Native T2 Predicts Myocardial Inflammation Irrespective of a Patient’s Volume Status
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. CMR Acquisitions
2.3. SSFP CINE Imaging
2.4. Native T1 Mapping
2.5. T2 Mapping
2.6. Late Gadolinium Enhancement
2.7. Post-Processing
2.8. Calculation of PVS
2.9. Statistics
3. Results
3.1. Baseline Characteristics and CMR Findings
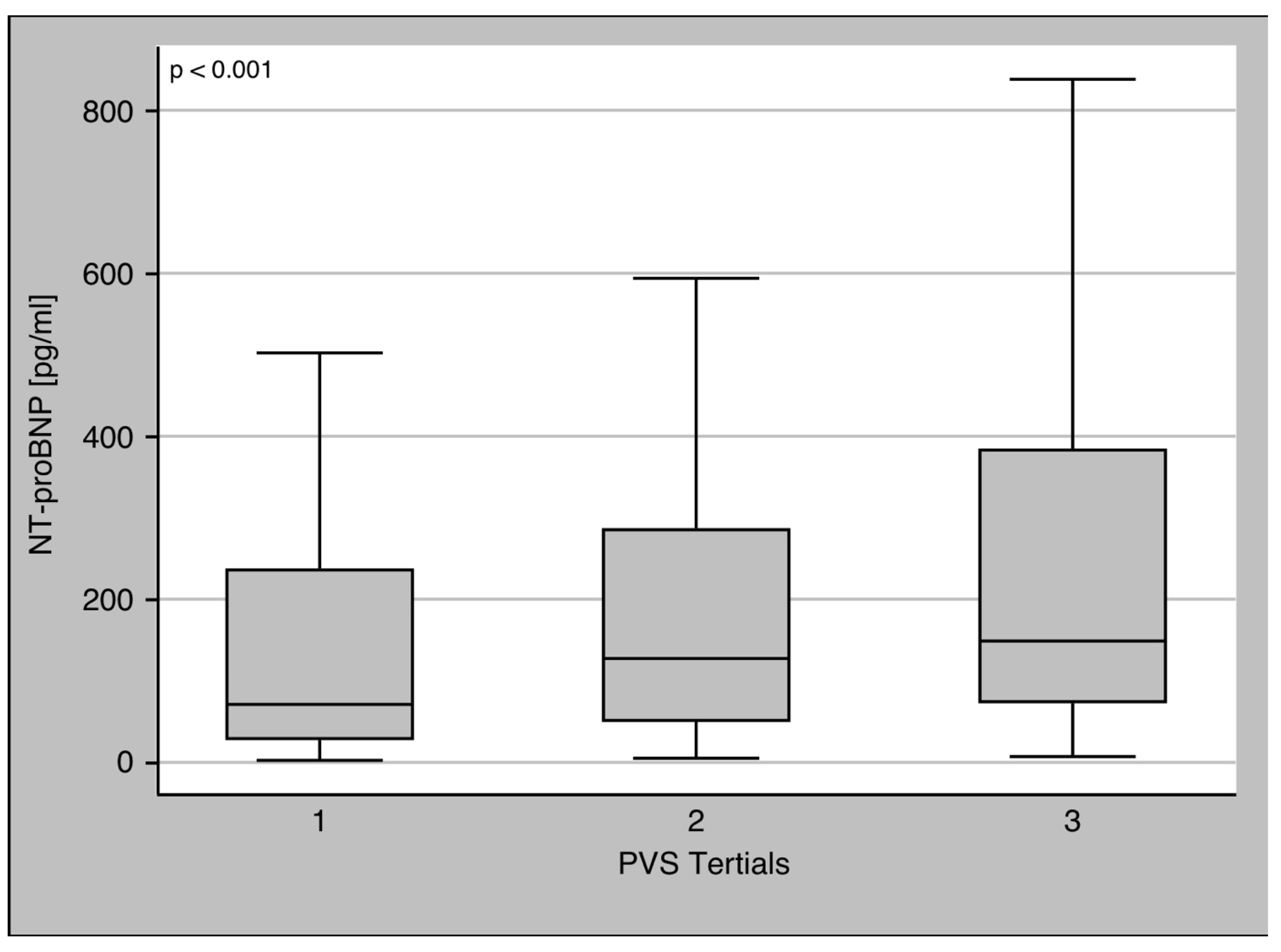
3.2. PVS and Biomarkers
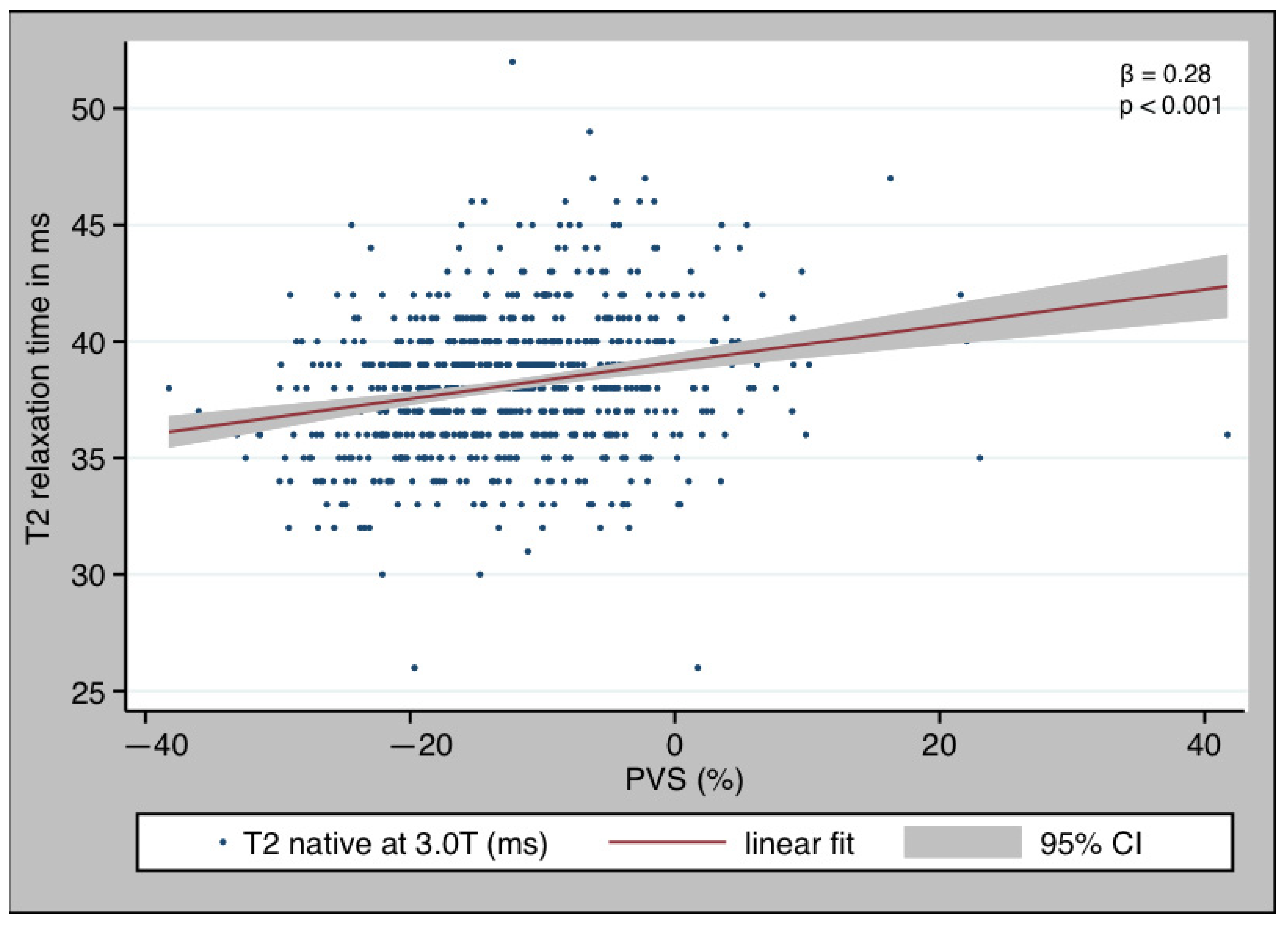
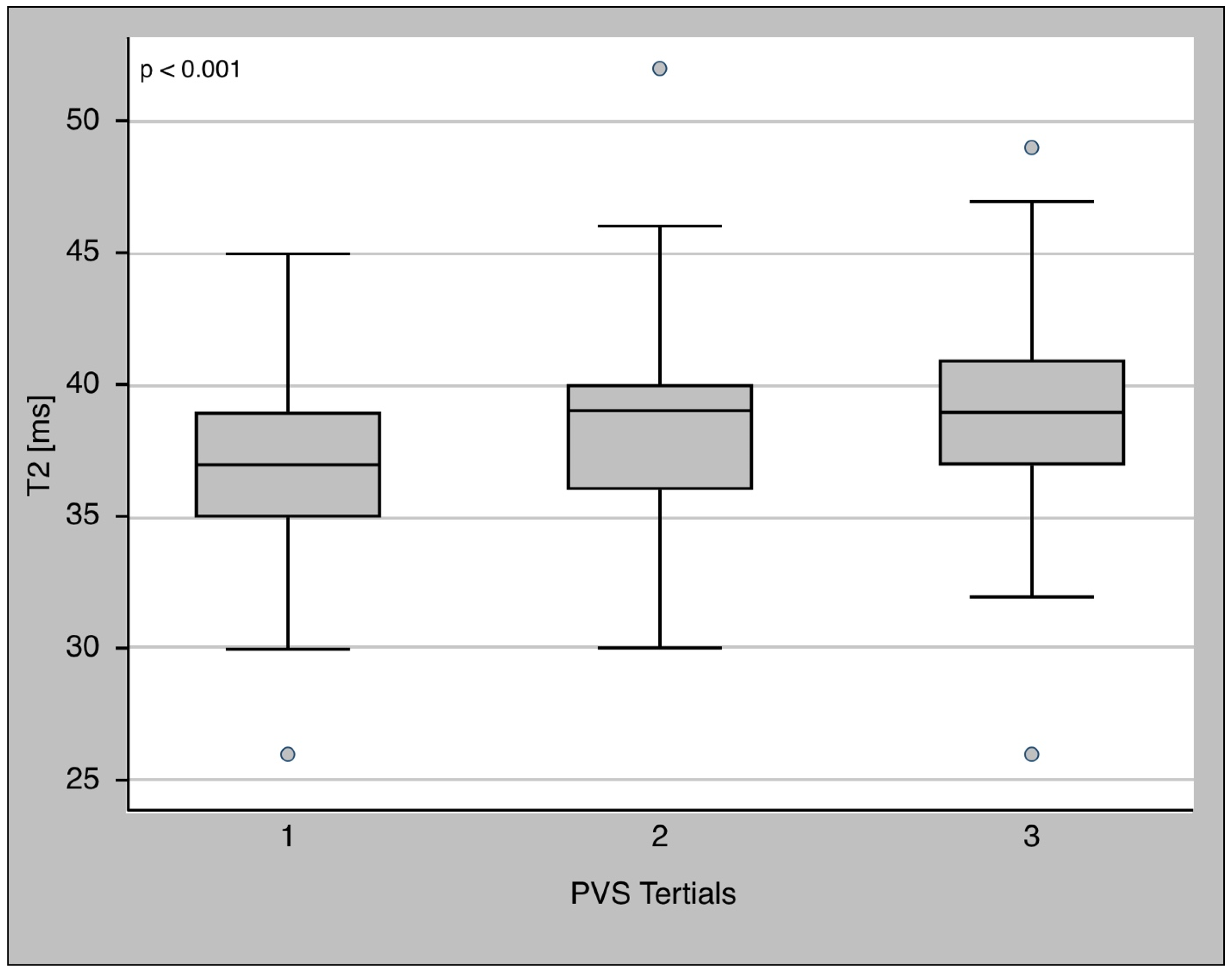
3.3. PVS and T2 Relaxation Time
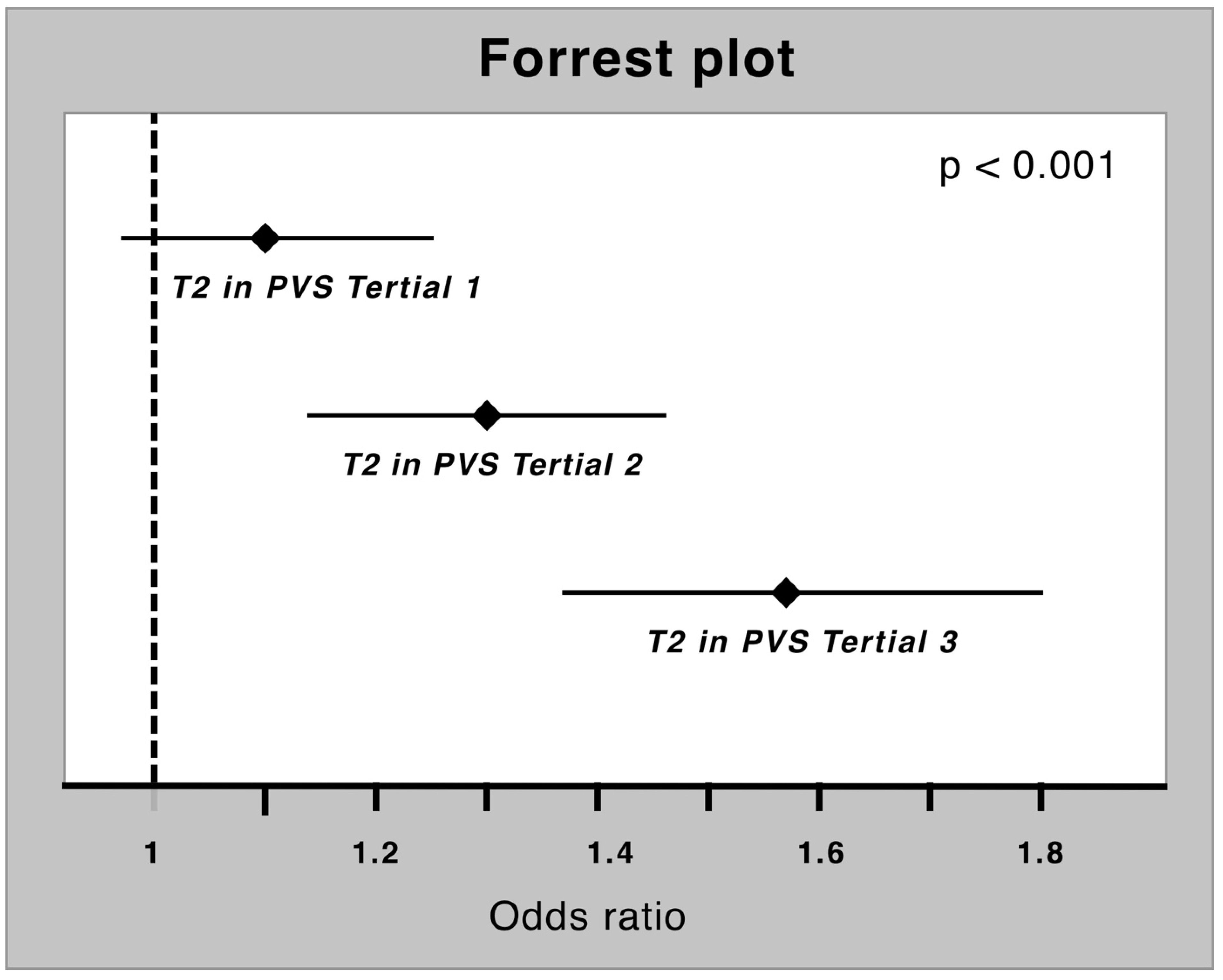
3.4. PVS and T2 as Diagnostic Tool in Myocardial Inflammation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Cooper, L.T.; Keren, A.; Sliwa, K.; Matsumori, A.; Mensah, G.A. The Global Burden of Myocarditis: Part 1: A Systematic Literature Review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 Study. Glob. Heart 2014, 9, 121–129. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990-2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [CrossRef]
- Tschöpe, C.; Cooper, L.T.; Torre-Amione, G.; Van Linthout, S. Management of Myocarditis-Related Cardiomyopathy in Adults. Circ. Res. 2019, 124, 1568–1583. [Google Scholar] [CrossRef]
- Ali-Ahmed, F.; Dalgaard, F.; Al-Khatib, S.M. Sudden Cardiac Death in Patients with Myocarditis: Evaluation, Risk Stratification, and Management. Am. Heart J. 2020, 220, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T. Ventricular Arrhythmias and Sudden Cardiac Death in Lymphocytic Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Hryniewiecki, T.; Zatorska, K.; Żebrowska, A.; Kuśmierczyk, M.; Szymański, P. High-sensitivity Troponin T as a Prognostic Marker in Patients Undergoing Aortic Valve Replacement. Pol. Arch. Intern. Med. 2017, 127, 628–630. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Ojeda, F.; Looft, Y.; Senel, M.; Radziwolek, L.; Avanesov, M.; Tahir, E.; Stehning, C.; et al. Tissue Characterization by T1 and T2 Mapping Cardiovascular Magnetic Resonance Imaging to Monitor Myocardial Inflammation in Healing Myocarditis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 744–751. [Google Scholar] [CrossRef]
- Hinojar, R.; Foote, L.; Sangle, S.; Marber, M.; Mayr, M.; Carr-White, G.; D’Cruz, D.; Nagel, E.; Puntmann, V.O. Native T1 and T2 Mapping by CMR in Lupus Myocarditis: Disease Recognition and Response to Treatment. Int. J. Cardiol. 2016, 222, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Hinojar, R.; Foote, L.; Ucar, E.; Dabir, D.; Schnackenburg, B. Myocardial T2 Mapping for Improved Detection of Inflammatory Myocardial Involvement in Acute and Chronic Myocarditis. J. Cardiovasc. Magn. Reson. 2014, 16, O63. [Google Scholar] [CrossRef]
- Lurz, P.; Luecke, C.; Eitel, I.; Föhrenbach, F.; Frank, C.; Grothoff, M.; De Waha, S.; Rommel, K.P.; Lurz, J.A.; Klingel, K.; et al. Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis: The MyoRacer-Trial. J. Am. Coll. Cardiol. 2016, 67, 1800–1811. [Google Scholar] [CrossRef]
- Bohnen, S.; Radunski, U.K.; Lund, G.K.; Kandolf, R.; Stehning, C.; Schnackenburg, B.; Adam, G.; Blankenberg, S.; Muellerleile, K. Performance of T1 and T2 Mapping Cardiovascular Magnetic Resonance to Detect Active Myocarditis in Patients with Recent-Onset Heart Failure. Circ. Cardiovasc. Imaging 2015, 8, e003073. [Google Scholar] [CrossRef] [PubMed]
- Treiber, J.; Hausmann, C.S.; Wolter, J.S.; Fischer-Rasokat, U.; Kriechbaum, S.D.; Hamm, C.W.; Nagel, E.; Puntmann, V.O.; Rolf, A. Native T1 Is Predictive of Cardiovascular Death/Heart Failure Events and All-Cause Mortality Irrespective of the Patient’s Volume Status. Front. Cardiovasc. Med. 2023, 10, 1091334. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Voigt, M.; Faron, A.; Isaak, A.; Mesropyan, N.; Dabir, D.; Sprinkart, A.M.; Pieper, C.C.; Chang, J.; Attenberger, U.; et al. Influence of Hydration Status on Cardiovascular Magnetic Resonance Myocardial T1 and T2 Relaxation Time Assessment: An Intraindividual Study in Healthy Subjects. J. Cardiovasc. Magn. Reson. 2020, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Yoowannakul, S.; Lambe, T.; Rezk, T.; Knight, D.S.; Hawkins, P.N.; Moon, J.C.; Muthurangu, V.; Kellman, P.; et al. Acute Changes in Cardiac Structural and Tissue Characterisation Parameters Following Haemodialysis Measured Using Cardiovascular Magnetic Resonance. Sci. Rep. 2019, 9, 1388. [Google Scholar] [CrossRef]
- Rankin, A.J.; Mangion, K.; Lees, J.S.; Rutherford, E.; Gillis, K.A.; Edy, E.; Dymock, L.; Treibel, T.A.; Radjenovic, A.; Patel, R.K.; et al. Myocardial Changes on 3T Cardiovascular Magnetic Resonance Imaging in Response to Haemodialysis with Fluid Removal. J. Cardiovasc. Magn. Reson. 2021, 23, 125. [Google Scholar] [CrossRef]
- Fudim, M.; Miller, W.L. Calculated Estimates of Plasma Volume in Patients With Chronic Heart Failure-Comparison With Measured Volumes. J. Card. Fail. 2018, 24, 553–560. [Google Scholar] [CrossRef]
- Ling, H.Z.; Flint, J.; Damgaard, M.; Bonfils, P.K.; Cheng, A.S.; Aggarwal, S.; Velmurugan, S.; Mendonca, M.; Rashid, M.; Kang, S.; et al. Calculated Plasma Volume Status and Prognosis in Chronic Heart Failure. Eur. J. Heart Fail. 2015, 17, 35–43. [Google Scholar] [CrossRef]
- Rogers, T.; Dabir, D.; Mahmoud, I.; Voigt, T.; Schaeffter, T.; Nagel, E.; Puntmann, V.O. Standardization of T1 Measurements with MOLLI in Differentiation between Health and Disease—The ConSept Study. J. Cardiovasc. Magn. Reson. 2013, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.; Fauci, A.; Hauser, S.; Longo, D.; Jameson, J.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 21st ed.; McGraw Hill: New York, NY, USA, 2015. [Google Scholar]
- Brown, J.J.; Andre, M.P.; Slutsky, R.A. Proton Nuclear Magnetic Resonance Tissue Analysis of Normal, Volume Overloaded, and Dehydrated Rabbit Myocardium. Am. Heart J. 1984, 108, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Bertrand, P.B.; Willems, E.; Gielen, E.; Mullens, W.; Giri, S.; Tang, W.H.W.; Raman, S.V.; Verhaert, D. Global Myocardial Oedema in Advanced Decompensated Heart Failure. Eur. Heart Cardiovasc. Imaging 2017, 18, 787–794. [Google Scholar] [CrossRef]
- Kindermann, I.; Barth, C.; Mahfoud, F.; Ukena, C.; Lenski, M.; Yilmaz, A.; Klingel, K.; Kandolf, R.; Sechtem, U.; Cooper, L.T.; et al. Update on Myocarditis. J. Am. Coll. Cardiol. 2012, 59, 779–792. [Google Scholar] [CrossRef]
- Fischer-Rasokat, U.; Renker, M.; Liebetrau, C.; Weferling, M.; Rieth, A.; Rolf, A.; Choi, Y.H.; Hamm, C.W.; Kim, W.K. Predictive Value of Overt and Non-Overt Volume Overload in Patients with High-or Low-Gradient Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Cardiovasc. Diagn. Ther. 2021, 11, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (n = 700) | Healthy (n = 551) | Myocardial Inflammation (n = 149) | p-Value |
|---|---|---|---|---|
| Age [years] | 56.31 (41.46–67.87) | 56.69 (41.57–67.68) | 55.38 (41.21–68.84) | 0.782 |
| Gender—female | 332 (47.43%) | 273 (49.55%) | 59 (39.6%) | <0.05 |
| CCS | 71 (10.26%) | 35 (6.45%) | 36 (24.16%) | <0.001 |
| Hypertonus | 343 (49.21%) | 272 (49.64%) | 71 (47.65%) | 0.668 |
| Diabetes mellitus | 78 (11.19%) | 52 (9.49%) | 26 (17.45%) | <0.01 |
| Renal insufficiency | 62 (8.91%) | 42 (7.66%) | 20 (13.51%) | <0.05 |
| Atrial fibrillation | 117 (16.79%) | 87 (15.88%) | 30 (20.31) | 0.218 |
| Heart failure | 74 (10.69%) | 18 (3.3%) | 56 (38.1%) | <0.001 |
| BMI [kg/m2] | 25.76 (22.75–29.2) | 25.71 (22.74–29.26) | 25.93 (22.64–29.06) | 0.786 |
| Heart rate [bpm] | 67 (60–76) | 68 (60–76) | 64 (56–77) | 0.101 |
| Systolic blood pressure [mmHg] | 121 (115–135) | 124 (117–137) | 120 (110–132) | 0.119 |
| Diastolic blood pressure [mmHg] | 80 (70–83) | 80 (70–85) | 72 (66–80) | 0.003 |
| Hematocrit [%] | 42.4 (39.2–45.2) | 42.4 (39.3–45) | 42.1 (38.4–45.8) | 0.760 |
| Hb [mg/dl] | 14.1 (13.1–15.1) | 14.05 (13–15.1) | 14.15 (13.35–15.33) | 0.398 |
| NT-proBNP [pg/mL] | 113.5 (45.4–297.3) | 95.1 (40.35–233.9) | 387 (90.33–1635.5) | <0.001 |
| hs-troponin T [ng/L] | 6.85 (3.7–14) | 6 (3.4–10.2) | 14.6 (5.2–30.1) | <0.001 |
| GFR [ml/min] | 96.33 (80.53–116.22) | 97.62 (81.93–117.08) | 92.44 (73.38–109.99) | <0.01 |
| CRP [mg/dl] | 0.1 (0.1–0.4) | 0.1 (0.1–0.4) | 0.2 (0.1–0.55) | 0.016 |
| LV-EF [%] | 59 (55–64) | 61 (56–65) | 53 (37–58) | <0.001 |
| T2 relaxation time [ms] | 38 (36–40) | 38 (36–39) | 40 (37–42) | <0.001 |
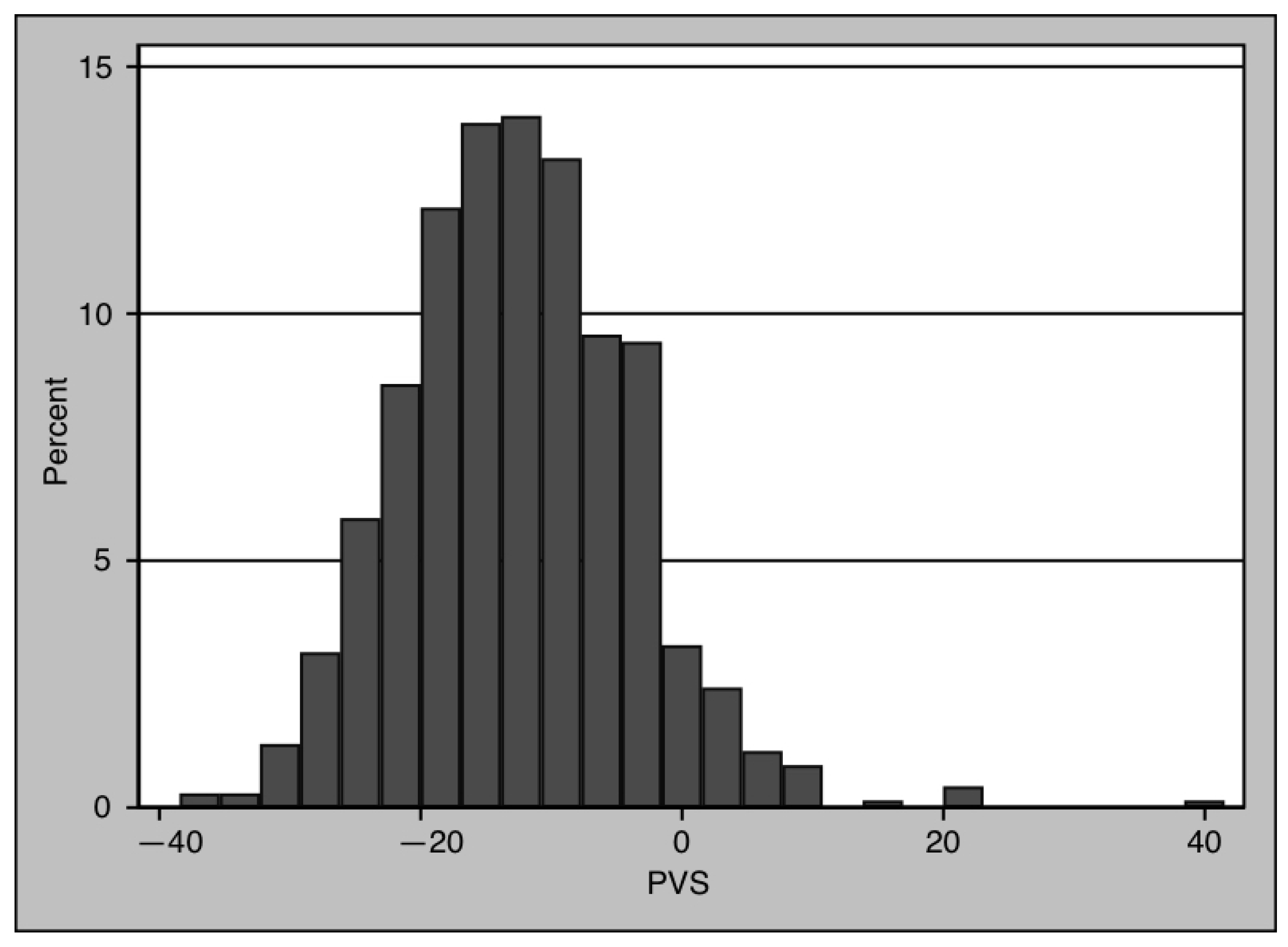
| PVS | −12.79 (−18.53–−6.95) | −12.94 (−18.40–−7.28) | −12.19 (−18.93–−5.87) | 0.384 |
| CMR Parameter | Healthy n = 551 Mean (± SD) | Inflammatory Disease n = 149 Mean (± SD) | p |
|---|---|---|---|
| LV-EDVi [mL/m2] | 75 (64–85) | 93 (74–108) | <0.001 |
| LV-ESVi [mL/m2] | 29 (23–35) | 42 (32.5–63) | <0.001 |
| RV-EDVi [mL/m2] | 75.5 (66–90) | 85 (65.5–103) | <0.001 |
| RV-ESVi [mL/m2] | 36 (28–45) | 44 (33–53.8) | <0.001 |
| LV-EF [%] | 61 (56–65) | 53 (37–58) | <0.001 |
| RV-EF [%] | 53 (48–59) | 49 (41–54) | <0.001 |
| GLS [%] | −18.81 (−20.71–−16.78) | −16.03 (−18.72–−11.33) | <0.001 |
| GCS [%] | −19.83 (−22.56–−17.77) | −16.23 (−18.34–−12.20) | <0.001 |
| GRS [%] | 35.71 (29.67–45.46) | 26.22 (17.9–32.46) | <0.001 |
| T1 [ms] | 1105 (1075–1136) | 1162 (1107–1211) | <0.001 |
| T2 [ms] | 38 (36–39) | 40 (37–42) | <0.001 |
| ECV | 0.24 (0.22–0.26) | 0.27 (0.23–0.31) | <0.001 |
| Parameter | PVS > −4% n = 107 | PVS ≤ −4% n = 593 | p |
|---|---|---|---|
| Inflammatory disease | 31 (28.97%) | 118 (28.97%) | <0.05 |
| T2 relaxation time [ms] | 39 (37–41) | 38 (36–40) | <0.01 |
| CRP [mg/dL] | 0.2 (0.1–0.5) | 0.1 (0.1–0.4) | 0.972 |
| NT-proBNP [pg/mL] | 214 (78–895) | 106 (41–269) | <0.001 |
| hs-troponin T [ng/L] | 6.4 (3.3–17.3) | 6.9 (3.7–13.3) | <0.05 |
| EF [%] | 60 (54–65) | 59 (55–64) | 0.959 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolter, J.S.; Treiber, J.M.; Fischer, S.; Fischer-Rasokat, U.; Kriechbaum, S.D.; Rieth, A.; Weferling, M.; von Jeinsen, B.; Hain, A.; Hamm, C.W.; et al. Native T2 Predicts Myocardial Inflammation Irrespective of a Patient’s Volume Status. Diagnostics 2023, 13, 2240. https://doi.org/10.3390/diagnostics13132240
Wolter JS, Treiber JM, Fischer S, Fischer-Rasokat U, Kriechbaum SD, Rieth A, Weferling M, von Jeinsen B, Hain A, Hamm CW, et al. Native T2 Predicts Myocardial Inflammation Irrespective of a Patient’s Volume Status. Diagnostics. 2023; 13(13):2240. https://doi.org/10.3390/diagnostics13132240
Chicago/Turabian StyleWolter, Jan Sebastian, Julia M. Treiber, Selina Fischer, Ulrich Fischer-Rasokat, Steffen D. Kriechbaum, Andreas Rieth, Maren Weferling, Beatrice von Jeinsen, Andreas Hain, Christian W. Hamm, and et al. 2023. "Native T2 Predicts Myocardial Inflammation Irrespective of a Patient’s Volume Status" Diagnostics 13, no. 13: 2240. https://doi.org/10.3390/diagnostics13132240
APA StyleWolter, J. S., Treiber, J. M., Fischer, S., Fischer-Rasokat, U., Kriechbaum, S. D., Rieth, A., Weferling, M., von Jeinsen, B., Hain, A., Hamm, C. W., Keller, T., & Rolf, A. (2023). Native T2 Predicts Myocardial Inflammation Irrespective of a Patient’s Volume Status. Diagnostics, 13(13), 2240. https://doi.org/10.3390/diagnostics13132240






