Pathomorphological Manifestations and the Course of the Cervical Cancer Disease Determined by Variations in the TLR4 Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Methods
2.3. SNP Selection
2.4. DNA Extraction and Genotyping
3. Results
3.1. Tumour Characteristics and SNP Frequencies
3.2. Association Analysis
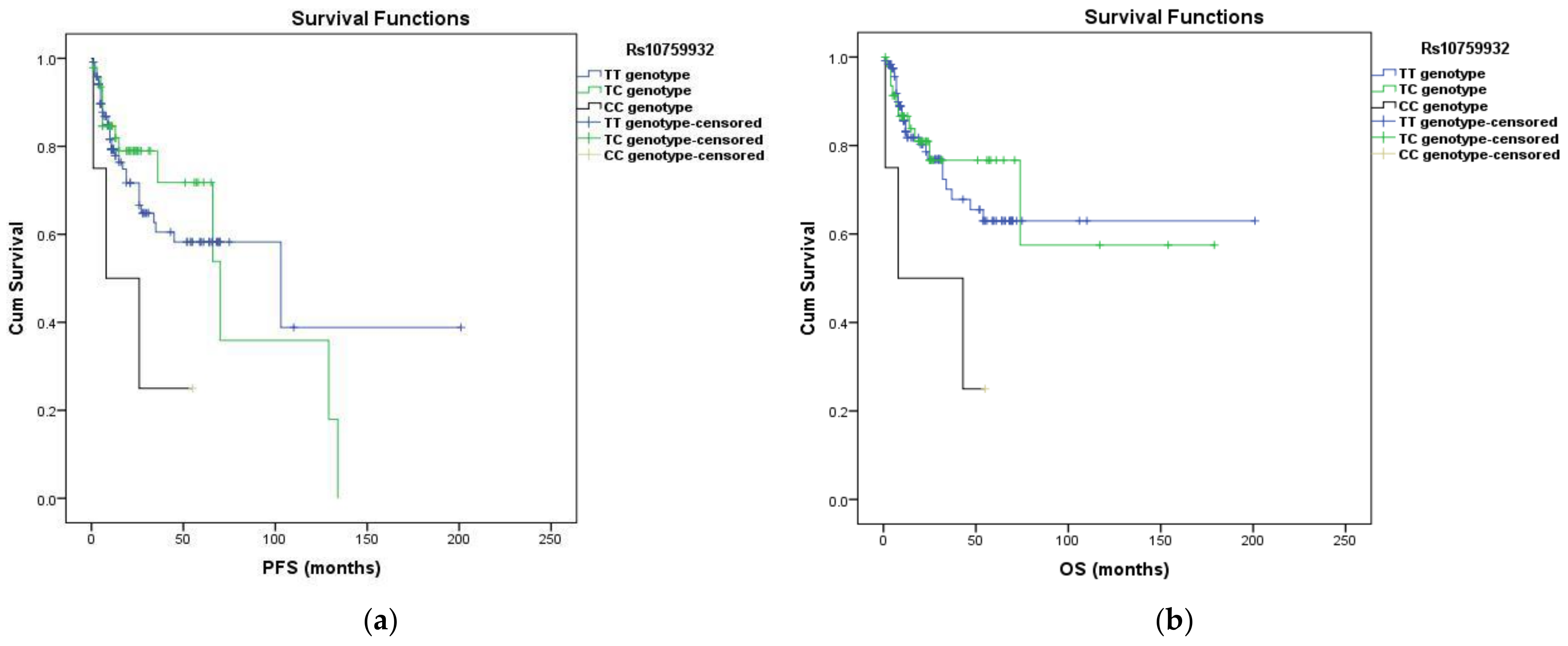
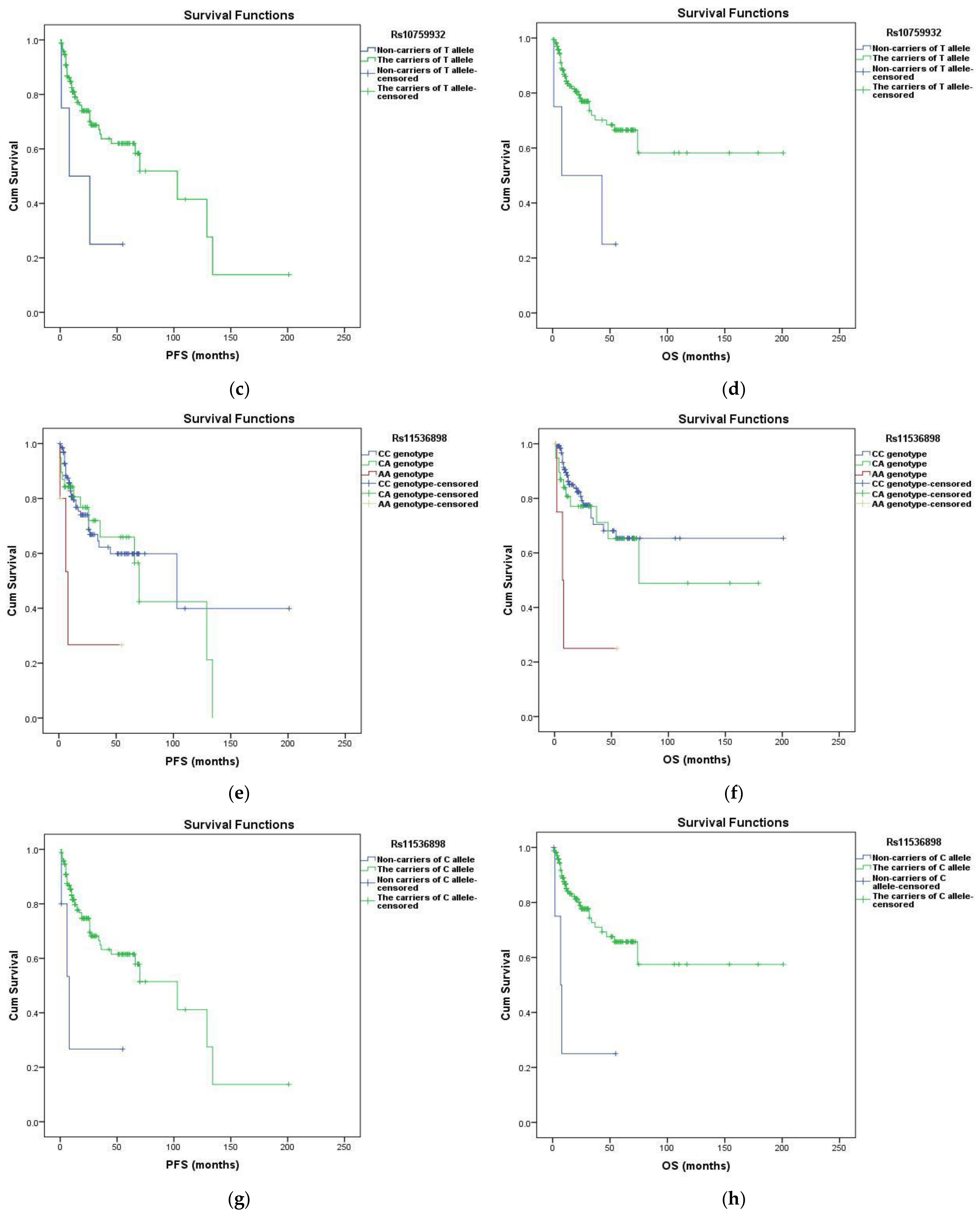
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Emily, S.; Sigmund, A.W. Chronic inflammation and cancer. Oncology 2002, 16, 217–222. Available online: https://pubmed.ncbi.nlm.nih.gov/11866137/ (accessed on 1 January 2022).
- Nitin, S.; Deepak, B.; Jagadish, P.R.; Pankaj, B.P.; Savita, S.T.; Veena, B.P. Inflammation and cancer Nitin Singh. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar]
- Goldszmid, R.S.; Trinchieri, G. The price of immunity. Nat. Immunol. 2012, 13, 932–938. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Erspectives Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Pradere, J.P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2013, 33, 3485–3495. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cen, X.; Liu, S.; Cheng, K. The Role of Toll-Like Receptor in Inflammation and Tumor Immunity. Front. Pharmacol. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lin, S.; Song, B.; Liu, J.; Lai, R.; Shao, L. The mechanisms of graphene-based materials-induced programmed cell death: A review of apoptosis, autophagy, and programmed necrosis. Int. J. Nanomed. 2017, 12, 6633–6646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Lin, Y.; Jiang, J.; Tang, Z.; Yang, S.; Lu, L.; Liang, Y.; Liu, X.; Tan, J.; Hu, X.G.; et al. High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. J. Pathol. 2017, 243, 376–389. [Google Scholar] [CrossRef]
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Bagheri, N.; Ghaffari, S.H.; Bashash, D. Toll-like receptors (TLRs) in cancer; with an extensive focus on TLR agonists and antagonists. IUBMB Life 2020, 73, 10–25. [Google Scholar] [CrossRef]
- Shi, M.; Chen, X.; Ye, K.; Yao, Y.; Li, Y. Application potential of toll-like receptors in cancer immunotherapy. Medicine 2016, 95, e3951. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Peng, G. TLR-mediated metabolic reprogramming in the tumor microenvironment: Potential novel strategies for cancer immunotherapy. Cell. Mol. Immunol. 2018, 15, 428–437. [Google Scholar] [CrossRef]
- Cheng, B.; Yuan, W.E.; Su, J.; Liu, Y.; Chen, J. Recent advances in small molecule based cancer immunotherapy. Eur. J. Med. Chem. 2018, 157, 582–598. [Google Scholar] [CrossRef]
- Braunstein, M.J.; Kucharczyk, J.; Adams, S. Targeting Toll-Like Receptors for Cancer Therapy. Target. Oncol. 2018, 13, 583–598. [Google Scholar] [CrossRef]
- Farooq, M.; Batool, M.; Kim, M.S.; Choi, S. Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies. Front. Cell Dev. Biol. 2021, 9, 756315. [Google Scholar] [CrossRef] [PubMed]
- Pahlavanneshan, S.; Sayadmanesh, A.; Ebrahimiyan, H.; Basiri, M. Toll-Like Receptor-Based Strategies for Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 9912188. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.T.; Larivière, L.; Leveque, G.; Clermont, S.; Moore, K.J.; Gros, P.; Malo, D. Endotoxin-tolerant Mice Have Mutations in Toll-like Receptor 4 (Tlr4). J. Exp. Med. 1999, 189, 615–625. [Google Scholar] [CrossRef]
- Arbour, N.C.; Lorenz, E.; Schutte, B.C.; Zabner, J.; Kline, J.N.; Jones, M.; Frees, K.; Watt, J.L.; Schwartz, D.A. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 2000, 25, 187–191. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bryant, C.E.; Doyle, S.L. Therapeutic Targeting of Toll-Like Receptors for Infectious and Inflammatory Diseases and Cancer. Pharmacol. Rev. 2009, 61, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, J.; Hume, A.J.; Mühlberger, E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018, 14, e1007390. [Google Scholar] [CrossRef]
- Shirey, K.A.; Blanco, J.C.G.; Vogel, S.N. Targeting TLR4 Signaling to Blunt Viral-Mediated Acute Lung Injury. Front. Immunol. 2021, 12, 705080. [Google Scholar] [CrossRef]
- Aguirre-García, M.; Rojas-Bernabé, A.; Gómez-García, A.R.; Escalona-Montaño, A. TLR-Mediated Host Immune Response to Parasitic Infectious Diseases. In Toll-Like Receptors; Rezaeli, N., Ed.; IntechOpen: London, UK, 2020; pp. 37–61. [Google Scholar] [CrossRef]
- Li, H.; Sun, B. Toll-like receptor 4 in atherosclerosis. J. Cell. Mol. Med. 2007, 11, 88–95. [Google Scholar] [CrossRef]
- Roshan, M.H.K.; Tambo, A.; Pace, N.P. The Role of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int. J. Inflamm. 2016, 2016, 1532832. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, R.; Dong, M.; Zheng, J.; Lin, H.; Lu, H. Contribution of TLR4 signaling in intermittent hypoxia-mediated atherosclerosis progression. J. Transl. Med. 2018, 16, 106. [Google Scholar] [CrossRef]
- Li, H.; Jiao, Y.; Xie, M. Paeoniflorin Ameliorates Atherosclerosis by Suppressing TLR4-Mediated NF-κB Activation. Inflammation 2017, 40, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Russo, M. Dual Role of Toll-like Receptors in Human and Experimental Asthma Models. Front. Immunol. 2018, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lessmann, A.; Mateus, E.; Vidal, S.; Ramos-Barbón, D.; Torrejón, M.; Giner, J.; Soto, L.; Juárez, C.; Plaza, V. Expression of toll-like receptors 2 and 4 in subjects with asthma by total serum IgE level. Respir. Res. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shang, H.; Cao, X.; Huang, Y.; Fang, X.; Zhang, S.; Xie, M.; Xie, J.; Liu, X. Association of polymorphisms in TLR2 and TLR4 with asthma risk. Medicine 2017, 96, e7909. [Google Scholar] [CrossRef]
- Li, Z.; Mao, X.; Liu, Q.; Song, H.; He, B.; Shi, P.; Zhang, Q.; Li, X.; Wang, J. Functional variations of the TLR4 gene in association with chronic obstructive pulmonary disease and pulmonary tuberculosis. BMC Pulm. Med. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, J.; Sun, Y. TLR4/MyD88/NF-κB-Mediated Inflammation Contributes to Cardiac Dysfunction in Rats of PTSD. Cell. Mol. Neurobiol. 2020, 40, 1029–1035. [Google Scholar] [CrossRef]
- Jia, S.J.; Niu, P.P.; Cong, J.Z.; Zhang, B.K.; Zhao, M. TLR4 signaling: A potential therapeutic target in ischemic coronary artery disease. Int. Immunopharmacol. 2014, 23, 54–59. [Google Scholar] [CrossRef]
- Yu, L.; Feng, Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediat. Inflamm. 2018, 2018, 9874109. [Google Scholar] [CrossRef]
- De Vicente, L.G.; Pinto, A.P.; da Rocha, A.L.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S. Role of TLR4 in physical exercise and cardiovascular diseases. Cytokine 2020, 136, 155273. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Huang, X.; Zhang, W.; Han, Z.; Liu, S. Association between TLR2 and TLR4 Gene Polymorphisms and the Susceptibility to Inflammatory Bowel Disease: A Meta-Analysis. PLoS ONE 2015, 10, e0126803. [Google Scholar] [CrossRef] [PubMed]
- Dejban, P.; Nikravangolsefid, N.; Chamanara, M.; Dehpour, A.; Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother. Res. 2020, 35, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, I.; Zidi, S.; Mouelhi, L.; Ghazoueni, E.; Brochot, E.; Almawi, W.; Loueslati, B. TLR3 and TLR4 SNP variants in the liver disease resulting from hepatitis B virus and hepatitis C virus infection. Br. J. Biomed. Sci. 2018, 76, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kapil, S.; Duseja, A.; Sharma, B.K.; Singla, B.; Chakraborti, A.; Das, A.; Ray, P.; Dhiman, R.K.; Chawla, Y. Genetic polymorphism in CD14 gene, a co-receptor of TLR4 associated with non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 9346. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.N.; Bohner, A.; Dapito, D.H.; Schwabe, R.F.; Lammert, F. TLR4 Deficiency Protects against Hepatic Fibrosis and Diethylnitrosamine-Induced Pre-Carcinogenic Liver Injury in Fibrotic Liver. PLoS ONE 2016, 11, e0158819. [Google Scholar] [CrossRef]
- Hritz, I.; Mandrekar, P.; Velayudham, A.; Catalano, D.; Dolganiuc, A.; Kodys, K.; Kurt-Jones, E.; Szabo, G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 2008, 48, 1224–1231. [Google Scholar] [CrossRef]
- Santos-Martins, M.; Sameiro-Faria, M.; Ribeiro, S.; Rocha-Pereira, P.; Nascimento, H.; Reis, F.; Miranda, V.; Quintanilha, A.; Belo, L.; Beirão, I.; et al. TLR4 and TLR9 Polymorphisms Effect on Inflammatory Response in End-Stage Renal Disease Patients. Eur. J. Inflamm. 2014, 12, 521–529. [Google Scholar] [CrossRef]
- Dessing, M.C.; Kers, J.; Damman, J.; Leuvenink, H.G.D.; van Goor, H.; Hillebrands, J.L.; Hepkema, B.G.; Snieder, H.; van den Born, J.; de Borst, M.H.; et al. Toll-Like Receptor Family Polymorphisms Are Associated with Primary Renal Diseases but Not with Renal Outcomes Following Kidney Transplantation. PLoS ONE 2015, 10, e0139769. [Google Scholar] [CrossRef]
- Taha, I.M.; Abdu Allah, A.M.; Abd El Gayed, E.M. Expression of toll-like receptor 4 and its connection with type 2 diabetes mellitus. Cell. Mol. Biol. 2018, 64, 15–20. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Fang, J.; Zhou, H.; Liu, X.; Su, S.B. High glucose induces and activates Toll-like receptor 4 in endothelial cells of diabetic retinopathy. Diabetol. Metab. Syndr. 2015, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, X.; Zhang, L.; Sun, L.; Zhu, X.; Zhou, Q.; Yang, Z.; Yuan, H. Toll-Like Receptor 4 and Inflammatory Micro-Environment of Pancreatic Islets in Type-2 Diabetes Mellitus: A Therapeutic Perspective. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Pope, R.M. The role of Toll-like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Li, Y.; Wang, M.; Li, Y.; Li, J. TlR2 and TlR4 are involved in the treatment of rheumatoid arthritis synovial fibroblasts with a medicated serum of asarinin through inhibition of Th1/Th17 cytokines. Exp. Ther. Med. 2020, 19, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.Q.; Jin, H.; Zhou, S.Y.; Shi, J.S.; Jin, F. TLR4 is a link between diabetes and Alzheimer’s disease. Behav. Brain Res. 2017, 316, 234–244. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K.I. TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Miron, J.; Picard, C.; Lafaille-Magnan, M.; Savard, M.; Labonté, A.; Breitner, J.; Rosa-Neto, P.; Auld, D.; Poirier, J. Association of TLR4 with Alzheimer’s disease risk and presymptomatic biomarkers of inflammation. Alzheimers Dement. 2019, 15, 951–960. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Perez-Pardo, P.; Dodiya, H.B.; Engen, P.A.; Forsyth, C.B.; Huschens, A.M.; Shaikh, M.; Voigt, R.M.; Naqib, A.; Green, S.J.; Kordower, J.H.; et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut 2018, 68, 829–843. [Google Scholar] [CrossRef]
- Zhao, J.; Han, X.; Xue, L.; Zhu, K.; Liu, H.; Xie, A. Association of TLR4 gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurol. Sci. 2015, 36, 1659–1665. [Google Scholar] [CrossRef]
- Kouli, A.; Horne, C.; Williams-Gray, C. Toll-like receptors and their therapeutic potential in Parkinson’s disease and α-synucleinopathies. Brain Behav. Immun. 2019, 81, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Paterniti, I.; Siracusa, R.; Filippone, A.; Esposito, E.; Cuzzocrea, S. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav. Immun. 2019, 76, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Obadia, N.; Andrade, G.; Leardini-Tristão, M.; Albuquerque, L.; Garcia, C.; Lima, F.; Daleprane, J.; Castro-Faria-Neto, H.C.; Tibiriçá, E.; Estato, V. TLR4 mutation protects neurovascular function and cognitive decline in high-fat diet-fed mice. J. Neuroinflamm. 2022, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh Manjili, F.; Yousefi-Ahmadipour, A.; Kazemi Arababadi, M. The roles played by TLR4 in the pathogenesis of multiple sclerosis; A systematic review article. Immunol. Lett. 2020, 220, 63–70. [Google Scholar] [CrossRef]
- Oliveira, I.; Gomes, R.; Gomides, L.; dos Santos, J.; Carneiro, M.; Ribeiro-Dias, F.; Diniz, D. Interferon-Beta Treatment Differentially Alters TLR2 and TLR4-Dependent Cytokine Production in Multiple Sclerosis Patients. Neuroimmunomodulation 2019, 26, 77–83. [Google Scholar] [CrossRef]
- Oliveira, J.; Busson, M.; Etain, B.; Jamain, S.; Hamdani, N.; Boukouaci, W.; Amokrane, K.; Bennabi, M.; Le Guen, E.; Dargél, A.A.; et al. Polymorphism of Toll-like receptor 4 gene in bipolar disorder. J. Affect. Disord. 2014, 152–154, 395–402. [Google Scholar] [CrossRef]
- Oblak, A.; Jerala, R. Toll-Like Receptor 4 Activation in Cancer Progression and Therapy. Clin. Dev. Immunol. 2011, 2011, 609579. [Google Scholar] [CrossRef]
- Ran, S.; Bhattarai, N.; Patel, R.; Volk-Draper, L. TLR4-Induced Inflammation Is a Key Promoter of Tumor Growth, Vascularization, and Metastasis. In Translational Studies on Inflammation; Nunes, A.C.F., Ed.; IntechOpen: London, UK, 2020; pp. 133–167. [Google Scholar] [CrossRef]
- Hao, B.; Chen, Z.; Bi, B.; Yu, M.; Yao, S.; Feng, Y.; Yu, Y.; Pan, L.; Di, D.; Luo, G.; et al. Role of TLR4 as a prognostic factor for survival in various cancers oncotarget: A meta-analysis. Oncotarget 2018, 9, 13088–13099. [Google Scholar] [CrossRef]
- Jiang, N.; Xie, F.; Chen, L.; Chen, F.; Sui, L. The effect of TLR4 on the growth and local inflammatory microenvironment of HPV-related cervical cancer in vivo. Infect. Agents Cancer 2020, 15, 10–12. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, Y.; Shi, Y.; Xia, X.; Wang, S.; Duan, H. Expression and Functional Analysis of Toll-like Receptor 4 in Human Cervical Carcinoma. J. Membr. Biol. 2014, 247, 591–599. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S. The expression of Foxp3 and TLR4 in cervical cancer: Association with immune escape and clinical pathology. Arch. Gynecol. Obstet. 2016, 295, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Pappa, K.; Kontostathi, G.; Lygirou, V.; Zoidakis, J.; Anagnou, N. Novel structural approaches concerning HPV proteins: Insight into targeted therapies for cervical cancer (Review). Oncol. Rep. 2018, 39, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.; Li, C. The role of TLRs in cervical cancer with HPV infection: A review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, B.; Wang, Y.; Rao, L.; Zhang, L. The TLR4 gene polymorphisms and susceptibility to cancer: A systematic review and meta-analysis. Eur. J. Cancer 2013, 49, 946–954. [Google Scholar] [CrossRef]
- Ding, L.; Jiang, Q.; Li, G.; Shen, J.; Du, J.; Lu, X.; Xiong, X. Comprehensive assessment of association between TLR4 gene polymorphisms and cancer risk: A systematic meta-analysis. Oncotarget 2017, 8, 100593–100602. [Google Scholar] [CrossRef]
- Kutikhin, A.G. Impact of Toll-like receptor 4 polymorphisms on risk of cancer. Hum. Immunol. 2011, 72, 193–206. [Google Scholar] [CrossRef]
- Pandey, N.; Chauhan, A.; Jain, N. TLR4 Polymorphisms and Expression in Solid Cancers. Mol. Diagn. Ther. 2018, 22, 683–702. [Google Scholar] [CrossRef]
- Chauhan, A.; Pandey, N.; Desai, A.; Raithatha, N.; Patel, P.; Choxi, Y.; Kapadia, R.; Khandelwal, R.; Jain, N. Association of TLR4 and TLR9 gene polymorphisms and haplotypes with cervicitis susceptibility. PLoS ONE 2019, 14, e0220330. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.; Zhong, X.; Wang, Y.; Ye, X.; Jing, L.; Huang, S.; Yin, Q.; Miao, Z.; Zhou, Z.; et al. Association between polymorphisms in TLR4 gene targeted by microRNA-140 and cervical precancerous lesion in south Chinese women: A case control study. Genet. Mol. Res. 2017, 16, gmr16039831. [Google Scholar] [CrossRef][Green Version]
- Nath, N.; Mishra, P.; Panda, A.K.; Mishra, R. Polymorphisms and haplotypes of TLR4, TLR9 and CYP1A1 genes possibly interfere with high-risk human papillomavirus infection and cervical cancer susceptibility in Jharkhand, India. Int. Immunopharmacol. 2020, 88, 106925. [Google Scholar] [CrossRef]
- Pandey, N.O.; Chauhan, A.V.; Raithatha, N.S.; Patel, P.K.; Khandelwal, R.; Desai, A.N.; Choxi, Y.; Kapadia, R.S.; Jain, N.D. Association of TLR4 and TLR9 polymorphisms and haplotypes with cervical cancer susceptibility. Sci. Rep. 2019, 9, 9729. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Chauhan, A.; Raithatha, N.; Patel, P.; Khandelwal, R.; Desai, A.; Choxi, Y.; Kapadia, R.; Jain, N. Influence of TLR4 and TLR9 polymorphisms and haplotypes on multiple hrHPV infections and HPV16 copy number in cervical cancer and cervicitis. Microb. Pathog. 2021, 159, 105149. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, J.; Jin, G.; Zhang, H.; Ding, Y.; Hua, Z.; Zhou, Y.; Xue, Y.; Lu, Y.; Hu, Z.; et al. A 5′-flanking region polymorphism in toll-like receptor 4 is associated with gastric cancer in a Chinese population. J. Biomed. Res. 2010, 24, 100–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tongtawee, T.; Simawaranon, T.; Wattanawongdon, W.; Dechsukhum, C.; Leeanansaksiri, W. Toll-like receptor 2 and 4 polymorphisms associated with Helicobacter pylori susceptibility and gastric cancer. Turk. J. Gastroenterol. 2018, 30, 15–20. [Google Scholar] [CrossRef]
- Cheng, I.; Plummer, S.J.; Casey, G.; Witte, J.S. Toll-Like Receptor 4 Genetic Variation and Advanced Prostate Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 352–355. [Google Scholar] [CrossRef]
- Luo, B.; Han, L.; Liu, S.; Wang, X.; Shi, Y.; Zhao, Z.; Zhao, C. Toll-like receptor gene polymorphisms and susceptibility to Epstein-Barr virus-associated and -negative gastric carcinoma in Northern China. Saudi J. Gastroenterol. 2015, 21, 95. [Google Scholar] [CrossRef]
- Suzuki, T.; Meguro, A.; Matsushima, M.; Masui, A.; Tsuda, S.; Nakamura, J.; Nishina, R.; Uchida, T.; Yuhara, H.; Igarashi, M.; et al. Investigation of the Association of TLR2 and TLR4 Polymorphisms with Susceptibility to Helicobacter pylori-Related Gastrointestinal Diseases. Open J. Intern. Med. 2014, 4, 52496. [Google Scholar]
- Semlali, A.; Jalouli, M.; Parine, N.R.; Al Amri, A.; Arafah, M.; Al Naeem, A.; Abdullah, S.; Rouabhia, M.; Alanazi, M. Toll-like receptor-4 as a predictor of clinical outcomes of estrogen receptor-negative breast cancer in Saudi women. OncoTargets Ther. 2017, 10, 1207–1216. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.A.; Choi, I.J.; Lee, Y.S.; Kim, S.Y.; Hwang, J.A.; Cho, S.J.; Kook, M.C.; Kim, C.G.; Kim, Y.W. Effects of Polymorphisms of Innate Immunity Genes and Environmental Factors on the Risk of Noncardia Gastric Cancer. Cancer Res. Treat. 2013, 45, 313–324. [Google Scholar] [CrossRef]
- Proença, M.A. TLR2 and TLR4 polymorphisms influence mRNA and protein expression in colorectal cancer. World J. Gastroenterol. 2015, 21, 7730. [Google Scholar] [CrossRef]
- Shui, I.M.; Stark, J.R.; Penney, K.L.; Schumacher, F.R.; Epstein, M.M.; Pitt, M.J.; Stampfer, M.J.; Tamimi, R.M.; Lindstrom, S.; Sesso, H.D.; et al. Genetic variation in the toll-like receptor 4 and prostate cancer incidence and mortality. Prostate 2011, 72, 209–216. [Google Scholar] [CrossRef]
- Chen, Y.C.; Giovannucci, E.; Lazarus, R.; Kraft, P.; Ketkar, S.; Hunter, D.J. Sequence Variants of Toll-Like Receptor 4 and Susceptibility to Prostate Cancer. Cancer Res. 2005, 65, 11771–11778. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Bondurant, K.L.; Wolff, R.K. Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int. J. Cancer 2011, 130, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Helzlsouer, K.J.; Smith, M.W.; Hoffman-Bolton, J.A.; Clipp, S.L.; Grinberg, V.; De Marzo, A.M.; Isaacs, W.B.; Drake, C.G.; Shugart, Y.Y.; et al. Association of IL10 and Other immune response- and obesity-related genes with prostate cancer in CLUE II. Prostate 2009, 69, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Lindström, S.; Hunter, D.J.; Grönberg, H.; Stattin, P.; Wiklund, F.; Xu, J.; Chanock, S.J.; Hayes, R.; Kraft, P. Sequence Variants in the TLR4 and TLR6-1-10 Genes and Prostate Cancer Risk. Results Based on Pooled Analysis from Three Independent Studies. Cancer Epidemiol. Biomark. Prev. 2010, 19, 873–876. [Google Scholar] [CrossRef]
- Zidi, S.; Verdi, H.; Yilmaz-Yalcin, Y.; Yazici, A.; Gazouani, E.; Mezlini, A.; Atac, F.B.; Yacoubi-Loueslati, B. Involvement of Toll-like receptors in cervical cancer susceptibility among Tunisian women. Bull. Du Cancer 2014, 101, E31–E35. [Google Scholar] [CrossRef] [PubMed]
- Zidi, S.; Sghaier, I.; Gazouani, E.; Mezlini, A.; Yacoubi-Loueslati, B. Evaluation of Toll-Like Receptors 2/3/4/9 Gene Polymorphisms in Cervical Cancer Evolution. Pathol. Oncol. Res. 2015, 22, 323–330. [Google Scholar] [CrossRef]
- Pandey, S.; Mittal, R.D.; Srivastava, M.; Srivastava, K.; Singh, S.; Srivastava, S.; Mittal, B. Impact of Toll-like receptors [TLR] 2 (−196 to −174 del) and TLR 4 (Asp299Gly, Thr399Ile) in cervical cancer susceptibility in North Indian women. Gynecol. Oncol. 2009, 114, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Association of toll-like receptor gene polymorphisms and its interaction with HPV infection in determining the susceptibility of cervical cancer in Chinese Han population. Mamm. Genome 2017, 28, 213–219. [Google Scholar] [CrossRef]
- Ashton, K.A.; Proietto, A.; Otton, G.; Symonds, I.; McEvoy, M.; Attia, J.; Scott, R.J. Toll-Like Receptor (TLR) and Nucleosome-binding Oligomerization Domain (NOD) gene polymorphisms and endometrial cancer risk. BMC Cancer 2010, 10, 382–387. [Google Scholar] [CrossRef]
- Wang, A.C.; Wu, F.X.; Gao, Y.S.; Sheng, X.G. Toll-like receptor 4 single-nucleotide polymorphisms Asp299Gly and Thr399Ile in ovarian cancers. Oncol. Lett. 2014, 8, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Kania, K.D.; Haręża, D.; Wilczyński, J.R.; Wilczyński, M.; Jarych, D.; Malinowski, A.; Paradowska, E. The Toll-like Receptor 4 Polymorphism Asp299Gly Is Associated with an Increased Risk of Ovarian Cancer. Cells 2022, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, G.E.; Saridakis, V.; Karantanos, T.; Michalopoulos, N.V.; Zagouri, F.; Kontogianni, P.; Lymperi, M.; Gazouli, M.; Zografos, G.C. Toll-like receptors gene polymorphisms may confer increased susceptibility to breast cancer development. Breast 2012, 21, 534–538. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.G.; Rossi, A.F.T.; Nizato, D.M.; Miyasaki, K.; Silva, A.E. Profiles of Gene Polymorphisms in Cytokines and Toll-Like Receptors with Higher Risk for Gastric Cancer. Dig. Dis. Sci. 2012, 58, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.G.D. (2012). Polymorphisms of the TLR2 and TLR4 genes are associated with risk of gastric cancer in a Brazilian population. World J. Gastroenterol. 2012, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- Garza-Gonzalez, E.; Bosques-Padilla, F.J.; Mendoza-Ibarra, S.I.; Flores-Gutierrez, J.P.; Maldonado-Garza, H.J.; Perez-Perez, G.I. Assessment of the toll-like receptor 4 Asp299Gly, Thr399Ile and interleukin-8 -251 polymorphisms in the risk for the development of distal gastric cancer. BMC Cancer 2007, 7, 70. [Google Scholar] [CrossRef]
- Trejo-de la, O.A.; Torres, J.; Pérez-Rodríguez, M.; Camorlinga-Ponce, M.; Luna, L.F.; Abdo-Francis, J.M.; Lazcano, E.; Maldonado-Bernal, C. TLR4 single-nucleotide polymorphisms alter mucosal cytokine and chemokine patterns in Mexican patients with Helicobacter pylori-associated gastroduodenal diseases. Clin. Immunol. 2008, 129, 333–340. [Google Scholar] [CrossRef]
- Santini, D.; Angeletti, S.; Ruzzo, A.; Dicuonzo, G.; Galluzzo, S.; Vincenzi, B.; Calvieri, A.; Pizzagalli, F.; Graziano, N.; Ferraro, E.; et al. Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in gastric cancer of intestinal and diffuse histotypes. Clin. Exp. Immunol. 2008, 154, 360–364. [Google Scholar] [CrossRef]
- Hold, G.L.; Rabkin, C.S.; Chow, W.; Smith, M.G.; Gammon, M.D.; Risch, H.A.; Vaughan, T.L.; McColl, K.E.; Lissowska, J.; Zatonski, W.; et al. A Functional Polymorphism of Toll-Like Receptor 4 Gene Increases Risk of Gastric Carcinoma and Its Precursors. Gastroenterology 2007, 132, 905–912. [Google Scholar] [CrossRef]
- Companioni, O.; Bonet, C.; Muñoz, X.; Weiderpass, E.; Panico, S.; Tumino, R.; Palli, D.; Agnoli, C.; Vineis, P.; Boutron-Ruault, M.C.; et al. Polymorphisms of Helicobacter pylori signaling pathway genes and gastric cancer risk in the European prospective investigation into cancer-eurgast cohort. Int. J. Cancer 2013, 134, 92–101. [Google Scholar] [CrossRef]
- Qadri, Q.; Rasool, R.; Afroze, D.; Naqash, S.; Gulzar, G.M.; Yousuf, A.; Siddiqi, M.A.; Shah, Z.A. Study of TLR4 and IL-8 Gene Polymorphisms in H.pylori-Induced Inflammation in Gastric Cancer in an Ethnic Kashmiri Population. Immunol. Investig. 2013, 43, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, C.; Wang, X.; Wu, X.; Zhu, Z.; Liu, B.; Su, L. Association between TLR4 (+896A/G and +1196C/T) Polymorphisms and Gastric Cancer Risk: An Updated Meta-Analysis. PLoS ONE 2014, 9, e109605. [Google Scholar] [CrossRef] [PubMed]
- Reilly, F.; Burke, J.P.; Lennon, G.; Kay, E.W.; McNamara, D.A.; Cullen, G.; Doherty, G.A.; Mulcahy, H.; Martin, S.; Winter, D.C.; et al. A case–control study examining the association of smad7 and TLR single nucleotide polymorphisms on the risk of colorectal cancer in ulcerative colitis. Color. Dis. 2021, 23, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Nowras, T.; Tavakoli, T.; Fakharian, T.; Razavi, F.E.; Soltaninejad, E.; Naseri, M. Association between rs4986790 and rs4986791 Polymorphisms in TLR4 with Colorectal Cancer Risk in Iranian Population. Russ. J. Genet. 2021, 57, 740–744. [Google Scholar] [CrossRef]
- Kopp, T.I.; Andersen, V.; Tjonneland, A.; Vogel, U. Polymorphisms in NFKB1 and TLR4 and Interaction with Dietary and Life Style Factors in Relation to Colorectal Cancer in a Danish Prospective Case-Cohort Study. PLoS ONE 2015, 10, e0116394. [Google Scholar] [CrossRef]
- Li, X.X.; Sun, G.P.; Meng, J.; Li, X.; Tang, Y.X.; Li, Z.; Wang, M.F.; Liang, G.F.; Lu, X.B. Role of Toll-Like Receptor 4 in Colorectal Carcinogenesis: A Meta-Analysis. PLoS ONE 2014, 9, e93904. [Google Scholar] [CrossRef]
- Moaaz, M.; Youssry, S.; Moaz, A.; Abdelrahman, M. Study of Toll-Like Receptor 4 Gene Polymorphisms in Colorectal Cancer: Correlation with Clinicopathological Features. Immunol. Investig. 2020, 49, 571–584. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Yuzhalin, A.E.; Volkov, A.N.; Zhivotovskiy, A.S.; Brusina, E.B. Correlation between genetic polymorphisms within IL-1B and TLR4 genes and cancer risk in a Russian population: A case-control study. Tumor Biol. 2014, 35, 4821–4830. [Google Scholar] [CrossRef]
- Weng, P.H.; Huang, Y.L.; Page, J.H.; Chen, J.H.; Xu, J.; Koutros, S.; Berndt, S.; Chanock, S.; Yeager, M.; Witte, J.S.; et al. Polymorphisms of an Innate Immune Gene, Toll-Like Receptor 4, and Aggressive Prostate Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e110569. [Google Scholar] [CrossRef]
- Winchester, D.A.; Till, C.; Goodman, P.J.; Tangen, C.M.; Santella, R.M.; Johnson-Pais, T.L.; Leach, R.J.; Xu, J.; Zheng, S.L.; Thompson, I.M.; et al. Association between variants in genes involved in the immune response and prostate cancer risk in men randomized to the finasteride arm in the Prostate Cancer Prevention Trial. Prostate 2017, 77, 908–919. [Google Scholar] [CrossRef]
- Kurt, H.; Ozbayer, C.; Bayramoglu, A.; Gunes, H.V.; Degirmenci, R.; Oner, K.S.; Metintas, M. Determination of the Relationship Between rs4986790 and rs4986791 Variants of TLR4 Gene and Lung Cancer. Inflammation 2015, 39, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Bachmann, H.S.; Bankfalvi, A.; Lotfi, R.; Pütter, C.; Wild, C.A.; Schuler, P.J.; Greve, J.; Hoffmann, T.K.; Lang, S.; et al. Toll-like receptor 4 single-nucleotide polymorphisms Asp299Gly and Thr399Ile in head and neck squamous cell carcinomas. J. Transl. Med. 2011, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Srivastava, A.; Kumar, A.; Mittal, B. Significant association between toll-like receptor gene polymorphisms and gallbladder cancer. Liver Int. 2010, 30, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Gast, A.; Bermejo, J.L.; Claus, R.; Brandt, A.; Weires, M.; Weber, A.; Plass, C.; Sucker, A.; Hemminki, K.; Schadendorf, D.; et al. Association of Inherited Variation in Toll-Like Receptor Genes with Malignant Melanoma Susceptibility and Survival. PLoS ONE 2011, 6, e24370. [Google Scholar] [CrossRef]
- AlKhulaifi, F.M.; Alkhuriji, A.; Mansour, L.; Al-jurayyan, A.; Al-Mulhim, N.M.; Tashkandy, Y.A.; Aldossari, G.S.; Alomar, S. Association between Toll-like receptor 4 polymorphism and Acute Lymphoblastic Leukemia susceptibility in Saudi Arabian patients. J. King Saud Univ.—Sci. 2022, 34, 101985. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Jiang, T.; Wang, R.; Ma, H.; Zhang, S. Association of TLR2 and TLR4 Polymorphisms with Risk of Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e82858. [Google Scholar] [CrossRef]
- Li, P.; He, C.Y.; Xu, Q.; Sun, L.P.; Ha, M.W.; Yuan, Y. Effect of the −2081G/A Polymorphism of the TLR4 Gene and Its Interaction with Helicobacter pylori Infection on the Risk of Gastric Cancer in Chinese Individuals. Genet. Test. Mol. Biomark. 2014, 18, 610–615. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.H.; Zhao, T.C.; Jia, Z.F.; Cao, D.H.; Yang, N.; Wang, Y.Q.; Cao, X.Y.; Jiang, J. Single-nucleotide polymorphisms in Toll-like receptor genes are associated with the prognosis of gastric cancer and are not associated with Helicobacter pylori infection. Infect. Genet. Evol. 2019, 73, 384–389. [Google Scholar] [CrossRef]
- Oetting, W.S.; Guan, W.; Schladt, D.P.; Leduc, R.E.; Jacobson, P.A.; Matas, A.J.; Chinnakotla, S.; Schröppel, B.; Murphy, B.T.; Israni, A.K. Donor polymorphisms of toll-like receptor 4 associated with graft failure in liver transplant recipients. Liver Transplant. 2012, 18, 1399–1405. [Google Scholar] [CrossRef]
- Shen, Y.; Bu, M.; Zhang, A.; Liu, Y.; Fu, B. Toll-like receptor 4 gene polymorphism downregulates gene expression and involves in susceptibility to bladder cancer. Tumor Biol. 2014, 36, 2779–2784. [Google Scholar] [CrossRef]
- Song, J.; Kim, D.Y.; Kim, C.S.; Kim, H.J.; Lee, D.H.; Lee, H.; Ko, W.; Lee, G. The association between Toll-like receptor 4 (TLR4) polymorphisms and the risk of prostate cancer in Korean men. Cancer Genet. Cytogenet. 2009, 190, 88–92. [Google Scholar] [CrossRef] [PubMed]
| SNP | Genomic Position in chr9 | Region/Location | MAF/Highest Population MAF |
|---|---|---|---|
| rs10983755 G > A | 117702392 | Promoter, 5′-UTR, intergenic variant −2081 | 0.07/0.31 (A) |
| rs10759932 T > C | 117702866 | Promoter, 5′-UTR, −1607 | 0.18/0.35 (C) |
| rs11536865 G > C | 117703745 | Promoter, 5′-UTR, regulatory region variant −729 | 0.04/0.24 (C) |
| rs4986790 A > G Asp299Gly | 117713024 | Exon, 3′-UTR, missense variant 896 | 0.06/0.14 (G)s |
| rs4986791 C > T Thr399Ile | 117713324 | Exon, 3′-UTR, missense variant 1196 | 0.04/0.17 (T) |
| rs11536897 G > A | 117717732 | 3′-UTR 3084 | 0.04/0.11 (A) |
| rs1927906 T > C | 117717837 | 3′-UTR 3189 | 0.21/0.49 (C) |
| rs11536898 C > A | 117717932 | 3′-UTR 3284 | 0.13/0.27 (A) |
| Variables | Subgroups | Frequencies (Count/%) |
|---|---|---|
| Age (years) (mean ± SD: 55.4 ± 13.5) | ≥50 | 123/71.5% |
| <50 | 49/28.5% | |
| Histology | Squamous | 157/92.3% |
| Non-squamous | 15/8.7% | |
| Tumor size (T) | T1A | 1/0.6% |
| T1B | 25/14.6% | |
| T2A | 4/2.3% | |
| T2B | 80/46.5% | |
| T3A | 13/7.6% | |
| T3B | 38/22.1% | |
| T3C | 4/2.3% | |
| T4A | 4/2.3% | |
| T4B | 3/1.7% | |
| Pathological regional lymph nodes status | N0 | 95/55.2% |
| N1 | 77/44.8% | |
| Distant metastases | M0 | 162/94.2% |
| M1 | 10/5.8% | |
| Stage | IA | 1/0.6% |
| IB | 15/8.7% | |
| IIA | 5/2.9% | |
| IIB | 12/7.0% | |
| IIIA | 9/5.2% | |
| IIIB | 12/7.0% | |
| IIIC1 | 53/31.0% | |
| IIIC2 | 9/5.2% | |
| IVA | 3/1.7% | |
| IVB | 10/5.8% | |
| Grade | 1 | 13/7.6% |
| 2 | 113/65.7% | |
| 3 | 46/26.7% |
| SNP | Genotypes Frequencies | Alleles Frequencies | |||
|---|---|---|---|---|---|
| Rs10759932 T > C | TT | 121 | 0.704 | T | 0.841 |
| TC | 47 | 0.273 | C | 0.159 | |
| CC | 4 | 0.023 | |||
| Rs1927906 T > C | TT | 133 | 0.773 | T | 0.883 |
| TC | 38 | 0.221 | C | 0.117 | |
| CC | 1 | 0.006 | |||
| Rs11536898 C > A | CC | 129 | 0.750 | C | 0.861 |
| CA | 38 | 0.221 | A | 0.139 | |
| AA | 5 | 0.029 | |||
| Rs11536865 G > C | GG | 172 | 1.000 | G | 1.000 |
| GC | 0 | 0 | |||
| CC | 0 | 0 | |||
| Rs10983755 G > A | GG | 159 | 0.924 | G | 0.962 |
| GA | 13 | 0.076 | A | 0.038 | |
| AA | 0 | 0 | |||
| Rs4986790 A > G | AA | 148 | 0.860 | A | 0.927 |
| AG | 23 | 0.134 | G | 0.073 | |
| GG | 1 | 0.006 | |||
| Rs4986791 C > T | CC | 147 | 0.854 | C | 0.924 |
| CT | 24 | 0.140 | T | 0.076 | |
| TT | 1 | 0.006 | |||
| Rs11536897 G > A | GG | 165 | 0.959 | G | 0.979 |
| GA | 7 | 0.041 | A | 0.021 | |
| AA | 0 | 0 | |||
| Possitive T3–T4 Versus T1–2 | Possitive N1 Versus N0 | Possitive M1 Versus M0 | Possitive G3 Versus G1 + G2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Genotype, alleles | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Rs10759932 | TC vs. TT | 1.276 | 0.639–2.551 | 0.490 | 1.272 | 0.647–2.499 | 0.486 | 2.158 | 0.554–8.413 | 0.268 | 1.110 | 0.520–2.370 | 0.787 |
| CC vs. TT | 1.881 | 0.256–13.834 | 0.535 | 1.327 | 0.181–9.734 | 0.781 | 7.733 | 0.678–88.176 | 0.099 | 2.903 | 0.392–21.495 | 0.297 | |
| T allele + vs. T - | 0.570 | 0.078–4.150 | 0.574 | 0.806 | 0.111–5.861 | 0.831 | 0.170 | 0.016–1.800 | 0.097 | 0.355 | 0.049–2.596 | 0.290 | |
| C allele + vs. C - | 1.317 | 0.6673–2.577 | 0.421 | 1.276 | 0.662–2.460 | 0.467 | 2.522 | 0.697–9.122 | 0.147 | 1.210 | 0.584–2.504 | 0.608 | |
| Rs1927906 | TC vs. TT | 1.033 | 0.489–2.183 | 0.932 | 0.668 | 0.318–1.403 | 0.567 | 1.543 | 0.379–6.278 | 0.545 | 1.919 | 0.887–4.154 | 0.098 |
| CC vs. TT | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| T allele + vs. T - | 2.758 | 2.261–3.364 | 0.187 | 2.250 | 1.903–2.660 | 0.265 | 1.062 | 1.023–1.103 | 0.803 | 3.800 | 2.957–4.883 | 0.097 | |
| C allele + vs. C - | 1.107 | 0.530–2.310 | 0.787 | 0.716 | 0.345–1.484 | 0.368 | 1.500 | 0.369–6.097 | 0.325 | 2.056 | 0.962–4.398 | 0.060 | |
| Rs11536898 | CA vs. CC | 1.405 | 0.671–2.944 | 0.368 | 1.543 | 0.746–3.191 | 0.242 | 4.735 | 1.204–18.626 | 0.026 | 1.576 | 0.722–3.439 | 0.253 |
| AA vs. CC | 2.898 | 0.469–17.989 | 0.253 | 2.083 | 0.337–12.898 | 0.430 | 7.812 | 0.704–86.710 | 0.094 | 0.758 | 0.082–7.030 | 0.807 | |
| C allele + vs. C - | 0.374 | 0.061–2.300 | 0.271 | 0.530 | 0.086–3.258 | 0.487 | 0.228 | 0.023–2.254 | 0.169 | 1.475 | 0.161–13.555 | 0.730 | |
| A allele + vs. A - | 1.529 | 0.757–3.090 | 0.235 | 1.597 | 0.798–3.197 | 0.184 | 5.068 | 1.357–18.918 | 0.008 | 1.463 | 0.689–3.106 | 0.320 | |
| Rs10983755 | AG vs. GG | 1.088 | 0.340–3.482 | 0.887 | 1.483 | 0.477–4.613 | 0.496 | 1.389 | 0.162–11.900 | 0.764 | 2.550 | 0.809–8.035 | 0.110 |
| AA vs. GG | * | * | * | * | * | * | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 1.088 | 0.340–3.482 | 0.887 | 1.483 | 0.477–4.613 | 0.494 | 1.389 | 0.162–11.900 | 0.763 | 2.550 | 0.809–8.035 | 0.110 | |
| Rs4986790 | AG vs. AA | 1.152 | 0.467–2.841 | 0.758 | 0.501 | 0.195–1.289 | 0.152 | 1.667 | 0.331–8.388 | 0.536 | 1.600 | 0.628–4.077 | 0.325 |
| GG vs. AA | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 2.758 | 2.261–3.364 | 0.187 | 2.250 | 1.903–2.660 | 0.265 | 1.062 | 1.023–1.103 | 0.803 | 3.800 | 2.957–4.883 | 0.267 | |
| G allele + vs. G - | 1.280 | 0.532–3.082 | 0.581 | 0.572 | 0.231–1.419 | 0.225 | 1.591 | 0.317–7.986 | 0.570 | 1.800 | 0.727–4.455 | 0.199 | |
| Rs4986791 | TC vs. CC | 1.305 | 0.542–3.143 | 0.553 | 0.581 | 0.234–1.441 | 0.241 | 1.580 | 0.315–7.929 | 0.580 | 1.486 | 0.588–3.756 | 0.402 |
| TT vs. CC | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| C allele + vs. C - | 2.758 | 2.261–3.364 | 0.187 | 2.250 | 1.903–2.660 | 0.265 | 1.062 | 1.023–1.103 | 0.803 | 3.800 | 2.957–4.883 | 0.267 | |
| T allele + vs. T - | 1.435 | 0.608–3.389 | 0.408 | 0.653 | 0.271–1.573 | 0.340 | 1.511 | 0.302–7.567 | 0.613 | 1.672 | 0.682–4.103 | 0.258 | |
| Rs11536897 | AG vs. GG | 0.682 | 0.128–3.623 | 0.653 | 0.922 | 0.200–4.251 | 0.917 | 0.000 | 0.000 | 1.000 | 1.100 | 0.206–5.877 | 0.911 |
| AA vs. GG | * | * | * | * | * | * | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 0.682 | 0.128–3.623 | 0.651 | 0.922 | 0.200–4.251 | 0.917 | 0.939 | 0.904–0.977 | 0.502 | 1.100 | 0.206–5.877 | 0.911 | |
| Possitive Stage III–IV Versus Stage I–II | Possitive Worse Prognosis: T3–T4 + G3 Versus T1–T2 + G1–G2 | Age (Years): ≤50 vs. >50 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Genotype, Alleles | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Rs10759932 | TC vs. TT | 1.088 | 0.551–2.148 | 0.808 | 1.143 | 0.396–3.298 | 0.805 | 1.575 | 0.793–3.134 | 0.195 |
| CC vs. TT | 0.806 | 0.110–5.911 | 0.832 | 4.000 | 0.237–67.473 | 0.336 | 5.854 | 0.590–58.052 | 0.131 | |
| T allele + vs. T - | 1.270 | 0.175–9.232 | 0.813 | 0.259 | 0.016–4.304 | 0.346 | 0.195 | 0.020–1.915 | 0.120 | |
| C allele + vs. C - | 1.062 | 0.549–2.055 | 0.857 | 1.273 | 0.463–3.500 | 0.640 | 1.734 | 0.891–3.377 | 0.104 | |
| Rs1927906 | TC vs. TT | 0.655 | 0.317–1.350 | 0.251 | 2.159 | 0.785–5.940 | 0.136 | 0.896 | 0.393–1.178 | 0.642 |
| CC vs. TT | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| T allele + vs. T - | 1.800 | 1.574–2.058 | 0.372 | 0.000 | 0.000 | 1.000 | 2.672 | 2.201–3.243 | 0.378 | |
| C allele + vs. C - | 0.691 | 0.338–1.414 | 0.310 | 2.429 | 0.907–6.506 | 0.078 | 0.900 | 0.429–1.891 | 0.852 | |
| Rs11536898 | CA vs. CC | .1.123 | 0.541–2.334 | 0.755 | 1.725 | 0.579–5.140 | 0.328 | 1.227 | 0.588–2.563 | 0.586 |
| AA vs. CC | 1.225 | 0.198–7.582 | 0.827 | 2.156 | 0.184–25.271 | 0.541 | 0.422 | 0.046–3.885 | 0.446 | |
| C allele + vs. C - | 0.838 | 0.136–5.146 | 0.848 | 0.524 | 0.045–6.045 | 0.604 | 2.485 | 0.272–22.734 | 0.651 | |
| A allele + vs. A - | 1.135 | 0.564 | 2.281 | 1.776 | 0.631–4.997 | 0.277 | 1.103 | 0.544–2.240 | 0.856 | |
| Rs10983755 | AG vs. GG | 1.291 | 0.405–4.119 | 0.666 | 2.430 | 0.535–11.030 | 0.250 | 1.453 | 0.466–4.528 | 0.520 |
| AA vs. GG | * | * | * | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 1.291 | 0.405–4.119 | 0.666 | 2.430 | 0.535–11.030 | 0.250 | 1.453 | 0.366–4.528 | 0.520 | |
| Rs4986790 | AG vs. AA | 0.570 | 0.235–1.383 | 0.214 | 2.005 | 0.616–6.533 | 0.248 | 0.876 | 0.349–2.199 | 0.778 |
| GG vs. AA | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 1.800 | 1.574–2.058 | 0.372 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| G allele + vs. G - | 0.627 | 0.264–1.492 | 0.289 | 2.406 | 0.781–7.416 | 0.126 | 0.986 | 0.405–2.402 | 0.975 | |
| Rs4986791 | TC vs. CC | 0.635 | 0.267–1.510 | 0.304 | 2.005 | 0.616–6.533 | 0.248 | 0.812 | 0.327–2.022 | 0.655 |
| TT vs. CC | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| C allele + vs. C - | 1.800 | 1.574–2.058 | 0.372 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| T allele + vs. T - | 0.692 | 0.296–1.620 | 0.395 | 2.406 | 0.781–7.416 | 0.126 | 0.914 | 0.378–2.208 | 0.842 | |
| Rs11536897 | AG vs. GG | 0.581 | 0.126–2.677 | 0.486 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 |
| AA vs. GG | * | * | * | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 0.581 | 0.126–2.677 | 0.481 | * | * | * | * | * | * | |
| Model No.1 | Model No.2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Dependent | SNP | Covariates | Odds | 95%CI | p | Odds | 95%CI | p |
| Positive M | Rs11536898 | CA vs. CC | 4.609 | 1.166–18.212 | 0.029 | 4.419 | 1.111–17.576 | 0.035 |
| AA vs. CC | 9.452 | 0.803–111.217 | 0.074 | 9.871 | 0.827–117.76 | 0.070 | ||
| Age group | 0.977 | 0.928–1.028 | 0.370 | 0.977 | 0.928–1.029 | 0.376 | ||
| Possitive G3 vs. G1+G2 | 1.729 | 0.445–6.716 | 0.429 | |||||
| Model No.1 | Model No.2 | |||||||
| Dependent | SNP | Covariates | Odds | 95%CI | p | Odds | 95%CI | p |
| Positive M | Rs11536898 | A allele + vs. A - | 5.044 | 1.346–18.899 | 0.016 | 4.884 | 1.297–18.392 | 0.019 |
| Age group | 0.979 | 0.931–1.030 | 0.415 | 0.980 | 0.932–1.030 | 0.426 | ||
| Possitive G3 vs. G1+G2 | 1.670 | 0.433–6.439 | 0.456 | |||||
| Progression-Free Survivol | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|
| SNP | Genotype/Allele | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Rs10759932 | TC vs. TT | 0.884 | 0.472–1.653 | 0.699 | 0.818 | 0.382–1.752 | 0.606 |
| CC vs. TT | 2.918 | 0.894–9.530 | 0.049 | 3.340 | 1.006–11.095 | 0.048 | |
| T allele + vs. T - | 0.331 | 0.103–1.067 | 0.048 | 0.284 | 0.087–0.928 | 0.037 | |
| C allele + vs. C - | 1.012 | 0.564–1.816 | 0.967 | 1.012 | 0.509–2.010 | 0.973 | |
| Rs1927906 | TC vs. TT | 0.975 | 0.498–1.910 | 0.975 | 0.695 | 0.306–1.576 | 0.383 |
| CC vs. TT | 2.584 | 0.352–18.949 | 0.350 | 3.081 | 0.417–22.761 | 0.383 | |
| T allele + vs. T - | 0.385 | 0.053–2.807 | 0.346 | 0.301 | 0.041–2.216 | 0.239 | |
| C allele + vs. C - | 1.028 | 0.537–1.971 | 0.933 | 0.770 | 0.354–1.673 | 0.509 | |
| Rs11536898 | CA vs. CC | 1.103 | 0.586–2.073 | 0.762 | 1.294 | 0.636–2.633 | 0.476 |
| AA vs. CC | 3.926 | 1.201–12.837 | 0.024 | 5.057 | 1.522–16.802 | 0.008 | |
| C allele + vs. C - | 0.261 | 0.081–0.844 | 0.025 | 0.212 | 0.065–0.691 | 0.010 | |
| A allele + vs. A - | 1.274 | 0.707–2.295 | 0.420 | 1.545 | 0.803–2.971 | 0.193 | |
| Rs10983755 | AG vs. GG | 0.508 | 0.123–2.097 | 0.349 | 0.341 | 0.043–2.290 | 0.253 |
| AA vs. GG | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 0.508 | 0.123–2.097 | 0.349 | 0.341 | 0.043–2.290 | 0.253 | |
| Rs4986790 | AG vs. AA | 1.482 | 0.716–3.069 | 0.290 | 1.062 | 0.444–2.542 | 0.892 |
| GG vs. AA | 2.767 | 0.378–20.275 | 0.316 | 3.346 | 0.453–24.696 | 0.236 | |
| A allele + vs. A - | 0.385 | 0.053–2.807 | 0.346 | 0.301 | 0.041–2.216 | 0.239 | |
| G allele + vs. G - | 1.554 | 0.774–3.123 | 0.215 | 1.178 | 0.520–2.669 | 0.695 | |
| Rs4986791 | TC vs. CC | 1.426 | 0.689–2.952 | 0.339 | 1.029 | 0.430–2.461 | 0.950 |
| TT vs. CC | 2.752 | 0.376–20.165 | 0.319 | 3.331 | 0.451–24.582 | 0.238 | |
| C allele + vs. C - | 1.499 | 0.746–3.010 | 0.256 | 0.301 | 0.041–2.216 | 0.239 | |
| T allele + vs. T - | 0.385 | 0.053–2.807 | 0.346 | 1.142 | 0.504–2.587 | 0.750 | |
| Rs11536897 | AG vs. GG | 0.425 | 0.058–3.084 | 0.397 | 1.314 | 0.316–5.454 | 0.707 |
| AA vs. GG | * | * | * | * | * | * | |
| G allele + vs. G - | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | |
| A allele + vs. A - | 0.425 | 0.058–3.084 | 0.397 | 1.314 | 0.316–5.454 | 0.707 | |
| Variables | Progression-Free Survivol | Overall Survival | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Rs10759932 | TC vs. TT | 0.658 | 0.338–1.280 | 0.217 | 0.747 | 0.351–1.590 | 0.449 |
| CC vs. TT | 3.674 | 1.115–12.108 | 0.032 | 4.608 | 1.344–15.801 | 0.015 | |
| Age at diagnosis | 0.993 | 0.971–1.016 | 0.566 | 1.017 | 0.991–1.043 | 0.199 | |
| T3-T4 vs. T1-T2 | 5.540 | 2.870–10.694 | <0.001 | 8.178 | 3.489–19.167 | <0.001 | |
| N1 vs. N0 | 1.340 | 0.709–2.534 | 0.368 | 1.775 | 0.854–3.689 | 0.124 | |
| G3 vs. G1-2 | 0.913 | 0.490–1.704 | 0.776 | 0.773 | 0.384–1.556 | 0.471 | |
| Rs10759932 | T allele + vs. T - | 0.244 | 0.075–0.795 | 0.019 | 0.200 | 0.059–0.674 | 0.009 |
| Age at diagnosis | 0.996 | 0.973–1.018 | 0.697 | 1.018 | 0.993–1.044 | 0.163 | |
| T3-T4 vs. T1-T2 | 5.298 | 2.750–10.206 | <0.001 | 8.045 | 3.430–18.871 | <0.001 | |
| N1 vs. N0 | 1.291 | 0.684–2.439 | 0.431 | 1.735 | 0.835–3.604 | 0.140 | |
| G3 vs. G1-2 | 0.962 | 0.520–1.779 | 0.902 | 0.797 | 0.399–1.593 | 0.521 | |
| Rs11536898 | CA vs. CC | 0.858 | 0.440–1.675 | 0.654 | 1.090 | 0.522–2.277 | 0.819 |
| AA vs. CC | 3.306 | 0.967–11.299 | 0.057 | 3.735 | 1.051–13.278 | 0.042 | |
| Age at diagnosis | 0.993 | 0.971–1.017 | 0.578 | 1.018 | 0.992–1.045 | 0.171 | |
| T3-T4 vs. T1-T2 | 5.158 | 2.675–9.947 | <0.001 | 7.658 | 3.280–17.876 | <0.001 | |
| N1 vs. N0 | 1.241 | 0.653–2.360 | 0.510 | 1.686 | 0.805–3.530 | 0.166 | |
| G3 vs. G1-2 | 1.009 | 0.538–1.894 | 0.977 | 0.819 | 0.405–1.654 | 0.577 | |
| Rs11536898 | C allele + vs. C - | 0.291 | 0.086–0.987 | 0.048 | 0.274 | 0.078–0.959 | 0.043 |
| Age at diagnosis | 0.994 | 0.971–1.017 | 0.612 | 1.018 | 0.992–1.044 | 0.176 | |
| T3-T4 vs. T1-T2 | 5.077 | 2.645–9.747 | 0.000 | 7.694 | 3.298–17.951 | 0.000 | |
| N1 vs. N0 | 1.232 | 0.648–2.342 | 0.525 | 1.694 | 0.810–3.540 | 0.161 | |
| G3 vs. G1-2 | 1.018 | 0.543–1.907 | 0.955 | 0.817 | 0.405–1.651 | 0.574 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žilienė, E.; Inčiūra, A.; Ugenskienė, R.; Juozaitytė, E. Pathomorphological Manifestations and the Course of the Cervical Cancer Disease Determined by Variations in the TLR4 Gene. Diagnostics 2023, 13, 1999. https://doi.org/10.3390/diagnostics13121999
Žilienė E, Inčiūra A, Ugenskienė R, Juozaitytė E. Pathomorphological Manifestations and the Course of the Cervical Cancer Disease Determined by Variations in the TLR4 Gene. Diagnostics. 2023; 13(12):1999. https://doi.org/10.3390/diagnostics13121999
Chicago/Turabian StyleŽilienė, Eglė, Arturas Inčiūra, Rasa Ugenskienė, and Elona Juozaitytė. 2023. "Pathomorphological Manifestations and the Course of the Cervical Cancer Disease Determined by Variations in the TLR4 Gene" Diagnostics 13, no. 12: 1999. https://doi.org/10.3390/diagnostics13121999
APA StyleŽilienė, E., Inčiūra, A., Ugenskienė, R., & Juozaitytė, E. (2023). Pathomorphological Manifestations and the Course of the Cervical Cancer Disease Determined by Variations in the TLR4 Gene. Diagnostics, 13(12), 1999. https://doi.org/10.3390/diagnostics13121999






