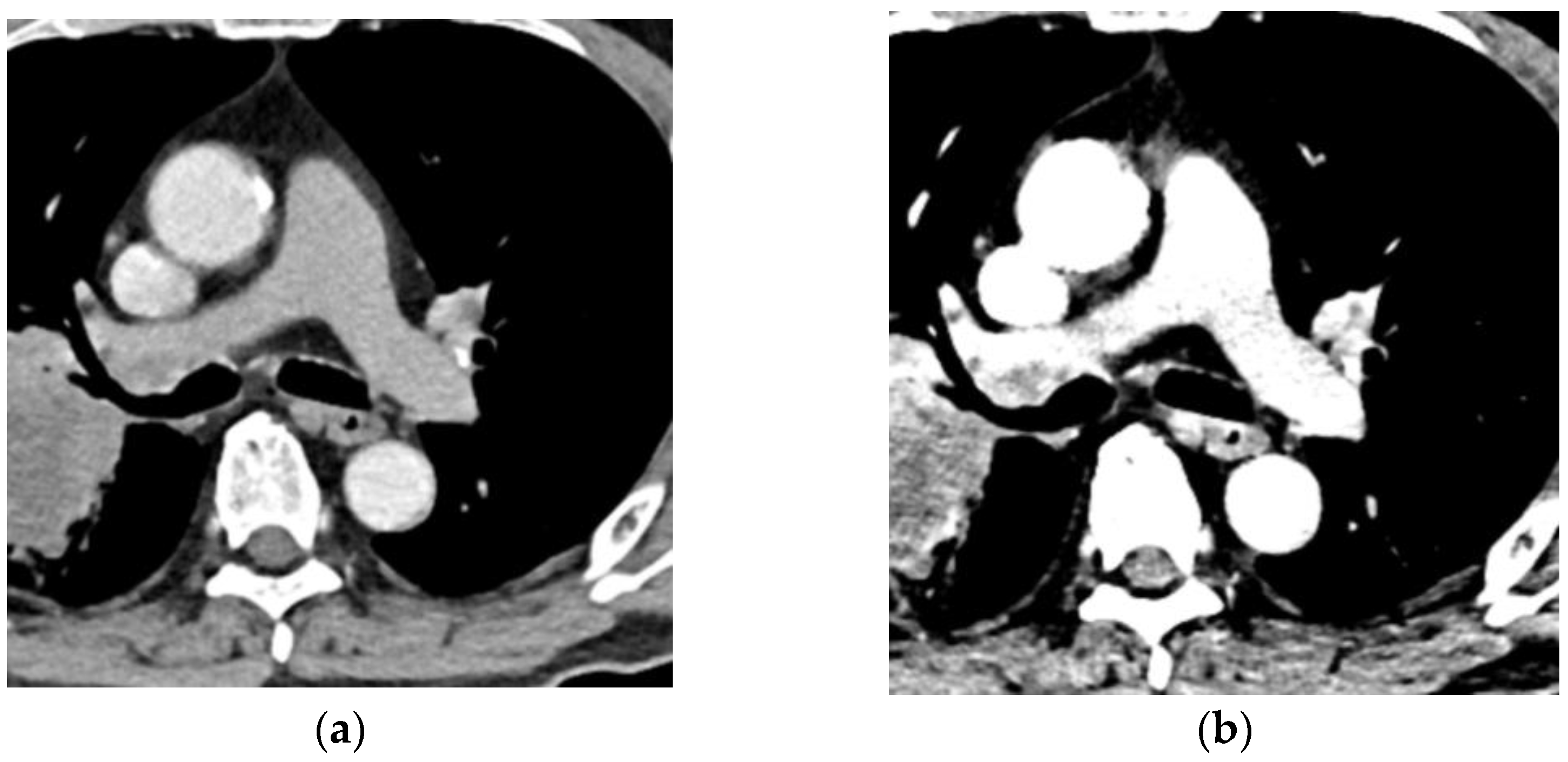
3.1.2. Virtual Monochromatic Imaging
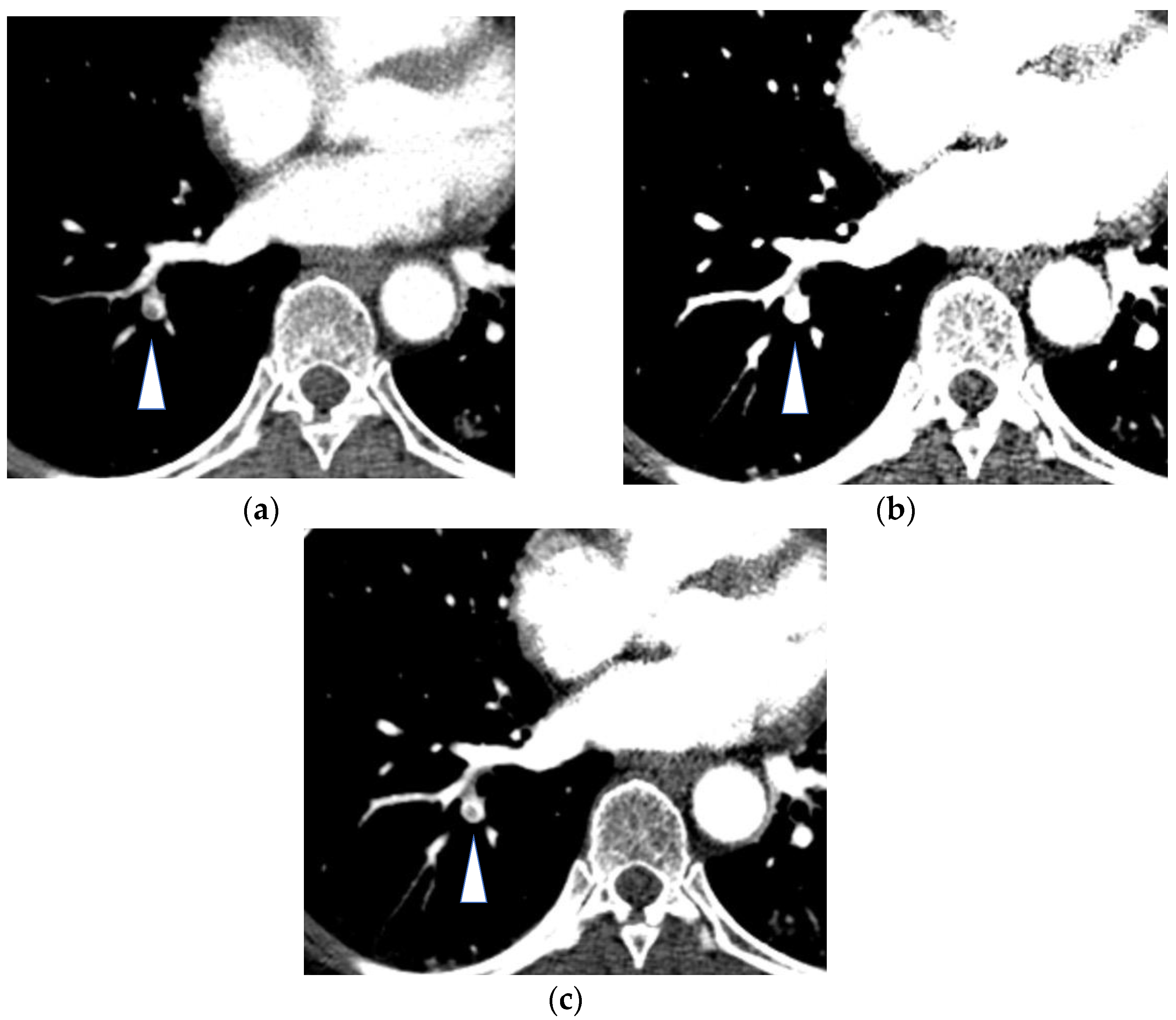
3.1.3. Iodine Imaging for Thoracic Oncology
3.2. Xenon Imaging
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flohr, T.G.; McCollough, C.H.; Bruder, H.; Petersilka, M.; Gruber, K.; Süss, C.; Grasruck, M.; Stierstorfer, K.; Krauss, B.; Raupach, R.; et al. First performance evaluation of a dual-source CT (DSCT) system. Eur. Radiol. 2006, 16, 256–268. [Google Scholar] [CrossRef]
- Johnson, T.R.; Krauss, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Honda, Y.; Awai, K. Dual-energy CT: Minimal essentials for radiologists. Jpn. J. Radiol. 2022, 40, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Koyama, H.; Lee, H.Y.; Miura, S.; Yoshikawa, T.; Sugimura, K. Contrast-enhanced CT- and MRI-based perfusion assessment for pulmonary diseases: Basics and clinical applications. Diagn. Interv. Radiol. 2016, 22, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Seo, J.B.; Parraga, G.; Lee, K.S.; Gefter, W.B.; Fain, S.B.; Schiebler, M.L.; Hatabu, H. Pulmonary Functional Imaging: Part 1-State-of-the-Art Technical and Physiologic Underpinnings. Radiology 2021, 299, 508–523. [Google Scholar] [CrossRef]
- Vlahos, I.; Jacobsen, M.C.; Godoy, M.C.; Stefanidis, K.; Layman, R.R. Dual-energy CT in pulmonary vascular disease. Br. J. Radiol. 2022, 95, 20210699. [Google Scholar] [CrossRef]
- Parakh, A.; Lennartz, S.; An, C.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Dual-Energy CT Images: Pearls and Pitfalls. Radiographics 2021, 41, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [Green Version]
- So, A.; Nicolaou, S. Spectral Computed Tomography: Fundamental Principles and Recent Developments. Korean J. Radiol. 2021, 22, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. Radiographics 2020, 40, 1284–1308. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kikano, E.G.; Bera, K.; Baruah, D.; Saboo, S.S.; Lennartz, S.; Hokamp, N.G.; Gholamrezanezhad, A.; Gilkeson, R.C.; Laukamp, K.R. Dual energy imaging in cardiothoracic pathologies: A primer for radiologists and clinicians. Eur. J. Radiol. Open 2021, 8, 100324. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.A. Origins of and lessons from quantitative functional X-ray computed tomography of the lung. Br. J. Radiol. 2022, 95, 20211364. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef]
- Hong, Y.J.; Shim, J.; Lee, S.M.; Im, D.J.; Hur, J. Dual-Energy CT for Pulmonary Embolism: Current and Evolving Clinical Applications. Korean J. Radiol. 2021, 22, 1555–1568. [Google Scholar] [CrossRef]
- Rapp, J.B.; Ho-Fung, V.M.; Ramirez, K.I.; White, A.M.; Otero, H.J.; Biko, D.M. Dual-source computed tomography protocols for the pediatric chest-scan optimization techniques. Pediatr. Radiol. 2022, 53, 1248–1259. [Google Scholar] [CrossRef]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in X-ray computerized tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef]
- Johnson, T.R. Dual-energy CT: General principles. AJR Am. J. Roentgenol. 2012, 199, S3–S8. [Google Scholar] [CrossRef]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and Minimizing Artifacts at Dual-Energy CT. Radiographics 2021, 41, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Leng, S.; McCollough, C.H. Dual-energy CT-based monochromatic imaging. AJR Am. J. Roentgenol. 2012, 199, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Christner, J.A.; Leng, S.; Wang, J.; Fletcher, J.G.; McCollough, C.H. Virtual monochromatic imaging in dual-source dual-energy CT: Radiation dose and image quality. Med. Phys. 2011, 38, 6371–6379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and avoidance. Radiographics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, S.; Lambert, J.W.; Sun, Y.; Wang, Z.J.; Sun, D.S.; Yeh, B.M. Pelvic Beam-Hardening Artifacts in Dual-Energy CT Image Reconstructions: Occurrence and Impact on Image Quality. AJR Am. J. Roentgenol. 2017, 208, 114–123. [Google Scholar] [CrossRef]
- Faby, S.; Kuchenbecker, S.; Sawall, S.; Simons, D.; Schlemmer, H.P.; Lell, M.; Kachelrieß, M. Performance of today’s dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: A simulation study. Med. Phys. 2015, 42, 4349–4366. [Google Scholar] [CrossRef]
- Petersilka, M.; Bruder, H.; Krauss, B.; Stierstorfer, K.; Flohr, T.G. Technical principles of dual source CT. Eur. J. Radiol. 2008, 68, 362–368. [Google Scholar] [CrossRef]
- So, A.; Lee, T.Y.; Imai, Y.; Narayanan, S.; Hsieh, J.; Kramer, J.; Procknow, K.; Leipsic, J.; Labounty, T.; Min, J. Quantitative myocardial perfusion imaging using rapid kVp switch dual-energy CT: Preliminary experience. J. Cardiovasc. Comput. Tomogr. 2011, 5, 430–442. [Google Scholar] [CrossRef]
- Almeida, I.P.; Schyns, L.E.; Öllers, M.C.; van Elmpt, W.; Parodi, K.; Landry, G.; Verhaegen, F. Dual-energy CT quantitative imaging: A comparison study between twin-beam and dual-source CT scanners. Med. Phys. 2017, 44, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.M.; Zhao, Y.; Zhang, L.J.; Schoepf, U.J. Dual-energy CT of the lung. AJR Am. J. Roentgenol. 2012, 199, S40–S53. [Google Scholar] [CrossRef]
- Thieme, S.F.; Johnson, T.R.; Lee, C.; McWilliams, J.; Becker, C.R.; Reiser, M.F.; Nikolaou, K. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am. J. Roentgenol. 2009, 193, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Thieme, S.F.; Graute, V.; Nikolaou, K.; Maxien, D.; Reiser, M.F.; Hacker, M.; Johnson, T.R. Dual Energy CT lung perfusion imaging—Correlation with SPECT/CT. Eur. J. Radiol. 2012, 81, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Masy, M.; Giordano, J.; Petyt, G.; Hossein-Foucher, C.; Duhamel, A.; Kyheng, M.; De Groote, P.; Fertin, M.; Lamblin, N.; Bervar, J.F.; et al. Dual-energy CT (DECT) lung perfusion in pulmonary hypertension: Concordance rate with V/Q scintigraphy in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH). Eur. Radiol. 2018, 28, 5100–5110. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Ohno, Y.; Kimura, H.; Kichikawa, K. Quantitative lung perfused blood volume imaging on dual-energy CT: Capability for quantitative assessment of disease severity in patients with acute pulmonary thromboembolism. Acta Radiol. 2015, 56, 284–293. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabari, A.; Lo Gullo, R.; Murugan, V.; Otrakji, A.; Digumarthy, S.; Kalra, M. Recent Advances in Computed Tomographic Technology: Cardiopulmonary Imaging Applications. J. Thorac. Imaging 2017, 32, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kazerooni, E.A.; Cascade, P.N. Pulmonary embolism: Optimization of small pulmonary artery visualization at multi-detector row CT. Radiology 2003, 227, 455–460. [Google Scholar] [CrossRef]
- Dournes, G.; Verdier, D.; Montaudon, M.; Bullier, E.; Rivière, A.; Dromer, C.; Picard, F.; Billes, M.A.; Corneloup, O.; Laurent, F.; et al. Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: Diagnostic accuracy and concordance with radionuclide scintigraphy. Eur. Radiol. 2014, 24, 42–51. [Google Scholar] [CrossRef]
- Moradi, F.; Morris, T.A.; Hoh, C.K. Perfusion Scintigraphy in Diagnosis and Management of Thromboembolic Pulmonary Hypertension. Radiographics 2019, 39, 169–185. [Google Scholar] [CrossRef]
- Worsley, D.F.; Palevsky, H.I.; Alavi, A. Ventilation-perfusion lung scanning in the evaluation of pulmonary hypertension. J. Nucl. Med. 1994, 35, 793–796. [Google Scholar]
- Tunariu, N.; Gibbs, S.J.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; Al-Nahhas, A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wu, D.; Ma, R.; Zhang, Z.; Zhang, H.; Han, K.; Xiong, C.; Wang, L.; Fang, W. Comparison of V/Q SPECT and CT Angiography for the Diagnosis of Chronic Thromboembolic Pulmonary Hypertension. Radiology 2020, 296, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Moreau-Triby, C.; Tylski, P.; Tatard-Leitman, V.; Wdowik, Q.; Boccalini, S.; Dessouky, R.; Douek, P.; Boussel, L. Head-to-head comparison of lung perfusion with dual-energy CT and SPECT-CT. Diagn. Interv. Imaging 2020, 101, 299–310. [Google Scholar] [CrossRef]
- Hasegawa, I.; Boiselle, P.M.; Hatabu, H. Bronchial artery dilatation on MDCT scans of patients with acute pulmonary embolism: Comparison with chronic or recurrent pulmonary embolism. AJR Am. J. Roentgenol. 2004, 182, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Leal, C.; Figueiredo, L. Evaluation of the bronchial arteries: Normal findings, hypertrophy and embolization in patients with hemoptysis. Insights Imaging 2020, 11, 70. [Google Scholar] [CrossRef]
- Remy-Jardin, M.; Duhamel, A.; Deken, V.; Bouaziz, N.; Dumont, P.; Remy, J. Systemic collateral supply in patients with chronic thromboembolic and primary pulmonary hypertension: Assessment with multi-detector row helical CT angiography. Radiology 2005, 235, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Mitzner, W.; Wagner, E.M. Vascular remodeling in the circulations of the lung. J. Appl. Physiol. 2004, 97, 1999–2004. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.J.; Kim, J.Y.; Choe, K.O.; Hur, J.; Lee, H.J.; Choi, B.W.; Kim, Y.J. Different perfusion pattern between acute and chronic pulmonary thromboembolism: Evaluation with two-phase dual-energy perfusion CT. AJR Am. J. Roentgenol. 2013, 200, 812–817. [Google Scholar] [CrossRef]
- Renard, B.; Remy-Jardin, M.; Santangelo, T.; Faivre, J.B.; Tacelli, N.; Remy, J.; Duhamel, A. Dual-energy CT angiography of chronic thromboembolic disease: Can it help recognize links between the severity of pulmonary arterial obstruction and perfusion defects? Eur. J. Radiol. 2011, 79, 467–472. [Google Scholar] [CrossRef]
- Bacon, J.L.; Madden, B.P.; Gissane, C.; Sayer, C.; Sheard, S.; Vlahos, I. Vascular and Parenchymal Enhancement Assessment by Dual-Phase Dual-Energy CT in the Diagnostic Investigation of Pulmonary Hypertension. Radiol. Cardiothorac. Imaging 2020, 2, e200009. [Google Scholar] [CrossRef]
- Sakamoto, A.; Sakamoto, I.; Nagayama, H.; Koike, H.; Sueyoshi, E.; Uetani, M. Quantification of lung perfusion blood volume with dual-energy CT: Assessment of the severity of acute pulmonary thromboembolism. AJR Am. J. Roentgenol. 2014, 203, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Iwano, S.; Okumura, N.; Adachi, S.; Abe, S.; Kondo, T.; Kato, K.; Naganawa, S. Assessment of Severity in Chronic Thromboembolic Pulmonary Hypertension by Quantitative Parameters of Dual-Energy Computed Tomography. J. Comput. Assist. Tomogr. 2020, 44, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Hur, J.; Han, K.H.; Lee, H.J.; Kim, Y.J.; Kwon, W.; Choi, B.W. Acute Pulmonary Embolism: Retrospective Cohort Study of the Predictive Value of Perfusion Defect Volume Measured with Dual-Energy CT. AJR Am. J. Roentgenol. 2017, 209, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Ameli-Renani, S.; Ramsay, L.; Bacon, J.L.; Rahman, F.; Nair, A.; Smith, V.; Baskerville, K.; Devaraj, A.; Madden, B.; Vlahos, I. Dual-energy computed tomography in the assessment of vascular and parenchymal enhancement in suspected pulmonary hypertension. J. Thorac. Imaging 2014, 29, 98–106. [Google Scholar] [CrossRef]
- Abozeed, M.; Conic, S.; Bullen, J.; Rizk, A.; Saeedan, M.B.; Karim, W.; Heresi, G.A.; Renapurkar, R.D. Dual energy CT based scoring in chronic thromboembolic pulmonary hypertension and correlation with clinical and hemodynamic parameters: A retrospective cross-sectional study. Cardiovasc. Diagn. Ther. 2022, 12, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Apfaltrer, P.; Bachmann, V.; Meyer, M.; Henzler, T.; Barraza, J.M.; Gruettner, J.; Walter, T.; Schoepf, U.J.; Schoenberg, S.O.; Fink, C. Prognostic value of perfusion defect volume at dual energy CTA in patients with pulmonary embolism: Correlation with CTA obstruction scores, CT parameters of right ventricular dysfunction and adverse clinical outcome. Eur. J. Radiol. 2012, 81, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Ota, H.; Sugimura, K.; Otani, K.; Tominaga, J.; Aoki, T.; Tatebe, S.; Miura, M.; Yamamoto, S.; Sato, H.; et al. Dual-energy CT to estimate clinical severity of chronic thromboembolic pulmonary hypertension: Comparison with invasive right heart catheterization. Eur. J. Radiol. 2016, 85, 1574–1580. [Google Scholar] [CrossRef]
- Swift, A.J.; Dwivedi, K.; Johns, C.; Garg, P.; Chin, M.; Currie, B.J.; Rothman, A.M.; Capener, D.; Shahin, Y.; Elliot, C.A.; et al. Diagnostic accuracy of CT pulmonary angiography in suspected pulmonary hypertension. Eur. Radiol. 2020, 30, 4918–4929. [Google Scholar] [CrossRef]
- Ema, R.; Sugiura, T.; Kawata, N.; Tanabe, N.; Kasai, H.; Nishimura, R.; Jujo, T.; Shigeta, A.; Sakao, S.; Tatsumi, K. The dilatation of main pulmonary artery and right ventricle observed by enhanced chest computed tomography predict poor outcome in inoperable chronic thromboembolic pulmonary hypertension. Eur. J. Radiol. 2017, 94, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Meinel, F.G.; Nance, J.W., Jr.; Schoepf, U.J.; Hoffmann, V.S.; Thierfelder, K.M.; Costello, P.; Goldhaber, S.Z.; Bamberg, F. Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am. J. Med. 2015, 128, 747–759.e2. [Google Scholar] [CrossRef]
- Kang, D.K.; Thilo, C.; Schoepf, U.J.; Barraza, J.M., Jr.; Nance, J.W., Jr.; Bastarrika, G.; Abro, J.A.; Ravenel, J.G.; Costello, P.; Goldhaber, S.Z. CT signs of right ventricular dysfunction: Prognostic role in acute pulmonary embolism. JACC Cardiovasc. Imaging 2011, 4, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, A.G.; Nansalmaa, B.; Kranz, J.; Taute, B.M.; Wienke, A.; Schramm, D.; Surov, A. CT pulmonary angiography findings that predict 30-day mortality in patients with acute pulmonary embolism. Eur. J. Radiol. 2015, 84, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Dane, B.; Patel, H.; O’Donnell, T.; Girvin, F.; Brusca-Augello, G.; Alpert, J.B.; Niu, B.; Attia, M.; Babb, J.; Ko, J.P. Image Quality on Dual-energy CTPA Virtual Monoenergetic Images: Quantitative and Qualitative Assessment. Acad. Radiol. 2018, 25, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jeon, K.N.; Cho, S.B.; Park, S.E.; Moon, J.I.; Baek, H.J.; Choi, B.H. Improved Opacification of a Suboptimally Enhanced Pulmonary Artery in Chest CT: Experience Using a Dual-Layer Detector Spectral CT. AJR Am. J. Roentgenol. 2018, 210, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, E.T.; Hague, C.; Manos, D.; Memauri, B.; Souza, C.; Taylor, J.; Dennie, C. Canadian Society of Thoracic Radiology/Canadian Association of Radiologists Best Practice Guidance for Investigation of Acute Pulmonary Embolism, Part 2, Technical Issues and Interpretation Pitfalls. Can. Assoc. Radiol. J. 2022, 73, 214–227. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- Ohana, M.; Jeung, M.Y.; Labani, A.; El Ghannudi, S.; Roy, C. Thoracic dual energy CT: Acquisition protocols, current applications and future developments. Diagn. Interv. Imaging 2014, 95, 1017–1026. [Google Scholar] [CrossRef]
- D’Angelo, T.; Bucher, A.M.; Lenga, L.; Arendt, C.T.; Peterke, J.L.; Caruso, D.; Mazziotti, S.; Blandino, A.; Ascenti, G.; Othman, A.E.; et al. Optimisation of window settings for traditional and noise-optimised virtual monoenergetic imaging in dual-energy computed tomography pulmonary angiography. Eur. Radiol. 2018, 28, 1393–1401. [Google Scholar] [CrossRef]
- Caruso, D.; Parinella, A.H.; Schoepf, U.J.; Stroebel, M.H.; Mangold, S.; Wichmann, J.L.; Varga-Szemes, A.; Ball, B.D., Jr.; De Santis, D.; Laghi, A.; et al. Optimization of window settings for standard and advanced virtual monoenergetic imaging in abdominal dual-energy CT angiography. Abdom. Radiol. 2017, 42, 772–780. [Google Scholar] [CrossRef]
- Wittram, C.; Maher, M.M.; Yoo, A.J.; Kalra, M.K.; Shepard, J.A.; McLoud, T.C. CT angiography of pulmonary embolism: Diagnostic criteria and causes of misdiagnosis. Radiographics 2004, 24, 1219–1238. [Google Scholar] [CrossRef]
- Ozawa, Y.; Hara, M.; Shibamoto, Y. The frequency of insufficient contrast enhancement of the pulmonary artery in routine contrast-enhanced chest CT and its improvement with an increased injection rate: A prospective study. J. Thorac. Imaging 2011, 26, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Nakaura, T.; Oda, S.; Utsunomiya, D.; Funama, Y.; Iyama, Y.; Taguchi, N.; Namimoto, T.; Yuki, H.; Kidoh, M.; et al. Dual-layer DECT for multiphasic hepatic CT with 50 percent iodine load: A matched-pair comparison with a 120 kVp protocol. Eur. Radiol. 2018, 28, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Takaoka, H.; Katahira, K.; Honda, K.; Nakaura, T.; Nagayama, Y.; Taguchi, N.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; et al. Low contrast material dose coronary computed tomographic angiography using a dual-layer spectral detector system in patients at risk for contrast-induced nephropathy. Br. J. Radiol. 2019, 92, 20180215. [Google Scholar] [CrossRef]
- Weiss, J.; Notohamiprodjo, M.; Bongers, M.; Schabel, C.; Mangold, S.; Nikolaou, K.; Bamberg, F.; Othman, A.E. Noise-optimized monoenergetic post-processing improves visualization of incidental pulmonary embolism in cancer patients undergoing single-pass dual-energy computed tomography. Radiol. Med. 2017, 122, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Geng, D.; Zhang, J. Noise-optimized virtual monoenergetic imaging technology of the third-generation dual-source computed tomography and its clinical applications. Quant. Imaging Med. Surg. 2021, 11, 4627–4643. [Google Scholar] [CrossRef]
- Grant, K.L.; Flohr, T.G.; Krauss, B.; Sedlmair, M.; Thomas, C.; Schmidt, B. Assessment of an advanced image-based technique to calculate virtual monoenergetic computed tomographic images from a dual-energy examination to improve contrast-to-noise ratio in examinations using iodinated contrast media. Investig. Radiol. 2014, 49, 586–592. [Google Scholar] [CrossRef]
- Siegel, M.J.; Bhalla, S.; Cullinane, M. Dual-energy CT material decomposition in pediatric thoracic oncology. Radiol. Imaging Cancer 2021, 15, e200097. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, G.F.; Wu, S.Y.; Xu, J.; Lu, G.M.; Schoepf, U.J. Dual-energy CT imaging of thoracic malignancies. Cancer Imaging 2013, 13, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Chae, E.J.; Song, J.W.; Seo, J.B.; Krauss, B.; Jang, Y.M.; Song, K.S. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: Initial experience. Radiology 2008, 249, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Pu, X.H.; Xu, H.; Yu, T.F.; Yuan, M. Dual-energy computed tomography iodine uptake in differential diagnosis of inflammatory and malignant pulmonary nodules. Diagn. Interv. Radiol. 2022, 28, 563–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, J.; Hua, X.; Yu, M.; Xu, C.; Zhang, F.; Xu, J.; Wu, H. Can Spectral CT Imaging Improve the Differentiation between Malignant and Benign Solitary Pulmonary Nodules? PLoS ONE 2016, 11, e0147537. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Choi, Y.L.; Lee, K.S.; Han, J.; Zo, J.I.; Shim, Y.M.; Moon, J.W. Pure ground-glass opacity neoplastic lung nodules: Histopathology, imaging, and management. AJR Am. J. Roentgenol. 2014, 202, W224–W233. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, K.; Sun, D.; Yu, J.; Cai, Z.; Cao, Y.; Wu, J. Invasive Pulmonary Adenocarcinomas Versus Preinvasive Lesions Appearing as Pure Ground-Glass Nodules: Differentiation Using Enhanced Dual-Source Dual-Energy CT. AJR Am. J. Roentgenol. 2019, 213, W114–W122. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, M.; Li, G.; Li, Z.; Liu, A.; Pu, R.; Cao, H.; Liu, Y. Assessing the Blood Supply Status of the Focal Ground-Glass Opacity in Lungs Using Spectral Computed Tomography. Korean J. Radiol. 2018, 19, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Meng, X.; Ye, Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: An initial experience. Eur. J. Radiol. 2016, 85, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, J.; Wang, X.; Fu, X.; Zhang, T. Non-small cell lung cancer: Spectral computed tomography quantitative parameters for preoperative diagnosis of metastatic lymph nodes. Eur. J. Radiol. 2017, 89, 129–135. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Ozawa, Y.; Hara, M.; Nakagawa, M.; Goto, T.; Shibamoto, Y. Visibility of the hilar lymph nodes using advanced virtual monoenergetic low-keV images for preoperative evaluation of lung cancer. Br. J. Radiol. 2019, 92, 20180734. [Google Scholar] [CrossRef]
- Baxa, J.; Vondráková, A.; Matoušková, T.; Růžičková, O.; Schmidt, B.; Flohr, T.; Sedlmair, M.; Ferda, J. Dual-phase dual-energy CT in patients with lung cancer: Assessment of the additional value of iodine quantification in lymph node therapy response. Eur. Radiol. 2014, 24, 1981–1988. [Google Scholar] [CrossRef]
- Honda, N.; Osada, H.; Watanabe, W.; Nakayama, M.; Nishimura, K.; Krauss, B.; Otani, K. Imaging of ventilation with dual-energy CT during breath hold after single vital-capacity inspiration of stable xenon. Radiology 2012, 262, 262–268. [Google Scholar] [CrossRef]
- Kong, X.; Sheng, H.X.; Lu, G.M.; Meinel, F.G.; Dyer, K.T.; Schoepf, U.J.; Zhang, L.J. Xenon-enhanced dual-energy CT lung ventilation imaging: Techniques and clinical applications. AJR Am. J. Roentgenol. 2014, 202, 309–317. [Google Scholar] [CrossRef]
- Park, E.A.; Goo, J.M.; Park, S.J.; Lee, H.J.; Lee, C.H.; Park, C.M.; Yoo, C.G.; Kim, J.H. Chronic obstructive pulmonary disease: Quantitative and visual ventilation pattern analysis at xenon ventilation CT performed by using a dual-energy technique. Radiology 2010, 256, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Chae, E.J.; Seo, J.B.; Lee, J.; Kim, N.; Goo, H.W.; Lee, H.J.; Lee, C.W.; Ra, S.W.; Oh, Y.M.; Cho, Y.S. Xenon ventilation imaging using dual-energy computed tomography in asthmatics: Initial experience. Investig. Radiol. 2010, 45, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.W.; Lee, C.H.; Goo, J.M.; Park, S.J.; Kim, J.H.; Park, E.A.; Cho, S.H. Xenon-enhanced dual-energy CT of patients with asthma: Dynamic ventilation changes after methacholine and salbutamol inhalation. AJR Am. J. Roentgenol. 2012, 199, 975–981. [Google Scholar] [CrossRef]
- Nambu, A.; Zach, J.; Schroeder, J.; Jin, G.; Kim, S.S.; Kim, Y.I.; Schnell, C.; Bowler, R.; Lynch, D.A. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: Relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur. J. Radiol. 2016, 85, 2144–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Techniques of DE Imaging | Dual-Source | Rapid kVp Switching | Dual-Layer Detector | Split-Filter |
|---|---|---|---|---|
| Number of X-ray sources | 2 | 1 | 1 | 1 |
| Number of detectors | 2 | 1 | 1, layered | 1 |
| FOV (cm) | 26, 33, 35.5 * | 50 | 50 | 50 |
| DECT analysis methods | Image-based | Raw-data-based | Raw-data-based | Image-based |
| Spectral separation | Good | Good | Good | Limited |
| Cross-scatter | Yes | No | No | Yes |
| Tube filtration | Yes | No | No | Yes |
| Tube current optimization for each energy bin | Yes | No | No | No |
| Temporal registration for DE imaging | Fair | Good | Excellent | Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozawa, Y.; Ohno, Y.; Nagata, H.; Tamokami, K.; Nishikimi, K.; Oshima, Y.; Hamabuchi, N.; Matsuyama, T.; Ueda, T.; Toyama, H. Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging. Diagnostics 2023, 13, 2295. https://doi.org/10.3390/diagnostics13132295
Ozawa Y, Ohno Y, Nagata H, Tamokami K, Nishikimi K, Oshima Y, Hamabuchi N, Matsuyama T, Ueda T, Toyama H. Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging. Diagnostics. 2023; 13(13):2295. https://doi.org/10.3390/diagnostics13132295
Chicago/Turabian StyleOzawa, Yoshiyuki, Yoshiharu Ohno, Hiroyuki Nagata, Keigo Tamokami, Keitaro Nishikimi, Yuka Oshima, Nayu Hamabuchi, Takahiro Matsuyama, Takahiro Ueda, and Hiroshi Toyama. 2023. "Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging" Diagnostics 13, no. 13: 2295. https://doi.org/10.3390/diagnostics13132295
APA StyleOzawa, Y., Ohno, Y., Nagata, H., Tamokami, K., Nishikimi, K., Oshima, Y., Hamabuchi, N., Matsuyama, T., Ueda, T., & Toyama, H. (2023). Advances for Pulmonary Functional Imaging: Dual-Energy Computed Tomography for Pulmonary Functional Imaging. Diagnostics, 13(13), 2295. https://doi.org/10.3390/diagnostics13132295






