Dynamic and Static 18F-FDG PET/CT Imaging in SMARCA4-Deficient Non-Small Cell Lung Cancer and Response to Therapy: A Case Report
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nambirajan, A.; Singh, V.; Bhardwaj, N.; Mittal, S.; Kumar, S.; Jain, D. SMARCA4/BRG1-Deficient Non-Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch. Pathol. Lab. Med. 2021, 145, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Fuchs, F.; Moskalev, E.A.; Sirbu, H.; Hartmann, A.; Haller, F. SMARCA4-deficient pulmonary adenocarcinoma: Clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017, 471, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Udagawa, H.; Umemura, S.; Sakai, T.; Zenke, Y.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Tsuboi, M.; et al. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden. Lung Cancer 2019, 138, 35–42. [Google Scholar] [CrossRef]
- Concepcion, C.P.; Ma, S.; LaFave, L.M.; Bhutkar, A.; Liu, M.; DeAngelo, L.P.; Kim, J.Y.; Del Priore, I.; Schoenfeld, A.J.; Mille, M.; et al. Smarca4 Inactivation Promotes Lineage-Specific Transformation and Early Metastatic Features in the Lung. Cancer Discov. 2022, 12, 562–585. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.V.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gao, X.; Wang, F.; Li, S.; Zhou, Y.; Guo, P.; Meng, Y.; Lu, T. Clinical characteristics and prognostic analysis of SMARCA4-deficient non-small cell lung cancer. Cancer Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Umemura, S.; Nakamura, H.; Zenke, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Motoi, N.; et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac. Cancer 2019, 10, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Rahmim, A.; Lodge, M.A.; Karakatsanis, N.A.; Panin, V.Y.; Zhou, Y.; McMillan, A.; Cho, S.; Zaidi, H.; Casey, M.E.; Wahl, R.L. Dynamic whole-body PET imaging: Principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging. 2019, 46, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.G.; Klippel, S.; Pan, L.; Schönleben, K.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Assessment of quantitative FDG PET data in primary colorectal tumours: Which parameters are important with respect to tumour detection? Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Wumener, X.; Zhang, Y.; Wang, Z.; Zhang, M.; Zang, Z.; Huang, B.; Liu, M.; Huang, S.; Huang, Y.; Wang, P.; et al. Dynamic FDG-PET imaging for differentiating metastatic from non-metastatic lymph nodes of lung cancer. Front Oncol. 2022, 12, 1005924. [Google Scholar] [CrossRef] [PubMed]
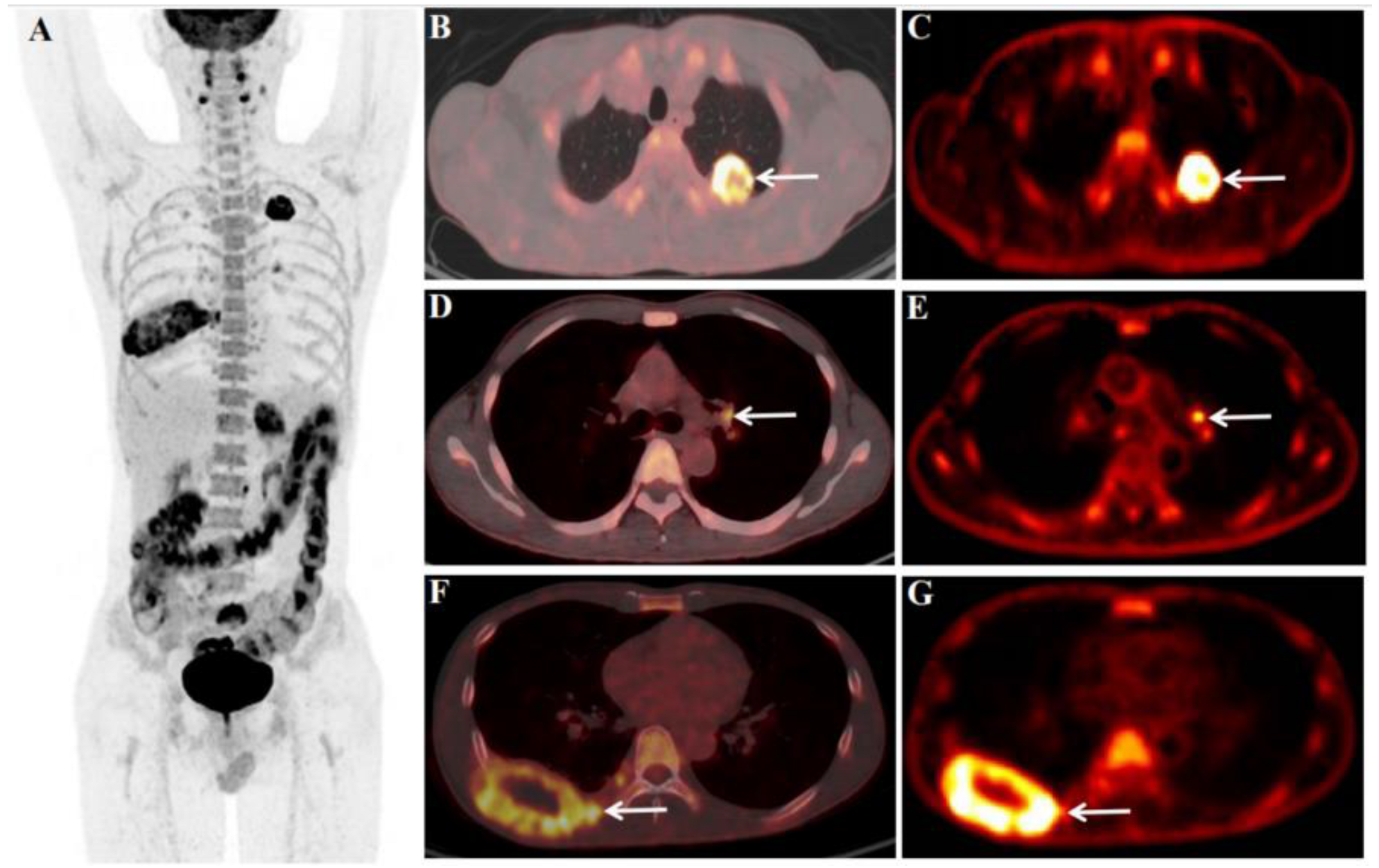
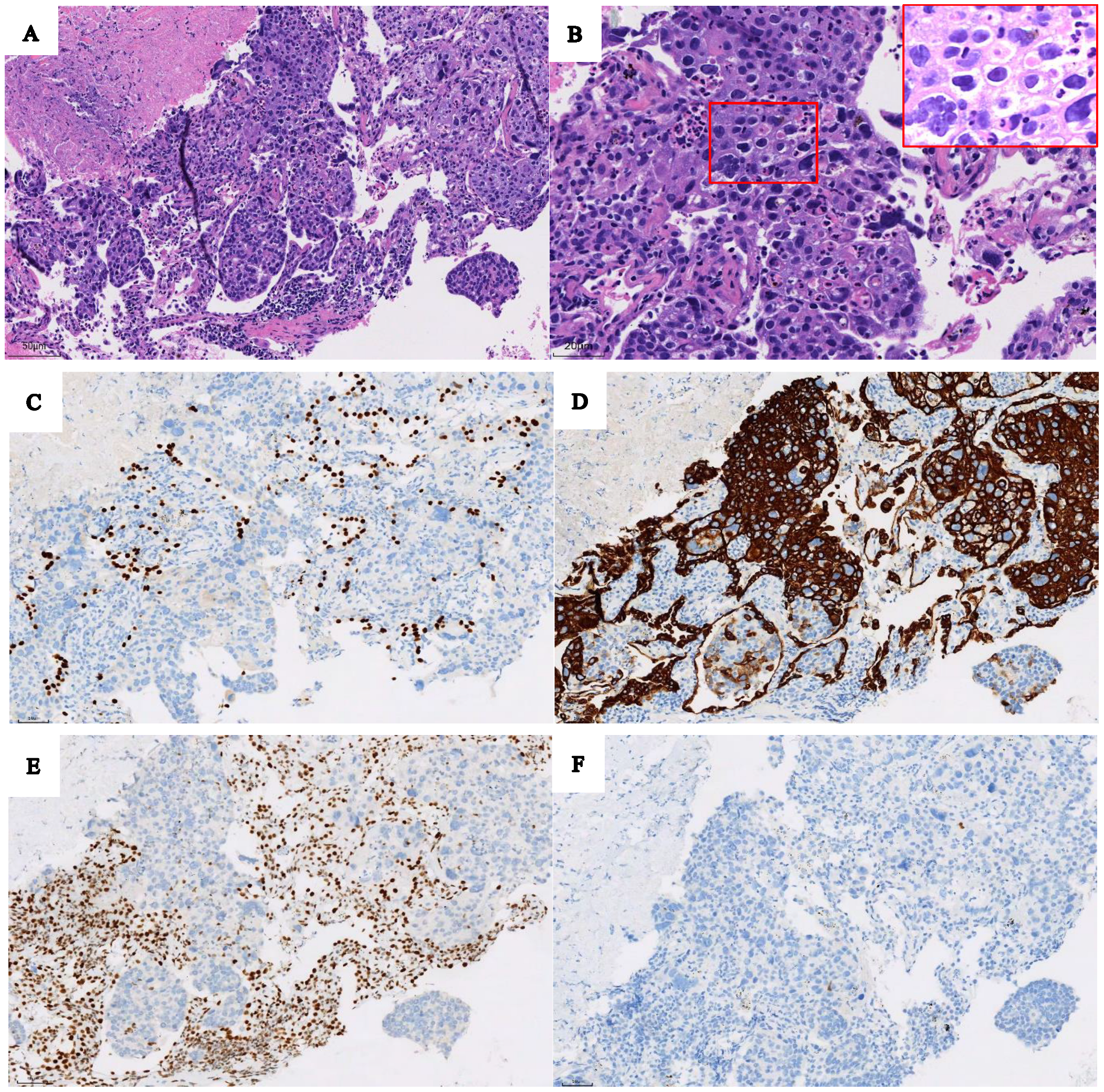

| Section | Before Treatment | After Treatment | Parameter Change | |||||
|---|---|---|---|---|---|---|---|---|
| Size | SUVmax | Ki | Size | SUVmax | Ki | ΔSUVmax 2 | ΔKi 3 | |
| Upper lobe of left lung | 3.4 × 3.0 | 22.4 | 0.0525 | 2.1 × 1.2 | 2.2 | 0.0070 | 90.18% | 86.67% |
| Mediastinal LN | - | - | - | 1.3 × 1.1 (zone 7) 1 | 3.8 | 0.0064 | - | - |
| Pulmonary hilar LN | 1.1 × 0.9 (left) | 5.7 | 0.0231 | 1.2 × 0.8 (left) | 4.0 | 0.0060 | 29.83% | 74.03% |
| - | - | - | 1.3 × 1.2 (right) 1 | 3.9 | - | - | - | |
| Retroperitoneal LN | 1.3 × 1.0 | 8.3 | - | Not clearly shown | - | - | - | - |
| Left adrenal gland | 3.8 × 3.5 | 8.4 | - | 1.8 × 1.2 | 2.1 | - | 75.0% | - |
| Brain | 1.9 × 1.8 | 9.1 | - | 1.0 × 0.8 | No uptake seen | - | - | - |
| Right 8th rib | 8.0 × 4.5 | 9.4 | 0.0214 | 4.5 × 1.8 | 2.8 | 0.0068 | 70.21% | 68.22% |
| Bone of the sacrum | 3.6 × 3.1 | 10.9 | - | 3.5 × 3.0 | 2.1 | - | 80.73% | - |
| Left subcutaneous lumbar region | 1.7 × 1.3 | 7.6 | - | 0.9 × 0.8 | 1.4 | - | 81.58% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wumener, X.; Ye, X.; Zhang, Y.; Jin, S.; Liang, Y. Dynamic and Static 18F-FDG PET/CT Imaging in SMARCA4-Deficient Non-Small Cell Lung Cancer and Response to Therapy: A Case Report. Diagnostics 2023, 13, 2048. https://doi.org/10.3390/diagnostics13122048
Wumener X, Ye X, Zhang Y, Jin S, Liang Y. Dynamic and Static 18F-FDG PET/CT Imaging in SMARCA4-Deficient Non-Small Cell Lung Cancer and Response to Therapy: A Case Report. Diagnostics. 2023; 13(12):2048. https://doi.org/10.3390/diagnostics13122048
Chicago/Turabian StyleWumener, Xieraili, Xiaoxing Ye, Yarong Zhang, Shi Jin, and Ying Liang. 2023. "Dynamic and Static 18F-FDG PET/CT Imaging in SMARCA4-Deficient Non-Small Cell Lung Cancer and Response to Therapy: A Case Report" Diagnostics 13, no. 12: 2048. https://doi.org/10.3390/diagnostics13122048
APA StyleWumener, X., Ye, X., Zhang, Y., Jin, S., & Liang, Y. (2023). Dynamic and Static 18F-FDG PET/CT Imaging in SMARCA4-Deficient Non-Small Cell Lung Cancer and Response to Therapy: A Case Report. Diagnostics, 13(12), 2048. https://doi.org/10.3390/diagnostics13122048





