Rapid Detection of SARS-CoV-2 Based on the LAMP Assay Associated with the CRISPRCas12a System
Abstract
:1. Introduction
2. Materials and Methods
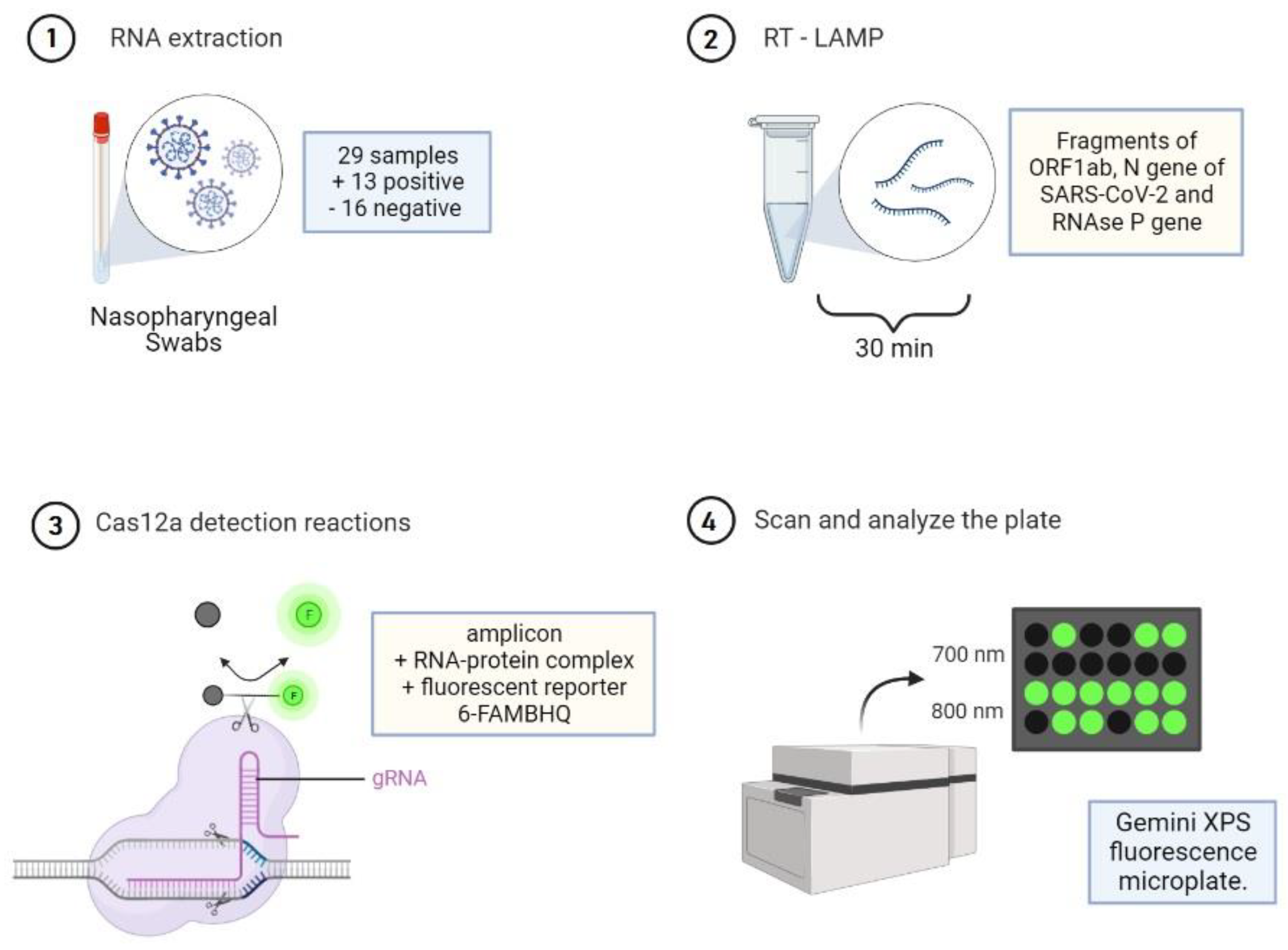
2.1. Ethical Statement and Samples
2.2. Nucleic Acid Preparation
2.3. Cas12a Detection Reactions
2.4. Statistical Analysis
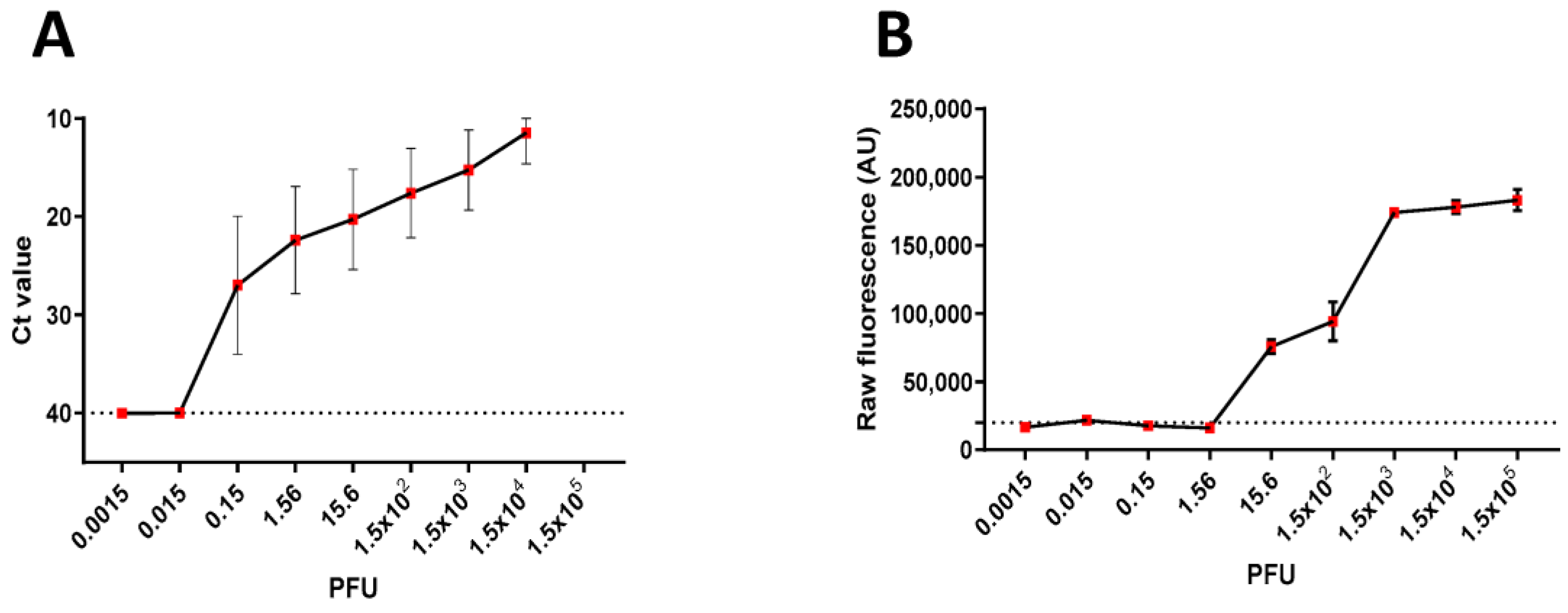
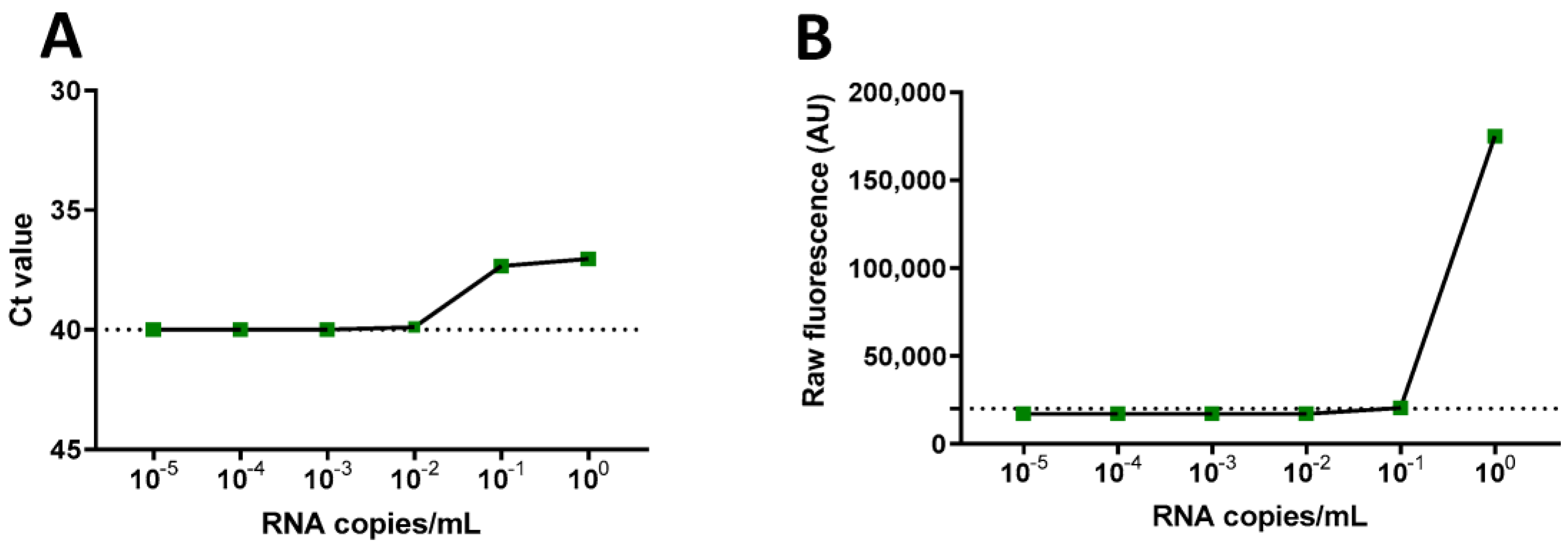
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Lf, A.-M.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [PubMed]
- World Health Organization Virtual Press Conference on COVID-19, 11 March 2020. Available online: https://www.who.int/docs/default-source/%0Acoronaviruse/transcripts/who-audioemergencies-coronavirus-press-conference-fulland-fnal-11mar2020.pdf?sfvrsn=cb432bb3_2 (accessed on 2 January 2022).
- Our World in Data COVID-19 Data Explorer. Available online: https://ourworldindata.org/explorers/coronavirus-data-explorer (accessed on 30 January 2023).
- Ji, T.; Liu, Z.; Wang, G.; Guo, X.; Khan, S.A.; Lai, C.; Chen, H.; Huang, S.; Xia, S.; Chen, B.; et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens. Bioelectron. 2020, 166, 112455. [Google Scholar] [CrossRef] [PubMed]
- Eis-Hübinger, A.M.; Hönemann, M.; Wenzel, J.J.; Berger, A.; Widera, M.; Schmidt, B.; Aldabbagh, S.; Marx, B.; Streeck, H.; Ciesek, S.; et al. Ad hoc laboratory-based surveillance of SARS-CoV-2 by real-time RT-PCR using minipools of RNA prepared from routine respiratory samples. J. Clin. Virol. 2020, 127, 104381. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Samadi, N.; Tabatabaei, N. Handheld thermo-photonic device for rapid, low-cost, and on-site detection and quantification of Anti-SARS-CoV-2 antibody. IEEE Sens. J. 2021, 21, 18504–18511. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.G.; Lee, M.-K.; Jorgensen, D.; Isaac-Renton, J.L. Molecular methods used in clinical laboratory: Prospects and pitfalls. FEMS Immunol. Med. Microbiol. 2007, 49, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-H.; Lin, Y.-C.; Teng, P.-H.; Chen, C.-L.; Lee, P.-Y. Real-time target-specific detection of loop-mediated isothermal amplification for white spot syndrome virus using fluorescence energy transfer-based probes. J. Virol. Methods 2011, 173, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Horita, N.; Yamamoto, M.; Tsukahara, T.; Nagakura, H.; Tashiro, K.; Shibata, Y.; Watanabe, H.; Nakashima, K.; Ushio, R.; et al. Diagnostic test accuracy of loop-mediated isothermal amplification assay for Mycobacterium tuberculosis: Systematic review and meta-analysis. Sci. Rep. 2016, 6, 39090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic Acid Detection Using CRISPR/Cas Biosensing Technologies. ACS Synth. Biol. 2020, 9, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization COVID-19 Strategy Update, 14 April 2020. Available online: https://www.who.int/publications/m/item/covid-19-strategyupdate (accessed on 15 April 2022).
- Pyrc, K.; Milewska, A.; Potempa, J. Development of loop-mediated isothermal amplification assay for detection of human coronavirus-NL63. J. Virol. Methods 2011, 175, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L.M.; Leung, C.S.W.; Tashiro, M.; Chan, K.H.; Wong, B.W.; Yuen, K.Y.; Guan, Y.; Peiris, J.S. Rapid Detection of the Severe Acute Respiratory Syndrome (SARS) Coronavirus by a Loop-Mediated Isothermal Amplification Assay. Clin. Chem. 2004, 50, 1050–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Deng, Q.; Li, J.; Chen, J.; Wang, Z.; Zhang, X.; Fang, Z.; Li, H.; Zhao, Y.; Yu, P.; et al. Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection. Msphere 2020, 5, e00808-20. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.T.C.; Le, M.Q.; Vuong, C.D.; Parida, M.; Minekawa, H.; Notomi, T.; Hasebe, F.; Morita, K. Development and Evaluation of a Novel Loop-Mediated Isothermal Amplification Method for Rapid Detection of Severe Acute Respiratory Syndrome Coronavirus. J. Clin. Microbiol. 2004, 42, 1956–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, P.; Guinea, J.; Muñoz-Gallego, I.; González-Donapetry, P.; Galán, J.C.; Antona, N.; Cilla, G.; Hernáez-Crespo, S.; Tuesta, J.L.D.-D.; Torrella, A.G.-D.; et al. Multicenter evaluation of the Panbio™ COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Hardick, J.; Morehead, E.; Miller, J.-A.; Gaydos, C.A.; Manabe, Y.C. Comparison of the analytical sensitivity of seven commonly used commercial SARS-CoV-2 automated molecular assays. J. Clin. Virol. 2020, 130, 104578. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, K.A.F.; Nonaka, C.K.V.; Khouri, R.; Gurgel Rocha, C.A.; Regis-Silva, C.G.; de Freitas Souza, B.S. Rapid Detection of SARS-CoV-2 Based on the LAMP Assay Associated with the CRISPRCas12a System. Diagnostics 2023, 13, 2233. https://doi.org/10.3390/diagnostics13132233
de Sousa KAF, Nonaka CKV, Khouri R, Gurgel Rocha CA, Regis-Silva CG, de Freitas Souza BS. Rapid Detection of SARS-CoV-2 Based on the LAMP Assay Associated with the CRISPRCas12a System. Diagnostics. 2023; 13(13):2233. https://doi.org/10.3390/diagnostics13132233
Chicago/Turabian Stylede Sousa, Karoline Almeida Felix, Carolina Kymie Vasques Nonaka, Ricardo Khouri, Clarissa Araújo Gurgel Rocha, Carlos Gustavo Regis-Silva, and Bruno Solano de Freitas Souza. 2023. "Rapid Detection of SARS-CoV-2 Based on the LAMP Assay Associated with the CRISPRCas12a System" Diagnostics 13, no. 13: 2233. https://doi.org/10.3390/diagnostics13132233
APA Stylede Sousa, K. A. F., Nonaka, C. K. V., Khouri, R., Gurgel Rocha, C. A., Regis-Silva, C. G., & de Freitas Souza, B. S. (2023). Rapid Detection of SARS-CoV-2 Based on the LAMP Assay Associated with the CRISPRCas12a System. Diagnostics, 13(13), 2233. https://doi.org/10.3390/diagnostics13132233




