Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. EUS Procedure
2.4. DFI-EUS
2.5. Contrast-Enhanced EUS
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Nishida, N.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig. Endosc. 2018, 30, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Seo, D.W.; Choi, J.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest. Endosc. 2013, 78, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Seo, D.W.; Choi, J.H.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg. Endosc. 2013, 27, 1414–1421. [Google Scholar]
- Yamashita, Y.; Kawaji, Y.; Shimokawa, T.; Yamazaki, H.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Kawai, M.; Kitano, M. Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics 2022, 12, 2141. [Google Scholar] [CrossRef]
- Fujita, M.; Itoi, T.; Ikeuchi, N.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Kamada, K.; Umeda, J.; Tanaka, R.; Tonozuka, R.; et al. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc. Ultrasound 2016, 5, 377–383. [Google Scholar]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6260. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kato, H.; Tomoda, T.; Matsumoto, K.; Sakakihara, I.; Noma, Y.; Horiguchi, S.; Harada, R.; Tsutsumi, K.; Hori, K.; et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy 2016, 48, 26–34. [Google Scholar] [CrossRef]
- Yashika, J.; Ohno, E.; Ishikawa, T.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yamada, K.; Yoshikawa, M.; Gibo, N.; Shimoyama, Y.; et al. Utility of multiphase contrast enhancement patterns on CEH-EUS for the differential diagnosis of IPMN-derived and conventional pancreatic cancer. Pancreatology 2021, 21, 390–396. [Google Scholar] [CrossRef]
- Ohno, E.; Itoh, A.; Kawashima, H.; Ishikawa, T.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; Miyahara, R.; et al. Malignant transformation of branch duct-type intraductal papillary mucinous neoplasms of the pancreas based on contrast-enhanced endoscopic ultrasonography morphological changes: Focus on malignant transformation of intraductal papillary mucinous neoplasm itself. Pancreas 2012, 41, 855–862. [Google Scholar]
- Leem, G.; Chung, M.J.; Park, J.Y.; Bang, S.; Song, S.Y.; Chung, J.B.; Park, S.W. Clinical Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography in the Differential Diagnosis of Pancreatic and Gallbladder Masses. Clin. Endosc. 2018, 51, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yoshikawa, T.; Yamazaki, H.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Ida, Y.; Maekita, T.; et al. A Novel Endoscopic Ultrasonography Imaging Technique for Depicting Microcirculation in Pancreatobiliary Lesions without the Need for Contrast-Enhancement: A Prospective Exploratory Study. Diagnostics 2021, 11, 2018. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yoshikawa, T.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ida, Y.; Maekita, T.; Iguchi, M.; Murata, S.I.; et al. Novel endoscopic ultrasonography imaging technique for visualizing microcirculation without contrast enhancement in subepithelial lesions: Prospective study. Dig. Endosc. 2021, 33, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L.; et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.A.; Adams, D.H. Gallbladder polyps: When to wait and when to act. Lancet 1997, 349, 817. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Chiam, K.H.; Shin, S.H.; Choi, K.C.; Leiria, F.; Militz, M.; Singh, R. Current Status of Mucosal Imaging with Narrow-Band Imaging in the Esophagus. Gut Liver 2021, 15, 492–499. [Google Scholar] [CrossRef]
- Inoue, H.; Kaga, M.; Ikeda, H.; Sato, C.; Sato, H.; Minami, H.; Santi, E.G.; Hayee, B.H.; Eleftheriadis, N. Magnification endoscopy in esophageal squamous cell carcinoma: A review of the intrapapillary capillary loop classification. Ann. Gastroenterol. 2015, 28, 41–48. [Google Scholar]
- Jensen, J.A. Estimation of Blood Velocities Using Ultrasound: A Signal Processing Approach; Cambridge University Press: New York, NY, USA, 1996. [Google Scholar]
- Lu, R.; Meng, Y.; Zhang, Y.; Zhao, W.; Wang, X.; Jin, M.; Guo, R. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med. Imaging 2017, 28, 65. [Google Scholar] [CrossRef]
- Bakdik, S.; Arslan, S.; Oncu, F.; Durmaz, M.S.; Altunkeser, A.; Eryilmaz, M.A.; Unlu, Y. Effectiveness of Superb Microvascular Imaging for the differentiation of intraductal breast lesions. Med. Ultrason. 2018, 20, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, T.J.; Revels, J.; Wang, S.; Toia, G.; Sonneborn, R.; Hippe, D.S.; Erpelding, T. Comparison of Superb Microvascular Imaging with Color Flow and Power Doppler Imaging of Small Hepatocellular Carcinomas. J. Ultrasound Med. 2018, 37, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Mu, J.; Zhao, J.; Zhao, L.; Xin, X. The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: A retrospective study. Br. J. Radiol. 2018, 91, 20170601. [Google Scholar] [CrossRef]
- Sim, J.K.; Lee, J.Y.; Hong, H.S. Differentiation Between Malignant and Benign Lymph Nodes: Role of Superb Microvascular Imaging in the Evaluation of Cervical Lymph Nodes. J. Ultrasound Med. 2019, 38, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, J.; Jin, C.; Chen, Y.; Chen, S.; Guo, G.; Gong, X. Diagnostic Value of Superb Microvascular Imaging in Differentiating Benign and Malignant Breast Tumors: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2648. [Google Scholar] [CrossRef]
- Bayramoglu, Z.; Kandemirli, S.G.; Akyol Sarı, Z.N.; Kardelen, A.D.; Poyrazoglu, S.; Bas, F.; Darendeliler, F.; Adaletli, I. Superb Microvascular Imaging in the Evaluation of Pediatric Graves Disease and Hashimoto Thyroiditis. J. Ultrasound Med. 2020, 39, 901–909. [Google Scholar] [CrossRef]
- Leong, J.Y.; Wessner, C.E.; Kramer, M.R.; Forsberg, F.; Halpern, E.J.; Lyshchik, A.; Torkzaban, M.; Morris, A.; Byrne, K.; VanMeter, M.; et al. Superb Microvascular Imaging Improves Detection of Vascularity in Indeterminate Renal Masses. J. Ultrasound Med. 2020, 39, 1947–1955. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.Y.; Yoon, R.G.; Kim, J.H.; Hong, H.S. The Value of Microvascular Imaging for Triaging Indeterminate Cervical Lymph Nodes in Patients with Papillary Thyroid Carcinoma. Cancers 2020, 12, 2839. [Google Scholar] [CrossRef]
- Kin, T.; Nagai, K.; Hayashi, T.; Takahashi, K.; Katanuma, A. Efficacy of superb microvascular imaging of ultrasound for diagnosis of gallbladder lesion. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 977–983. [Google Scholar] [CrossRef]
- Kuroda, H.; Abe, T.; Kakisaka, K.; Fujiwara, Y.; Yoshida, Y.; Miyasaka, A.; Ishida, K.; Ishida, H.; Sugai, T.; Takikawa, Y. Visualizing the hepatic vascular architecture using superb microvascular imaging in patients with hepatitis C virus: A novel technique. World J. Gastroenterol. 2016, 22, 6057–6064. [Google Scholar] [CrossRef]

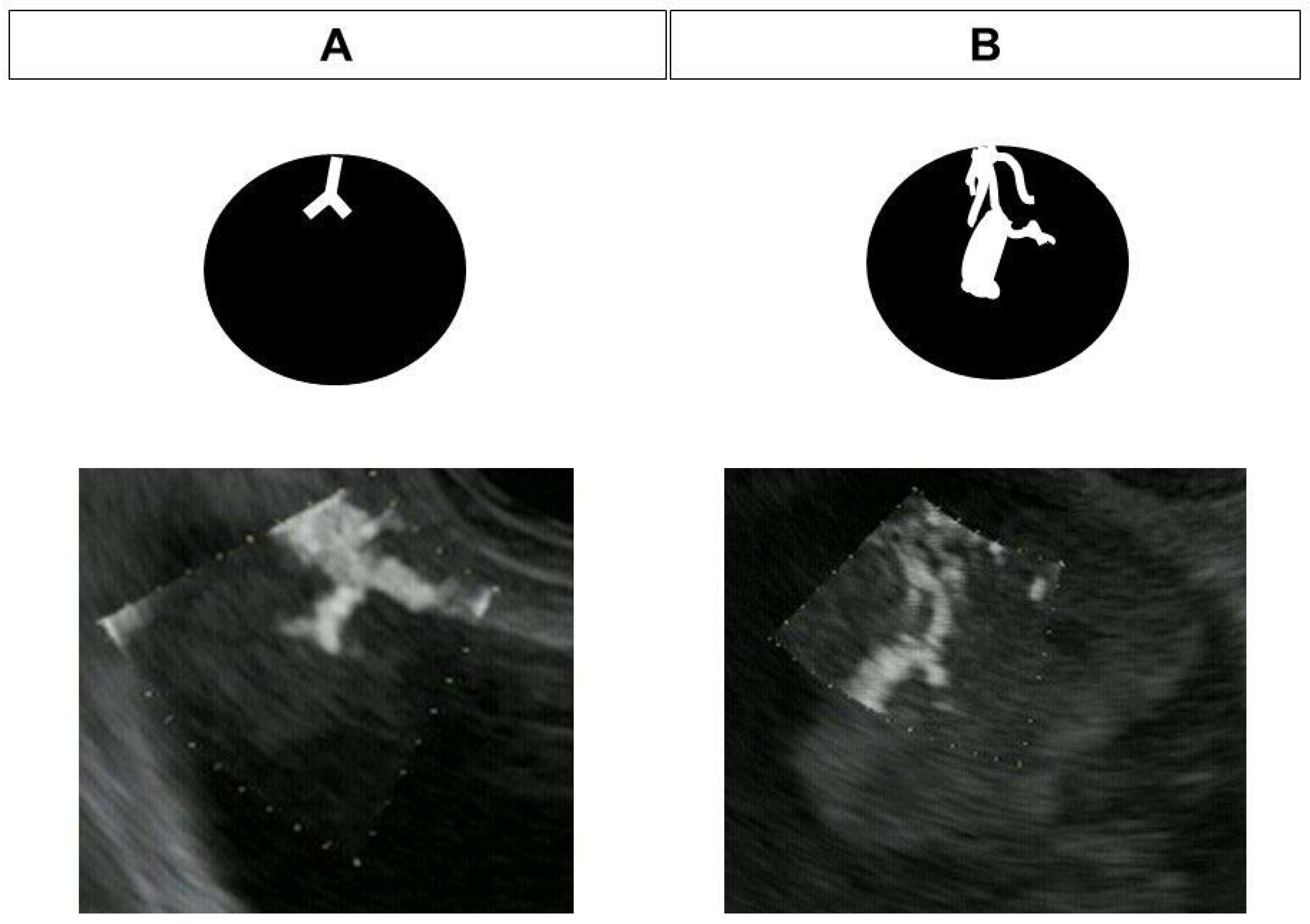


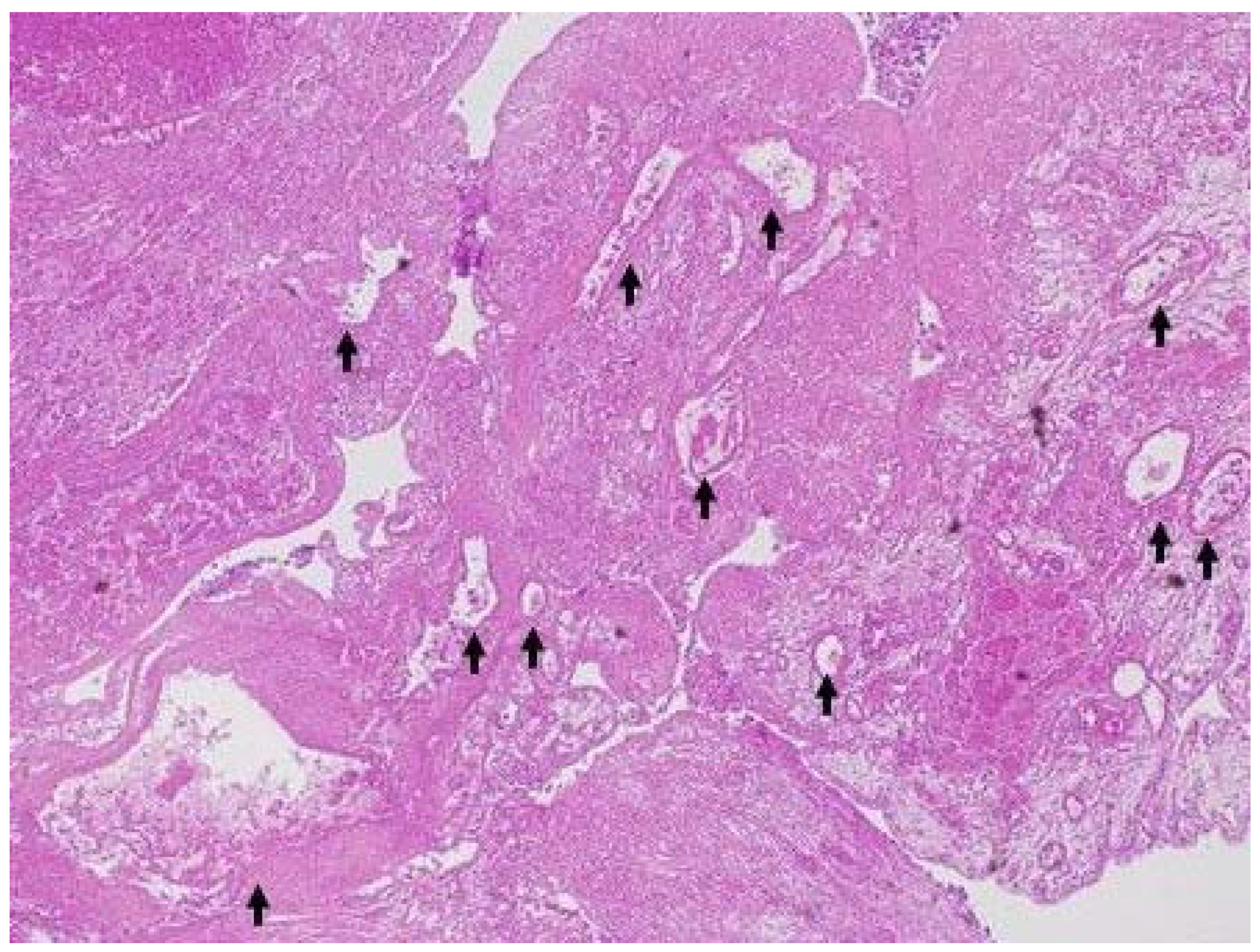

| IPMN (n = 13) | Gallbladder Lesion (n = 20) | |
|---|---|---|
| Age, years, mean ± SD | 66.9 ± 13.0 | 67.6 ± 10.5 |
| Sex, male/female | 6/7 | 13/7 |
| Final diagnosis | mucous clot (9), mural nodule (4) | sludge (8), solid lesion (12) |
| Mural nodule or solid gallbladder lesion size, median (range) | 8.5 (5–24) mm | 26 (10–44) mm |
| Final diagnosis of mural nodule or solid gallbladder lesion | IPMA (1), IPMC (3); stage 0 (1), stage IA (2) | carcinoma (8), metastasis of renal cancer (1), adenoma (1), cholesterol polyp (1), chronic cholecystitis (1) |
| Final Diagnosis (Pathology) | ||
|---|---|---|
| Benign (n = 3) | Malignant (n = 9) | |
| DFI-EUS finding | ||
| Regular vessel | 3 (100%) | 1 (11%) |
| Irregular vessel | 0 (0%) | 8 (89%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Ashida, R.; Tamura, T.; Shimokawa, T.; Yamazaki, H.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Kitano, M. Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study. Diagnostics 2023, 13, 2132. https://doi.org/10.3390/diagnostics13132132
Yamashita Y, Ashida R, Tamura T, Shimokawa T, Yamazaki H, Kawaji Y, Tamura T, Hatamaru K, Itonaga M, Kitano M. Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study. Diagnostics. 2023; 13(13):2132. https://doi.org/10.3390/diagnostics13132132
Chicago/Turabian StyleYamashita, Yasunobu, Reiko Ashida, Takaaki Tamura, Toshio Shimokawa, Hirofumi Yamazaki, Yuki Kawaji, Takashi Tamura, Keiichi Hatamaru, Masahiro Itonaga, and Masayuki Kitano. 2023. "Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study" Diagnostics 13, no. 13: 2132. https://doi.org/10.3390/diagnostics13132132
APA StyleYamashita, Y., Ashida, R., Tamura, T., Shimokawa, T., Yamazaki, H., Kawaji, Y., Tamura, T., Hatamaru, K., Itonaga, M., & Kitano, M. (2023). Novel Technique of Endoscopic Ultrasonography for the Differential Diagnosis of Gallbladder Lesions and Intraductal Papillary Mucinous Neoplasms: A Single-Center Prospective Study. Diagnostics, 13(13), 2132. https://doi.org/10.3390/diagnostics13132132








