Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic–Zooanthroponotic Transmission Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiology of Specimens and Antibiograms of Staphylococcus aureus Lineages from Patients
2.2. Standard Definitions for Classification of Resistance in S. aureus as Multi-Drug Resistant Bacteria (MDR)
2.3. Molecular Profiling of S. aureus Lineages by Multi-Gene Systems (GeneXpert)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2015. Available online: https://www.who.int/initiatives/glass (accessed on 8 May 2023).
- Fowler, V.G.; Miro, J.M.; Hoen, B.; Cabell, C.H.; Abrutyn, E.; Rubinstein, E.; Corey, G.R.; Spelman, D.; Bradley, S.F.; Barsic, B.; et al. Staphylococcus aureus Endocarditis: A Consequence of Medical Progress. JAMA 2005, 293, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Said, K.B.; Ismail, J.; Campbell, J.; Mulvey, M.R.; Bourgault, A.-M.; Messier, S.; Zhao, X. Regional Profiling for Determination of Genotype Diversity of Mastitis-Specific Staphylococcus aureus Lineage in Canada by Use of Clumping Factor A, Pulsed-Field Gel Electrophoresis, and spa Typing. J. Clin. Microbiol. 2010, 48, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Said, K.B.; Ramotar, K.; Zhu, G.; Zhao, X. Repeat-based subtyping and grouping of Staphylococcus aureus from human infections and bovine mastitis using the R-domain of the clumping factor A gene. Diagn. Microbiol. Infect. Dis. 2009, 63, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.; Francioli, P.; Blanc, D.S. Evidence for Clonal Evolution among Highly Polymorphic Genes in Methicillin-Resistant Staphylococcus aureus. J. Bacteriol. 2006, 188, 169–178. [Google Scholar] [CrossRef]
- Feil, E.J.; Cooper, J.E.; Grundmann, H.; Robinson, D.A.; Enright, M.C.; Berendt, T.; Peacock, S.J.; Smith, J.M.; Murphy, M.; Spratt, B.G.; et al. How Clonal Is Staphylococcus aureus? J. Bacteriol. 2003, 185, 3307–3316. [Google Scholar] [CrossRef]
- Said, K.B.; Zhu, G.; Zhao, X. Organ- and Host-Specific Clonal Groups of Staphylococcus aureus from Human Infections and Bovine Mastitis Revealed by the Clumping Factor A Gene. Foodborne Pathog. Dis. 2010, 7, 111–119. [Google Scholar] [CrossRef]
- Van Leeuwen, W.B.; Melles, D.C.; Alaidan, A.; Al-Ahdal, M.; Boelens, H.A.M.; Snijders, S.V.; Wertheim, H.; van Duijkeren, E.; Peeters, J.K.; van der Spek, P.J.; et al. Host- and Tissue-Specific Pathogenic Traits of Staphylococcus aureus. J. Bacteriol. 2005, 187, 4584–4591. [Google Scholar] [CrossRef]
- Josefsson, E.; Kubica, M.; Mydel, P.; Potempa, J.; Tarkowski, A. In vivo sortase A and clumping factor A mRNA expression during Staphylococcus aureus infection. Microb. Pathog. 2008, 44, 103–110. [Google Scholar] [CrossRef]
- Rossney, A.S.; Shore, A.C.; Morgan, P.M.; Fitzgibbon, M.M.; O’Connell, B.; Coleman, D.C. The Emergence and Importation of Diverse Genotypes of Methicillin-Resistant Staphylococcus aureus (MRSA) Harboring the Panton-Valentine Leukocidin Gene (pvl) Reveal that pvl Is a Poor Marker for Community-Acquired MRSA Strains in Ireland. J. Clin. Microbiol. 2007, 45, 2554–2563. [Google Scholar] [CrossRef]
- Li, M.; Diep, B.A.; Villaruz, A.E.; Braughton, K.R.; Jiang, X.; DeLeo, F.R.; Chambers, H.F.; Lu, Y.; Otto, M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 5883–5888. [Google Scholar] [CrossRef]
- Wardenburg, J.B.; Palazzolo-Ballance, A.M.; Otto, M.; Schneewind, O.; DeLeo, F.R. Panton-Valentine Leukocidin Is Not a Virulence Determinant in Murine Models of Community-Associated Methicillin-Resistant Staphylococcus aureus Disease. J. Infect. Dis. 2008, 198, 1166–1170. [Google Scholar] [CrossRef]
- Ogston, A. Report upon Micro-Organisms in Surgical Diseases. BMJ 1881, 1, 369–377. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Tenover, F.C.; Gorwitz, R.J. The Epidemiology of Staphylococcus Infections. Gram-Posit. Pathog. 2014, 526–534. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.E.O.; Jevons, M.P.; Shooter, R.A.; Hunter, C.J.W.; Girling, J.A.; Griffiths, J.D.; Taylor, G.W. Nasal Staphylococci and Sepsis in Hospital Patients. BMJ 1959, 2, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Kirby, W.M.M. Extraction of a Highly Potent Penicillin Inactivator from Penicillin Resistant Staphylococci. Science 1944, 99, 452–453. [Google Scholar] [CrossRef]
- Jevons, M.P. “Celbenin”—Resistant Staphylococci. BMJ 1961, 1, 124. [Google Scholar] [CrossRef]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Projan, S.J.; Ruzin, A. Antibiotic Resistance in the Staphylococci. Gram-Posit. Pathog. 2014, 587–597. [Google Scholar] [CrossRef]
- Bryskier, A. Penicillins. Antimicrob. Agents 2014, 113–162. [Google Scholar] [CrossRef]
- Ito, T.; Hiramatsu, K.; Oliveira, D.C.; de Lencastre, H.; Zhang, K.; Westh, H.; O’Brien, F.; Giffard, P.M.; Coleman, D.; Tenover, F.C.; et al. Classification of Staphylococcal Cassette Chromosome mec (SCC mec): Guidelines for Reporting Novel SCC mec Elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef]
- Robinson, D.A.; Enright, M.C. Evolutionary Models of the Emergence ofMethicillin-Resistant Staphylococcusaureus. Antimicrob. Agents Chemother. 2003, 47, 3926–3934. [Google Scholar] [CrossRef]
- Ito, T.; Okuma, K.; Ma, X.X.; Yuzawa, H.; Hiramatsu, K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist. Updat. 2003, 6, 41–52. [Google Scholar] [CrossRef]
- Shang, W.; Rao, Y.; Zheng, Y.; Yang, Y.; Hu, Q.; Hu, Z.; Yuan, J.; Peng, H.; Xiong, K.; Tan, L.; et al. β-Lactam Antibiotics Enhance the Pathogenicity of Methicillin-Resistant Staphylococcus aureus via SarA-Controlled Lipoprotein-Like Cluster Expression. mBio 2019, 10, e00880-19. [Google Scholar] [CrossRef]
- Halaby, T.; al Naiemi, N.; Kluytmans, J.; van der Palen, J.; Vandenbroucke-Grauls, C.M. Emergence of Colistin Resistance in Enterobacteriaceae after the Introduction of Selective Digestive Tract Decontamination in an Intensive Care Unit. Antimicrob. Agents Chemother. 2013, 57, 3224–3229. [Google Scholar] [CrossRef]
- Namdari, S.; Farhadi, A.; Khademalhoseini, A.; Behzad-Behbahani, A.; Moaddeb, A. Emergence of Highly Multidrug-Resistant Bacteria Isolated from Patients with Infections Admitted to Public Hospitals in Southwest Iran. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 5265379. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Awada, R.; Salami, A.; Bahmad, H.F.; Awad, A.; Joumaa, W.H.; El Roz, A. Prevalence, Laboratory Findings and Clinical Characteristics of Campylobacteriosis Agents among Hospitalized Children with Acute Gastroenteritis in Lebanon. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aires-De-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Corey, G.R. Epidemiology of Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. S5), S344–S349. [Google Scholar] [CrossRef] [PubMed]
- Kuehnert, M.J.; Hill, H.A.; Kupronis, B.A.; Tokars, J.I.; Solomon, S.L.; Jernigan, D.B. Methicillin-resistant–Staphylococcus aureus Hospitalizations, United States. Emerg. Infect. Dis. 2005, 11, 868–872. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef]
- Louise Gerberding, J.; Director, M.; Cohen, M.; Ronald Valdiserri, P.O.; Acting Director, M.; Janssen, R.S. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2004-vol-16.pdf (accessed on 7 June 2023).
- FastStats—Viral Hepatitis. Available online: https://www.cdc.gov/nchs/fastats/hepatitis.htm (accessed on 19 March 2022).
- Ito, T.; Iijima, M.; Fukushima, T.; Nonoyama, M.; Ishii, M.; Baranovich, T.; Otsuka, T.; Takano, T.; Yamamoto, T. Pediatric Pneumonia Death Caused by Community-acquired Methicillin-Resistant Staphylococcus aureus, Japan. Emerg. Infect. Dis. 2008, 14, 1312–1314. [Google Scholar] [CrossRef]
- Zetola, N.; Francis, J.S.; Nuermberger, E.L.; Bishai, W.R. Community-acquired meticillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect. Dis. 2005, 5, 275–286. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Stobberingh, E.E. The evolution of Staphylococcus aureus. Infect. Genet. Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hung, W.-C.; Takano, T.; Nishiyama, A. Genetic nature and virulence of community-associated methicillin-resistant Staphylococcus aureus. Biomed. Pharmacother. 2013, 3, 2–18. [Google Scholar] [CrossRef]
- Uhlemann, A.-C.; Otto, M.; Lowy, F.D.; DeLeo, F.R. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. 2014, 21, 563–574. [Google Scholar] [CrossRef]
- Diep, B.A.; Stone, G.G.; Basuino, L.; Graber, C.J.; Miller, A.; Etages, S.D.; Jones, A.; Palazzolo-Ballance, A.M.; Perdreau-Remington, F.; Sensabaugh, G.F.; et al. The Arginine Catabolic Mobile Element and Staphylococcal Chromosomal Cassette mec Linkage: Convergence of Virulence and Resistance in the USA300 Clone of Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 2008, 197, 1523–1530. [Google Scholar] [CrossRef]
- Levin, B.R. Minimizing Potential Resistance: A Population Dynamics View. Clin. Infect. Dis. 2001, 33 (Suppl. S3), S161–S169. [Google Scholar] [CrossRef] [PubMed]
- Aratani, T.; Tsukamoto, H.; Higashi, T.; Kodawara, T.; Yano, R.; Hida, Y.; Iwasaki, H.; Goto, N. Association of methicillin resistance with mortality of hospital-acquired Staphylococcus aureus bacteremia. J. Int. Med. Res. 2021, 49, 03000605211058872. [Google Scholar] [CrossRef]
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in Animals. Microbiol. Spectr. 2019, 7, GPP3-0060-2019. [Google Scholar] [CrossRef] [PubMed]
- Vandenesch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Liassine, N.; Bes, M.; Greenland, T.; Reverdy, M.-E.; et al. Community-Acquired Methicillin-Resistant Staphylococcus aureus Carrying Panton-Valentine Leukocidin Genes: Worldwide Emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Brumfitt, W.; Hamilton-Miller, J. Methicillin-Resistant Staphylococcus aureus. N. Engl. J. Med. 1989, 320, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Naimi, T.S.; LeDell, K.H.; Como-Sabetti, K.; Borchardt, S.M.; Boxrud, D.J.; Etienne, J.; Johnson, S.K.; Vandenesch, F.; Fridkin, S.; O’Boyle, C.; et al. Comparison of Community- and Health Care–Associated Methicillin-Resistant Staphylococcus aureus Infection. JAMA 2003, 290, 2976–2984. [Google Scholar] [CrossRef]
- Da Costa, T.M.; De Oliveira, C.R.; Chambers, H.F.; Chatterjee, S.S. PBP4: A New Perspective on Staphylococcus aureus β-Lactam Resistance. Microorganisms 2018, 6, 57. [Google Scholar] [CrossRef]
- Geisinger, E.; Isberg, R.R. Interplay Between Antibiotic Resistance and Virulence During Disease Promoted by Multidrug-Resistant Bacteria. J. Infect. Dis. 2017, 215 (Suppl. S1), S9–S17. [Google Scholar] [CrossRef]
- Kateete, D.P.; Bwanga, F.; Seni, J.; Mayanja, R.; Kigozi, E.; Mujuni, B.; Ashaba, F.K.; Baluku, H.; Najjuka, C.F.; Källander, K.; et al. CA-MRSA and HA-MRSA coexist in community and hospital settings in Uganda. Antimicrob. Resist. Infect. Control 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Al-Saleh, A.; Shahid, M.; Farid, E.; Bindayna, K. Trends in methicillin-resistant Staphylococcus aureus in the Gulf Cooperation Council countries: Antibiotic resistance, virulence factors and emerging strains. East. Mediterr. Health J. 2022, 28, 434–443. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Balkhy, H.H.; Assiri, A.; Al Mousa, H.; Al-Abri, S.S.; Al-Katheeri, H.; Alansari, H.; Abdulrazzaq, N.M.; Aidara-Kane, A.; Pittet, D.; Erlacher-Vindel, E.; et al. The strategic plan for combating antimicrobial resistance in Gulf Cooperation Council States. J. Infect. Public Health 2016, 9, 375–385. [Google Scholar] [CrossRef]
- Balkhy, H.H.; Zowawi, H.M.; Alshamrani, M.M.; Allegranzi, B.; Srinivasan, A.; Al-Abdely, H.M.; Somily, A.M.; Al-Quwaizani, M.A.; Al-Maani, A.S.; Al-Katheeri, H.A.; et al. Antimicrobial resistance: A round table discussion on the “One Health” concept from the Gulf Cooperation Council Countries. Part Two: A focus on Human Health. J. Infect. Public Health 2018, 11, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.E. Community-Acquired Methicillin-Resistant Staphylococcus aureus: The New Face of an Old Foe? Med. Princ. Pract. 2013, 22 (Suppl. S1), 20–29. [Google Scholar] [CrossRef]
- Vali, L.; Dashti, A.A.; Mathew, F.; Udo, E.E. Characterization of Heterogeneous MRSA and MSSA with Reduced Susceptibility to Chlorhexidine in Kuwaiti Hospitals. Front. Microbiol. 2017, 8, 1359. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, W.; Udo, E.E.; Modhaffer, A.; Alosaimi, A. Molecular Characterization of Methicillin- Resistant Staphylococcus aureus in a Tertiary Care hospital in Kuwait. Sci. Rep. 2019, 9, 18527. [Google Scholar] [CrossRef]
- Senok, A.; Nassar, R.; Celiloglu, H.; Nabi, A.; Alfaresi, M.; Weber, S.; Rizvi, I.; Müller, E.; Reissig, A.; Gawlik, D.; et al. Genotyping of methicillin resistant Staphylococcus aureus from the United Arab Emirates. Sci. Rep. 2020, 10, 18551. [Google Scholar] [CrossRef]
- Al Jalaf, M.; Fadali, H.; Alanee, R.; Najjar, F.; Al Deesi, Z.; Seliem, R.M.; Nilles, E.J. Methicillin resistant Staphylococcus Aureus in emergency department patients in the United Arab Emirates. BMC Emerg. Med. 2018, 18, 12. [Google Scholar] [CrossRef]
- Alrahmany, D.; Albeloushi, A.; Alreesi, I.; Alzaabi, A.; Alreesi, M.; Pontiggia, L.; Ghazi, I.M. Exploring bacterial resistance in Northern Oman, a foundation for implementing evidence-based antimicrobial stewardship program. Int. J. Infect. Dis. 2019, 83, 77–82. [Google Scholar] [CrossRef]
- Eed, E.M.; Ghonaim, M.M.; Hussein, Y.M.; Saber, T.M.; Khalifa, A.S. Phenotypic and molecular characterization of HA-MRSA in Taif hospitals, Saudi Arabia. J. Infect. Dev. Ctries. 2015, 9, 298–303. [Google Scholar] [CrossRef]
- Monecke, S.; Skakni, L.; Hasan, R.; Ruppelt, A.; Ghazal, S.S.; Hakawi, A.; Slickers, P.; Ehricht, R. Characterisation of MRSA strains isolated from patients in a hospital in Riyadh, Kingdom of Saudi Arabia. BMC Microbiol. 2012, 12, 146. [Google Scholar] [CrossRef]
- Alkharsah, K.R.; Rehman, S.; Alkhamis, F.; AlNimr, A.; Diab, A.; Al-Ali, A.K. Comparative and molecular analysis of MRSA isolates from infection sites and carrier colonization sites. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 7. [Google Scholar] [CrossRef]
- Sonnevend, Á.; Blair, I.; Alkaabi, M.; Jumaa, P.; al Haj, M.; Ghazawi, A.; Akawi, N.; Jouhar, F.S.; Hamadeh, M.B.; Pál, T. Change in meticillin-resistant Staphylococcus aureus clones at a tertiary care hospital in the United Arab Emirates over a 5-year period. J. Clin. Pathol. 2012, 65, 178–182. [Google Scholar] [CrossRef]
- Senok, A.; Somily, A.M.; Nassar, R.; Garaween, G.; Sing, G.K.; Müller, E.; Reissig, A.; Gawlik, D.; Ehricht, R.; Monecke, S. Emergence of novel methicillin-resistant Staphylococcus aureus strains in a tertiary care facility in Riyadh, Saudi Arabia. Infect. Drug Resist. 2019, 12, 2739–2746. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Udo, E.E.; Monecke, S.; Mathew, B.; Noronha, B.; Verghese, T.; Tappa, S.B. Emerging variants of methicillin-resistant Staphylococcus aureus genotypes in Kuwait hospitals. PLoS ONE 2018, 13, e0195933. [Google Scholar] [CrossRef]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [PubMed]
- De Jong, N.W.M.; van Kessel, K.P.M.; van Strijp, J.A.G. Immune Evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, GPP3-0061-2019. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing An Informational Supplement for Global Application Devel-Oped through the Clinical and Laboratory Standards Institute Consensus Process. M100; 26th ANSI Web Store. Available online: https://webstore.ansi.org/standards/clsi/clsim100s26 (accessed on 7 June 2023).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Suresh, K.P.; Shinduja, R.; Amachawadi, R.G.; Chandrashekar, S.; Pradeep, S.; Kollur, S.P.; Syed, A.; Sood, R.; Roy, P.; et al. Prevalence of Methicillin-resistant Staphylococcus Aureus in India: A Systematic Review and Meta-analysis. Oman Med. J. 2022, 37, e440. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Taha, A.E.; Al-Ruwaili, N.M.; El-Masry, E.A.; Saad, A.E.; Taher, I.A. MRSA as an indicator of infection control measures in Turaif General Hospital, Northern Area-Saudi Arabia. J. Infect. Dev. Ctries. 2022, 16, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Farook, N.A.; Argimón, S.; Abdul Samat, M.N.; Salleh, S.A.; Sulaiman, S.; Tan, T.L.; Periyasamy, P.; Lau, C.L.; Ismail, Z.; Muhammad Azami, N.A.; et al. Diversity and Dissemination of Methicillin-Resistant Staphylococcus aureus (MRSA) Genotypes in Southeast Asia. Trop. Med. Infect. Dis. 2022, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yin, Y.; van Dorp, L.; Shaw, L.P.; Gao, H.; Acman, M.; Yuan, J.; Chen, F.; Sun, S.; Wang, X.; et al. Drivers of methicillin-resistant Staphylococcus aureus (MRSA) lineage replacement in China. Genome Med. 2021, 13, 171. [Google Scholar] [CrossRef]
- Abroo, S.; Hosseini Jazani, N.; Sharifi, Y. Methicillin-resistant Staphylococcus aureus nasal carriage between healthy students of medical and nonmedical universities. Am. J. Infect. Control 2017, 45, 709–712. [Google Scholar] [CrossRef]
- Ahmadi, E.; Khojasteh, M.; Mortazavi, S.M.; Khan-Mohammadi, F.; Kazemnia, A.; Beheshtipour, J.; Raeeszadeh, M. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus nasal carriage in the West of Iran: A population-based cross-sectional study. BMC Infect. Dis. 2019, 19, 899. [Google Scholar] [CrossRef]
- Qodrati, M.; SeyedAlinaghi, S.A.; Dehghan Manshadi, S.A.; Abdollahi, A.; Dadras, O. Antimicrobial susceptibility testing of Staphylococcus aureus isolates from patients at a tertiary hospital in Tehran, Iran, 2018–2019. Eur. J. Med. Res. 2022, 27, 152. [Google Scholar] [CrossRef]
- El Amin, N.M.; Faidah, H.S. Methicillin-resistant Staphylococcus aureus in the western region of Saudi Arabia: Prevalence and antibiotic susceptibility pattern. Ann. Saudi Med. 2012, 32, 513–516. [Google Scholar] [CrossRef]
- Al Musawi, S.; Alkhaleefa, Q.; Alnassri, S.; Alamri, A.M.; Alnimr, A. Eleven-Year Surveillance of Methicillin-Resistant Staphylococcus aureus Infections at an Academic Health Centre. J. Prev. Med. Hyg. 2022, 63, E132–E138. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Jansen, A.; Lewitter, F.; Fink, G.R. Intragenic tandem repeats generate functional variability. Nat. Genet. 2005, 37, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Giddey, M.; Moreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus Host Range and Human-Bovine Host Shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Golubchik, T.; Batty, E.M.; Fung, R.; Larner-Svensson, H.; Votintseva, A.A.; Miller, R.R.; Godwin, H.; Knox, K.; Everitt, R.G.; et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc. Natl. Acad. Sci. USA 2012, 109, 4550–4555. [Google Scholar] [CrossRef] [PubMed]
- Seidl, K.; Müller, S.; François, P.; Kriebitzsch, C.; Schrenzel, J.; Engelmann, S.; Bischoff, M.; Berger-Bächi, B. Effect of a glucose impulse on the CcpA regulon in Staphylococcus aureus. BMC Microbiol. 2009, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Atkins, K.L.; Burman, J.D.; Chamberlain, E.S.; Cooper, J.E.; Poutrel, B.; Bagby, S.; Jenkins, A.T.A.; Feil, E.J.; van den Elsen, J.M.H.S. aureus IgG-binding proteins SpA and Sbi: Host specificity and mechanisms of immune complex formation. Mol. Immunol. 2008, 45, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.L.; Walsh, E.; Choudhurry, R.; Melles, D.C.; Boelens, H.A.M.; Miajlović, H.; Verbrugh, H.A.; Foster, T.; Van Belkum, A. Key Role for Clumping Factor B in Staphylococcus aureus Nasal Colonization of Humans. PLoS Med. 2008, 5, 0104–0112. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Paquette, K.M.; Tikh, I.B.; Volper, E.M.; Greenberg, E.P. Generation of Virulence Factor Variants in Staphylococcus aureus Biofilms. J. Bacteriol. 2007, 189, 7961–7967. [Google Scholar] [CrossRef]

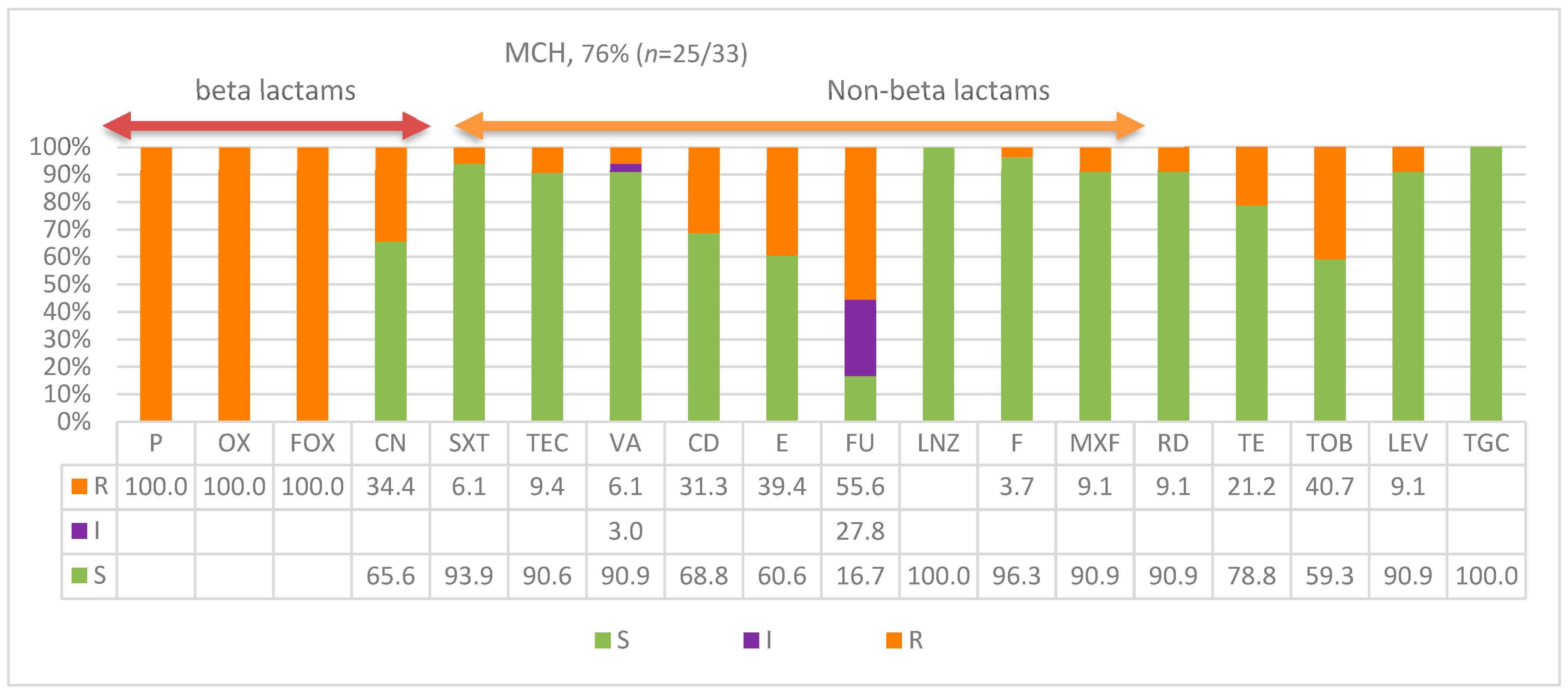
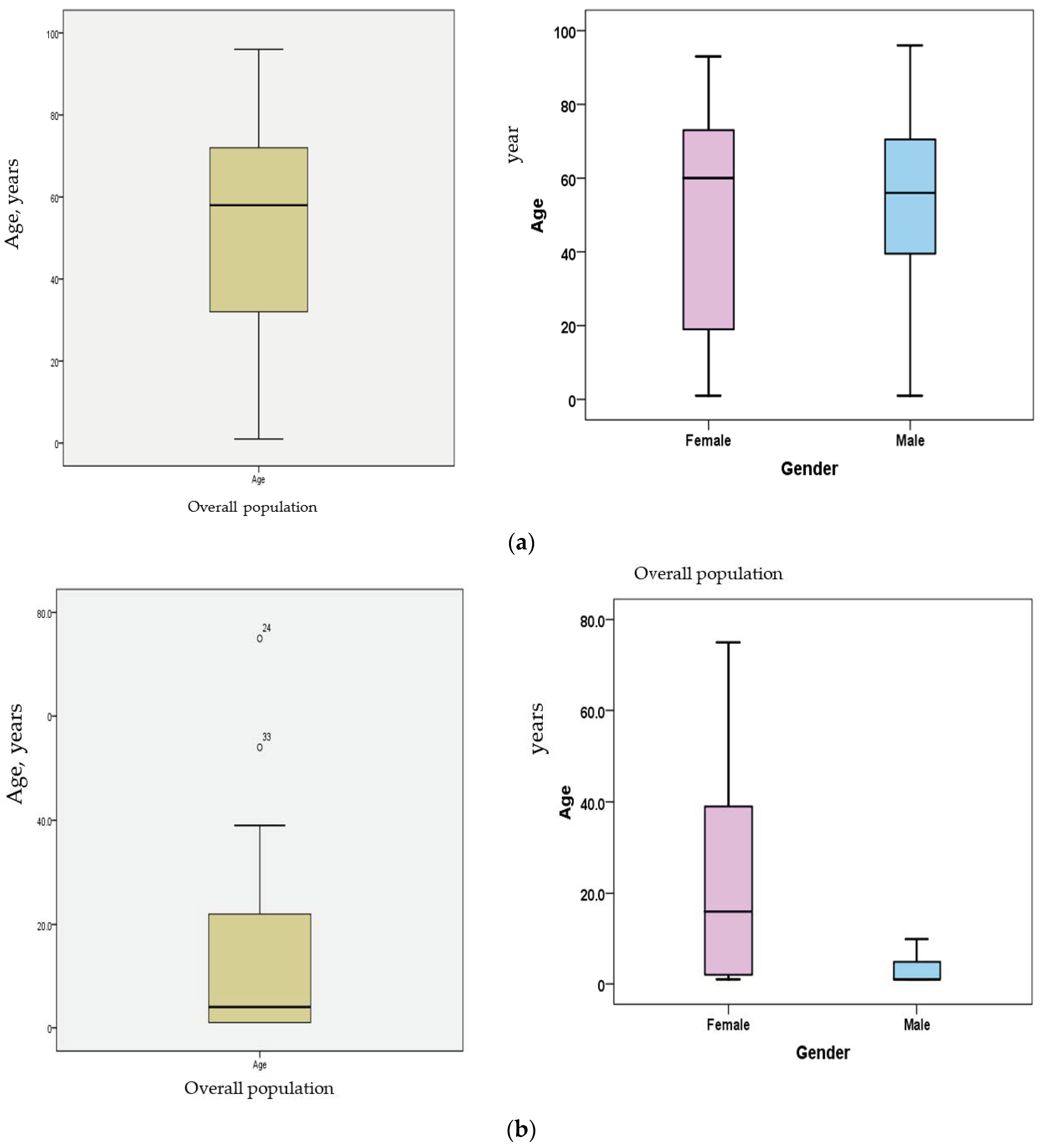
| Patient Age | MCH | KKH | Totals | Frequency |
|---|---|---|---|---|
| 0 to 20 | 23 (70%) | 19 (13%) | 42 | 23% |
| 21 to 50 | 7 | 41 (28%) | 48 | 27% |
| >50 | 2 | 87 (59%) | 89 | 49% |
| Not mentioned | 1 | 1 | 2 | |
| Total | 33 (76% CA-MRSA, n = 25) | 148 (80% CA-MRSA, n = 119) | 181 | 80% overall CA-MRSA, n = 144 |
| Gender | Total | |||
| Male | 15 | 87 | 102 | 70% |
| Female | 18 | 60 | 78 | 53% |
| Not mentioned | 0 | 1 | 1 | |
| Total | 33 | 148 | 181 | |
| Ward KKH (n = 148) | Total | |||
| ICU | 40 | 40/148 | 27% | |
| Men surgical ward | 16 | 16/148 | 11% | |
| Female surgical ward | 15 | 15/148 | 10% | |
| Female medical ward | 6 | 6/148 | 4% | |
| Medical neuropathy | 1 | 1 | ||
| Men medical ward | 16 | 16/148 | 11% | |
| Alkabtonuria | 7 | 7/148 | 5% | |
| ER | 21 | 21/148 | 14% | |
| PSW | 2 | 2 | ||
| PIW | 1 | 1 | ||
| Pediatric specimens | 33 | 33 | ||
| Total | 33 | 148 | 181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsolami, A.; ALGhasab, N.S.; Alharbi, M.S.M.; Bashir, A.I.; Saleem, M.; Syed Khaja, A.S.; Aldakheel, D.F.; Rakha, E.; Alshammari, J.A.; Taha, T.E.; et al. Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic–Zooanthroponotic Transmission Dynamics. Diagnostics 2023, 13, 2089. https://doi.org/10.3390/diagnostics13122089
Alsolami A, ALGhasab NS, Alharbi MSM, Bashir AI, Saleem M, Syed Khaja AS, Aldakheel DF, Rakha E, Alshammari JA, Taha TE, et al. Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic–Zooanthroponotic Transmission Dynamics. Diagnostics. 2023; 13(12):2089. https://doi.org/10.3390/diagnostics13122089
Chicago/Turabian StyleAlsolami, Ahmed, Naif Saad ALGhasab, Mohammed S. M. Alharbi, Abdelhafiz I. Bashir, Mohd Saleem, Azharuddin Sajid Syed Khaja, Dakheel F. Aldakheel, Ehab Rakha, Jabar Aziz Alshammari, Taha E. Taha, and et al. 2023. "Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic–Zooanthroponotic Transmission Dynamics" Diagnostics 13, no. 12: 2089. https://doi.org/10.3390/diagnostics13122089
APA StyleAlsolami, A., ALGhasab, N. S., Alharbi, M. S. M., Bashir, A. I., Saleem, M., Syed Khaja, A. S., Aldakheel, D. F., Rakha, E., Alshammari, J. A., Taha, T. E., Melibari, Z., Alharbi, Y. H., Almutlag, A. A., & Said, K. B., on behalf of the Ha’il COM Research Unit Group. (2023). Community-Acquired Methicillin-Resistant Staphylococcus aureus in Hospitals: Age-Specificity and Potential Zoonotic–Zooanthroponotic Transmission Dynamics. Diagnostics, 13(12), 2089. https://doi.org/10.3390/diagnostics13122089





