An ELISA Test Able to Predict the Development of Oral Cancer: The Significance of the Interplay between Steroid Receptors and the EGF Receptor for Early Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies
2.2. Cell Culture
2.3. Western Blot
2.4. PVDF Strip Preparation Protocol
2.5. Device Design
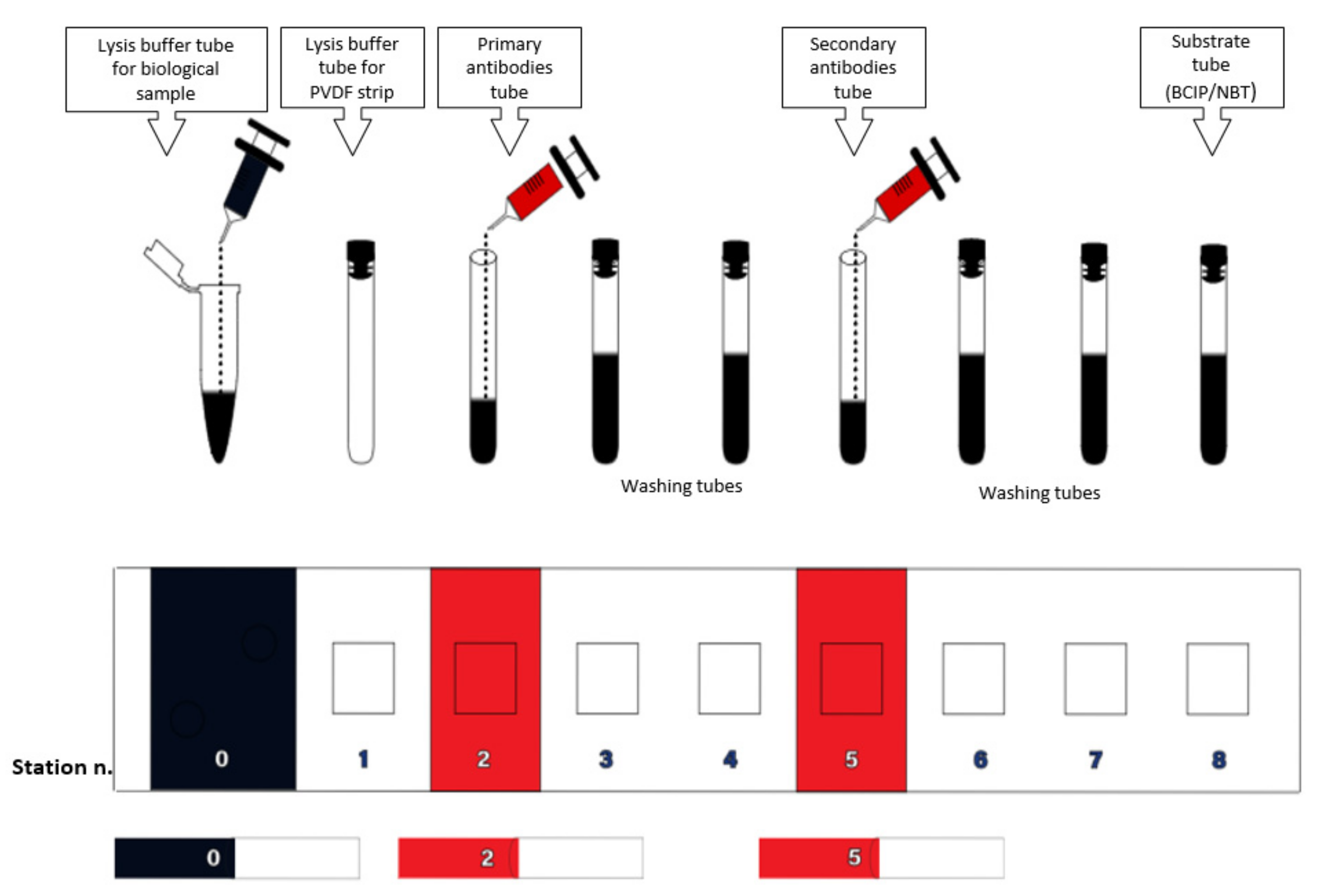
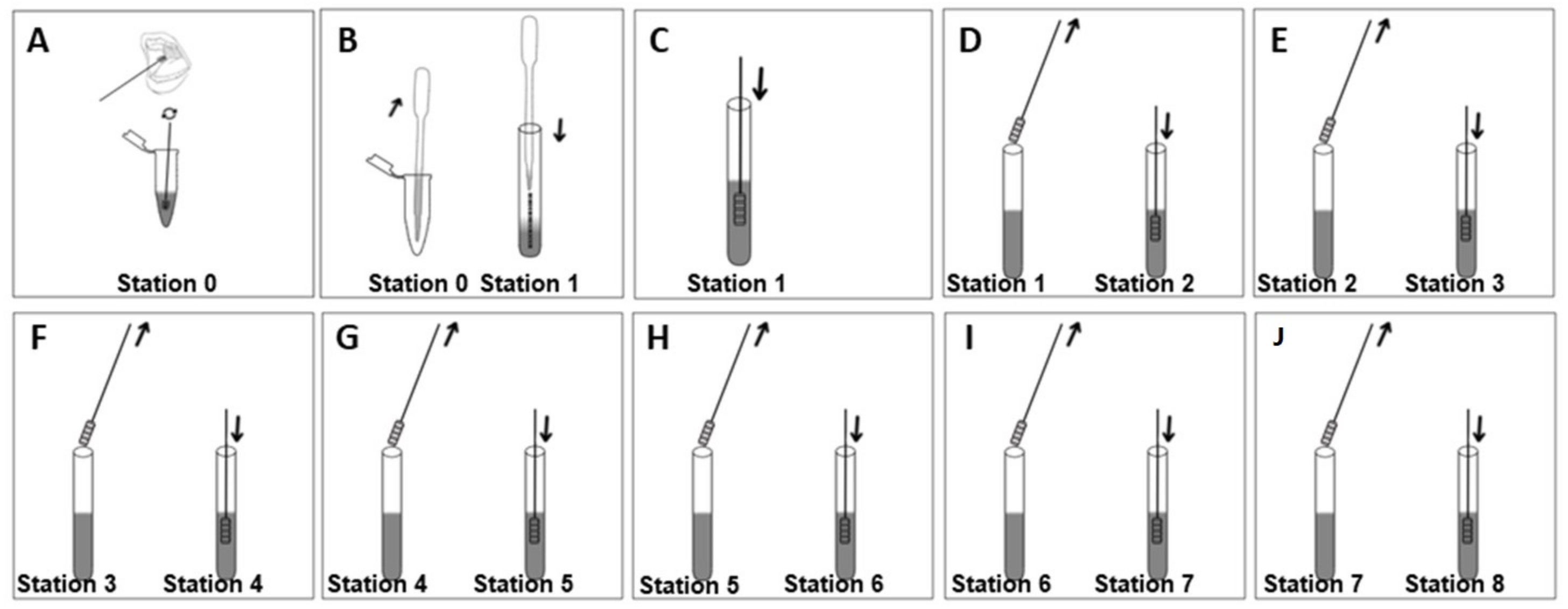
2.6. Description of the Device
2.7. Test Procedure
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- The Global Cancer Observatory (Globocan). Lip, Oral Cavity. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf (accessed on 27 January 2023).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Caraglia, M.; Iorio, B.; Abate, T.; Boccellino, M.; Colella, G.; Oliva, F.; Ferrise, P.; Zappavigna, S.; Faenza, M.; et al. Aggressiveness pattern and second primary tumor risk associated with basaloid squamous cell carcinoma of the larynx. Oncotarget 2017, 8, 95791–95798. [Google Scholar] [CrossRef]
- Menditti, D.; Laino, L.; Milano, M.; Caputo, C.; Boccellino, B.; D’Avino, A.; Baldi, A. Intraoral lymphoepithelial carcinoma of the minor salivary glands. In Vivo 2012, 26, 1087–1089. [Google Scholar]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Mehrtash, H.; Duncan, K.; Parascandola, M.; David, A.; Gritz, E.R.; Gupta, P.C.; Mehrotra, R.; Amer Nordin, A.S.; Pearlman, P.C.; Warnakulasuriya, S.; et al. Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 2017, 18, e767–e775. [Google Scholar] [CrossRef]
- Winn, D.M.; Lee, Y.C.; Hashibe, M.; Boffetta, P. The INHANCE consortium: Toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. 2015, 21, 685–693. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- van der Waal, I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e33–e37. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Ang, K.K.; Berkey, B.A.; Tu, X.; Zhang, H.-Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of Epidermal Growth Factor Receptor Expression on Survival and Pattern of Relapse in Patients with Advanced Head and Neck Carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar]
- Soulieres, D.; Senzer, N.N.; Vokes, E.E.; Hidalgo, M.; Agarwala, S.S.; Siu, L.L. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 2004, 22, 77–85. [Google Scholar] [CrossRef]
- Marocchio, L.S.; Giudice, F.; Corrêa, L.; Ddos, S.P.J.; de Sousa, S.O.M. Oestrogens and androgen receptors in oral squamous cell carcinoma. Acta Odontol. Scand. 2013, 71, 1513–1519. [Google Scholar] [CrossRef]
- Egloff, A.M.; Rothstein, M.E.; Seethala, R.; Siegfried, J.M.; Grandis, J.R.; Stabile, L.P. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin. Cancer Res. 2009, 15, 6529–6540. [Google Scholar] [CrossRef]
- Contaldo, M.; Boccellino, M.; Zannini, G.; Romano, A.; Sciarra, A.; Sacco, A.; Settembre, G.; Coppola, M.; Di Carlo, A.; D’Angelo, L.; et al. Sex Hormones and Inflammation Role in Oral Cancer Progression: A Molecular and Biological Point of View. J. Oncol. 2020, 2020, 9587971. [Google Scholar] [CrossRef]
- Boccellino, M.; Giuberti, G.; Quagliuolo, L.; Marra, M.; D’Alessandro, A.M.; Fujita, H.; Giovane, A.; Abbruzzese, A.; Caraglia, M. Apoptosis induced by interferon-alpha and antagonized by EGF is regulated by caspase-3-mediated cleavage of gelsolin in human epidermoid cancer cells. J. Cell. Physiol. 2004, 201, 71–83. [Google Scholar] [CrossRef]
- Caraglia, M.; Marra, M.; Giuberti, G.; D’Alessandro, A.M.; Beninati, S.; Lentini, A.; Pepe, S.; Boccellino, M.; Abbruzzese, A. Theophylline-induced apoptosis is paralleled by protein kinase A-dependent tissue transglutaminase activation in cancer cells. J. Biochem. 2002, 132, 45–52. [Google Scholar] [CrossRef]
- Toldo, S.; Boccellino, M.; Rinaldi, B.; Seropian, I.M.; Mezzaroma, E.; Severino, A.; Quagliuolo, L.; Van Tassell, B.W.; Marfella, R.; Paolisso, G.; et al. Altered oxido-reductive state in the diabetic heart: Loss of cardioprotection due to protein disulfide isomerase. Mol. Med. 2011, 17, 1012–1021. [Google Scholar] [CrossRef]
- Boccellino, M.; Cuccovillo, F.; Napolitano, M.; Sannolo, N.; Balestrieri, C.; Acampora, A.; Giovane, A.; Quagliuolo, L. Styrene-7,8-oxide activates a complex apoptotic response in neuronal PC12 cell line. Carcinogenesis 2003, 24, 535–540. [Google Scholar] [CrossRef]
- Pieri, M.; Quagliuolo, L.; La Porta, R.; Silvestre, A.; Miraglia, N.; Pedata, P.; Acampora, A.; Castiglia, L.; Sannolo, N.; Boccellino, M. Epirubicin permeation of personal protective equipment can induce apoptosis in keratinocytes. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 428–434. [Google Scholar] [CrossRef]
- Buommino, E.; Boccellino, M.; De Filippis, A.; Petrazzuolo, M.; Cozza, V.; Nicoletti, R.; Ciavatta, M.L.; Quagliuolo, L.; Tufano, M.A. 3-O-methylfunicone produced by penicillium pinophilum affects cell motility of breast cancer cells, downregulating alphavbeta5 integrin and inhibiting metalloproteinase-9 secretion. Mol. Carcinog. 2007, 46, 930–940. [Google Scholar] [CrossRef]
- Khan, A.R.; Anwar, N.; Manan, A.H.; Narayan, K.A. Case series analysis of oral cancer and their risk factors. Malays. Dent. J. 2008, 29, 46–50. [Google Scholar]
- Fierro-Garibay, C.; Almendros-Marques, N.; Berini-Aytes, L.; Gay-Escoda, C. Prevalence of biopsied oral lesions in a department of oral surgery. J. Clin. Exp. Dent. 2011, 3, e73–e77. [Google Scholar] [CrossRef]
- Wamakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Boccellino, M.; Di Stasio, D.; Dipalma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and growth factors in oral squamous cell carcinoma: Useful source of dental-derived stem cells to develop a steroidogenic model in new clinical strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740. [Google Scholar]
- Cossu, A.M.; Mosca, L.; Zappavigna, S.; Misso, G.; Bocchetti, M.; De Micco, F.; Quagliuolo, L.; Porcelli, M.; Caraglia, M.; Boccellino, M. Long Non-coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int. J. Mol. Sci. 2019, 20, 3444. [Google Scholar] [CrossRef]
- Boccellino, M.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Lucchese, A.; Serpico, R.; Frati, L.; Di Domenico, M. Lichen planus: Molecular pathway and clinical implications in oral disorders. J. Biol. Regul. Homeost. Agents. 2018, 32 (Suppl. S1), 135–138. [Google Scholar]
- Piao, Y.; Jung, S.N.; Lim, M.A.; Oh, C.; Jin, Y.L.; Kim, H.J.; Nguyen, Q.K.; Chang, J.W.; Won, H.R.; Koo, B.S. A circulating microRNA panel as a novel dynamic monitor for oral squamous cell carcinoma. Sci. Rep. 2023, 13, 2000. [Google Scholar] [CrossRef]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef]
- Sarkis, S.A.; Abdullah, B.H.; Majeed, B.A.; Talabani, N.G. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol. 2010, 2, 13. [Google Scholar] [CrossRef]
- Pidone Ribeiro, F.A.; Noguti, J.; Fujiyama Oshima, C.T.; Ribeiro, D.A. Effective Targeting of the Epidermal Growth Factor Receptor (EGFR) for Treating Oral Cancer: A Promising Approach. Ant. Res. 2014, 34, 1547–1552. [Google Scholar]
- Thomas, C.; Gustafsson, J.A. The different roles of ER subtypes in-cancer biology and therapy. Nat. Rev. Cancer 2011, 11, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Akyu Takei, R.; Tomihara, K.; Yamazaki, M.; Moniruzzaman, R.; Heshiki, W.; Sekido, K.; Tachinami, H.; Sakurai, K.; Yonesi, A.; Imaue, S.; et al. Protumor role of estrogen receptor expression in oral squamous cell carcinoma cells. Oral Surg. Oral. Med. Oral Pathol. Oral Radiol. 2021, 132, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019, 120, 6813–6819. [Google Scholar] [CrossRef]
- Stabile, L.P.; Lyker, J.S.; Gubish, C.T.; Zhang, W.; Grandis, J.R.; Siegfried, J.M. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005, 65, 1459–1470. [Google Scholar] [CrossRef]
- Wu, T.F.; Luo, F.J.; Chang, Y.L.; Huang, C.M.; Chiu, W.J.; Weng, C.F.; Hsu, Y.K.; Yuan, T.C. The oncogenic role of androgen receptors in promoting the growth of oral squamous cell carcinoma cells. Oral Dis. 2012, 1, 320–327. [Google Scholar] [CrossRef]
- Mandel, A.; Larsson, P.; Sarwar, M.; Semenas, J.; Syed Khaja, A.S.; Persson, J.L. The interplay between AR, EGF receptor and MMP-9 signaling pathways in invasive prostate cancer. Mol. Med. 2018, 24, 34. [Google Scholar] [CrossRef]
- Boccellino, M.; Alaia, C.; Misso, G.; Cossu, A.M.; Facchini, G.; Piscitelli, R.; Quagliuolo, L.; Caraglia, M. Gene interference strategies as a new tool for the treatment of prostate cancer. Endocrine 2015, 49, 588–605. [Google Scholar] [CrossRef]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrom, M.J.; Bofin, A.M. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; Ciociola, A.; Lombardi, M.; De Falco, A.; Nanayakkara, M.; Bottero, D.; De Stasio, R.; Varricchio, L.; et al. Crosstalk between EGFR and extranuclear steroid receptors. Ann. N. Y. Acad. Sci. 2006, 1089, 194–200. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccellino, M.; De Rosa, A.; Di Domenico, M. An ELISA Test Able to Predict the Development of Oral Cancer: The Significance of the Interplay between Steroid Receptors and the EGF Receptor for Early Diagnosis. Diagnostics 2023, 13, 2001. https://doi.org/10.3390/diagnostics13122001
Boccellino M, De Rosa A, Di Domenico M. An ELISA Test Able to Predict the Development of Oral Cancer: The Significance of the Interplay between Steroid Receptors and the EGF Receptor for Early Diagnosis. Diagnostics. 2023; 13(12):2001. https://doi.org/10.3390/diagnostics13122001
Chicago/Turabian StyleBoccellino, Mariarosaria, Alfredo De Rosa, and Marina Di Domenico. 2023. "An ELISA Test Able to Predict the Development of Oral Cancer: The Significance of the Interplay between Steroid Receptors and the EGF Receptor for Early Diagnosis" Diagnostics 13, no. 12: 2001. https://doi.org/10.3390/diagnostics13122001
APA StyleBoccellino, M., De Rosa, A., & Di Domenico, M. (2023). An ELISA Test Able to Predict the Development of Oral Cancer: The Significance of the Interplay between Steroid Receptors and the EGF Receptor for Early Diagnosis. Diagnostics, 13(12), 2001. https://doi.org/10.3390/diagnostics13122001






