Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases
2.2. Immunohistochemistry
2.3. Immunohistochemical Classification
2.4. Statistics
3. Results
3.1. Immunohistochemical Results
3.2. Clinicopathological Characteristics of ICEC
3.3. Intra-Tumoral and Peri-Tumoral CD8-Positive TCs
3.4. Clinical Outcomes and ICEC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Global Cancer Observatory, Globocan 2020. International Agency for Research on Cancer 2020. Available online: http://gco.iarc.fr (accessed on 13 December 2021).
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.A.; Felix, A.S.; Cohn, D.E. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol. Oncol. 2017, 144, 243–249. [Google Scholar] [CrossRef]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Soslow, R.A. Endometrial carcinomas with ambiguous features. Semin. Diagn. Pathol. 2010, 27, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Murali, R.; Delair, D.F.; Bean, S.M.; Abu-Rustum, N.R.; Soslow, R.A. Evolving Roles of Histologic Evaluation and Molecular/Genomic Profiling in the Management of Endometrial Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 201–209. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit from Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Li, B.; Wan, X. Prognostic significance of immune landscape in tumour microenvironment of endometrial cancer. J. Cell. Mol. Med. 2020, 24, 7767–7777. [Google Scholar] [CrossRef]
- Liu, W.; Sun, L.; Zhang, J.; Song, W.; Li, M.; Wang, H. The landscape and prognostic value of immune characteristics in uterine corpus endometrial cancer. Biosci. Rep. 2021, 41, BSR20202321. [Google Scholar] [CrossRef]
- Fan, C.-T.; Hsu, S.-T.; Sun, L.; Hwang, S.-F.; Liu, C.-K.; Shih, Y.-H.; Chen, M.-J.; Li, H.-N.; Wang, J.-S.; Wen, M.-C.; et al. Improved Progression-Free Survival Associated with Tumor-Infiltrating Lymphocytes in High-Grade Endometrial Cancer. J. Clin. Med. 2023, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of polymerase e–mutated and microsatellite instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Suemori, T.; Susumu, N.; Iwata, T.; Banno, K.; Yamagami, W.; Hirasawa, A.; Sugano, K.; Matsumoto, E.; Aoki, D. Intratumoral CD8+ Lymphocyte Infiltration as a Prognostic Factor and Its Relationship with Cyclooxygenase 2 Expression and Microsatellite Instability in Endometrial Cancer. Int. J. Gynecol. Cancer 2015, 25, 1165–1172. [Google Scholar] [CrossRef]
- van Gool, I.C.; Eggink, F.A.; Freeman-Mills, L.; Stelloo, E.; Marchi, E.; de Bruyn, M.; Palles, C.; Nout, R.A.; de Kroon, C.D.; Osse, E.M.; et al. POLE proofreading mutations elicit an anti-tumor immune response in endometrial cancer. Clin. Cancer Res. 2015, 21, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Mascolo, M.; Carbone, L.; Guida, M.; Insabato, L.; Zullo, F. TCGA molecular groups of endometrial cancer: Pooled data about prognosis. Gynecol. Oncol. 2019, 155, 374–383. [Google Scholar] [CrossRef]
- Ramchander, N.C.; Ryan, N.A.J.; Walker, T.D.J.; Harries, L.; Bolton, J.; Bosse, T.; Evans, D.G.; Crosbie, E.J. Distinct Immunological Landscapes Characterize Inherited and Sporadic Mismatch Repair Deficient Endometrial Cancer. Front. Immunol. 2020, 10, 3023. [Google Scholar] [CrossRef]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Köbel, M. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J. Pathol. 2020, 250, 336–345. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.M.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- López-Reig, R.; Fernández-Serra, A.; Romero, I.; Zorrero, C.; Illueca, C.; García-Casado, Z.; Poveda, A.; López-Guerrero, J.A. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci. Rep. 2019, 12, 18093. [Google Scholar] [CrossRef]
- de Biase, D.; Maloberti, T.; Corradini, A.G.; Rosini, F.; Grillini, M.; Ruscelli, M.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Sanza, V.; et al. Integrated clinicopathologic and molecular analysis of endometrial carcinoma: Prognostic impact of the new ESGO-ESTRO-ESP endometrial cancer risk classification and proposal of histopathologic algorithm for its implementation in clinical practice. Front. Med. 2023, 10, 1146499. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Ding, J.; Cheang, M.C.; Wiegand, K.C.; Senz, J.; Tone, A.A.; Yang, W.; Prentice, L.M.; Tse, K.; Zeng, T.; et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012, 228, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Kinloch, M.A.; Leo, J.M.; Grondin, K.; Lee, C.-H.; Ewanowich, C.; Köbel, M.; Cheng, A.B.; Talhouk, A.; McConechy, M.; et al. Interobserver Agreement in Endometrial Carcinoma Histotype Diagnosis Varies Depending on the Cancer Genome Atlas (TCGA)-based Molecular Subgroup. Am. J. Surg. Pathol. 2017, 41, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Huvila, J.; Pors, J.; Thompson, E.F.; Gilks, C.B. Endometrial carcinoma: Molecular subtypes, precursors and the role of pathology in early diagnosis. J. Pathol. 2021, 253, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, L.; Liu, X.; Ou, K.; Yang, L. POLE/POLD1 mutation and tumor immunotherapy. J. Exp. Clin. Cancer Res. 2022, 41, 216. [Google Scholar] [CrossRef]
- Sari, A.; Pollett, A.; Eiriksson, L.R.; Lumsden-Johanson, B.; Van De Laar, E.; Kazerouni, H.; Salehi, A.; Sur, M.; Lytwyn, A.; Ferguson, S.E. Interobserver Agreement for Mismatch Repair Protein Immunohistochemistry in Endometrial and Nonserous, Nonmucinous Ovarian Carcinomas. Am. J. Surg. Pathol. 2019, 43, 591–600. [Google Scholar] [CrossRef]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Meng, B.; Hoang, L.N.; McIntyre, J.B.; Duggan, M.A.; Nelson, G.S.; Lee, C.-H.; Köbel, M. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol. Oncol. 2014, 134, 15–19. [Google Scholar] [CrossRef]
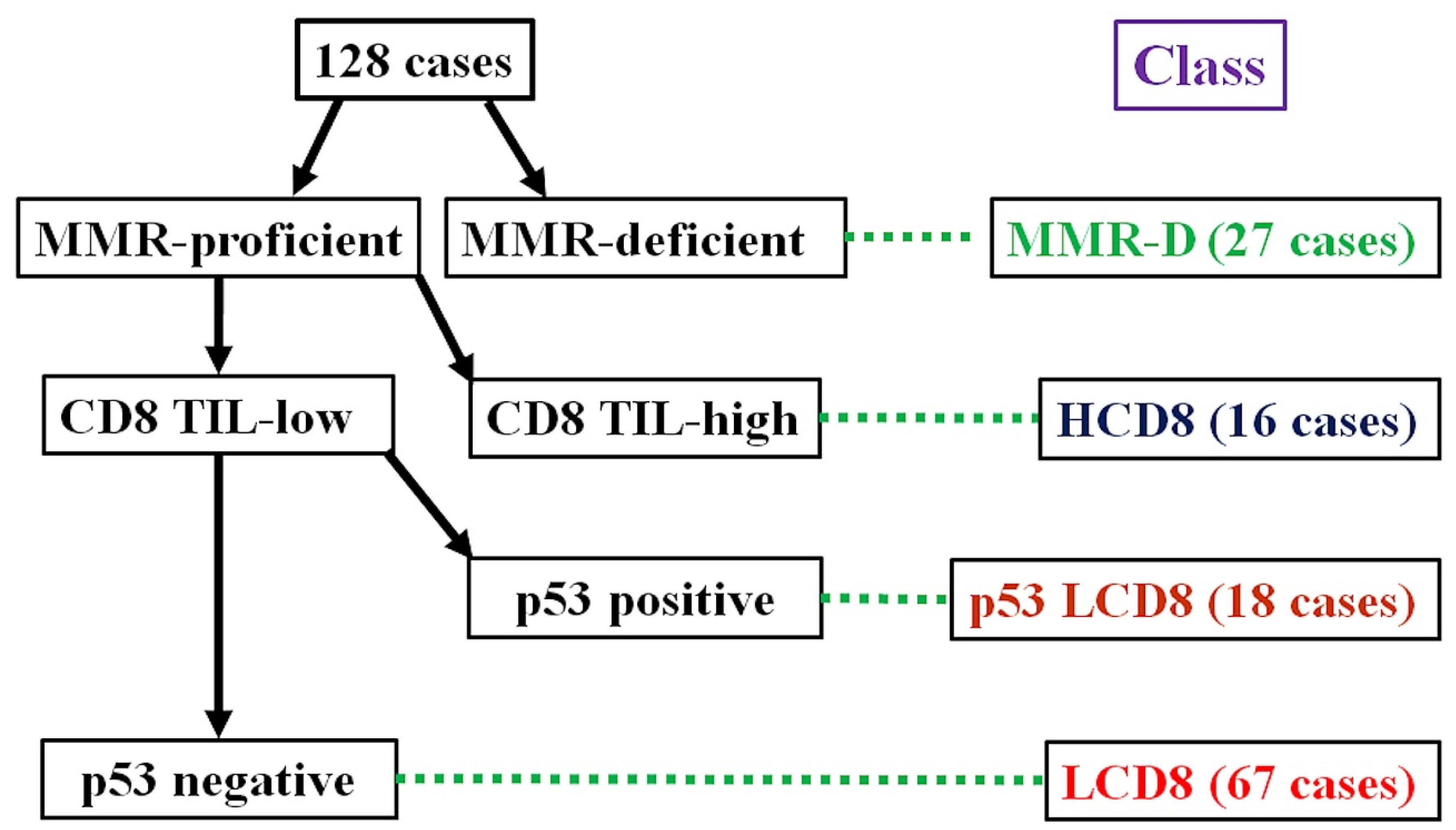
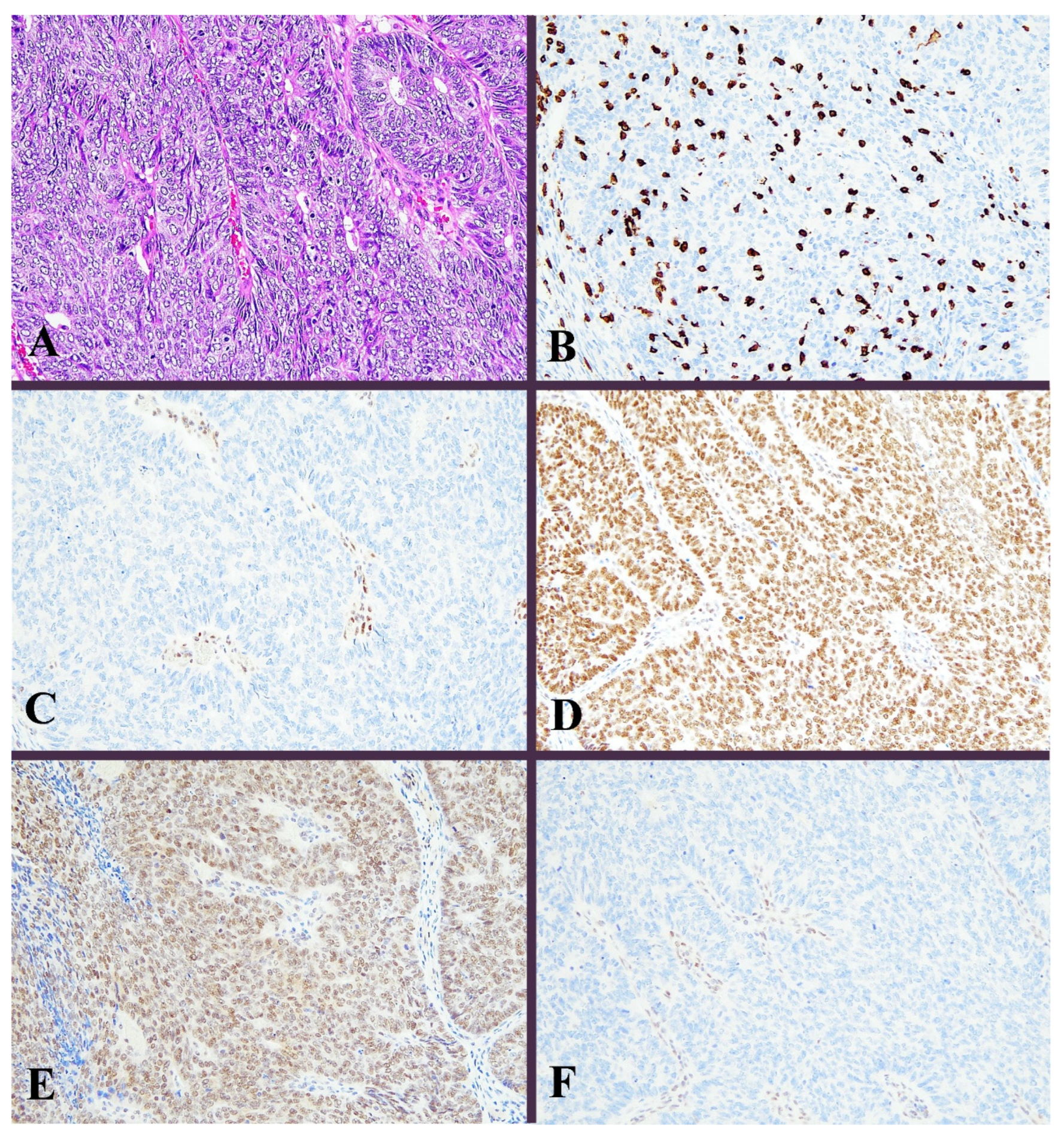

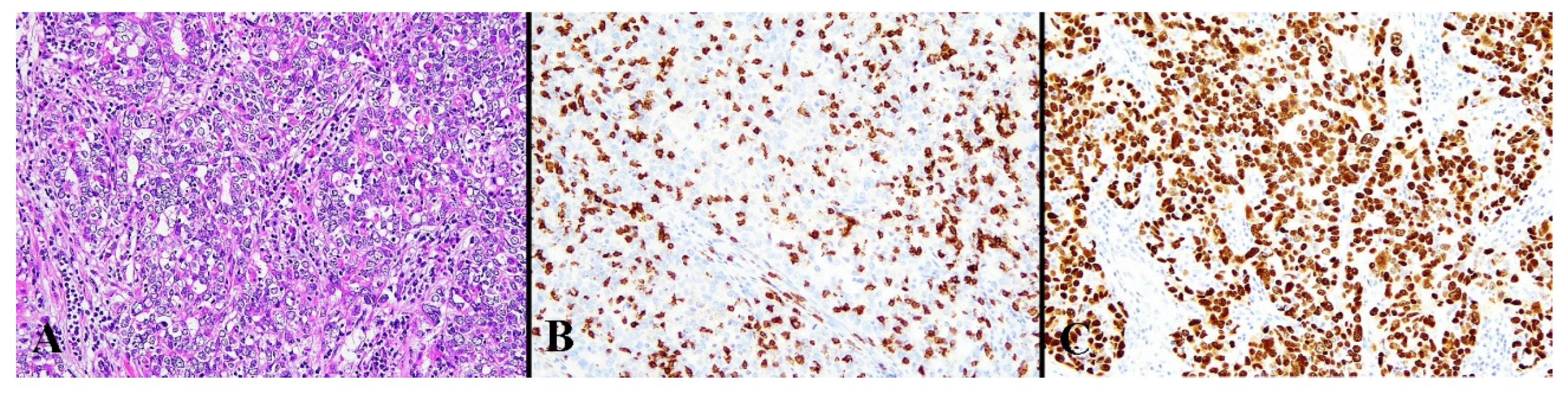
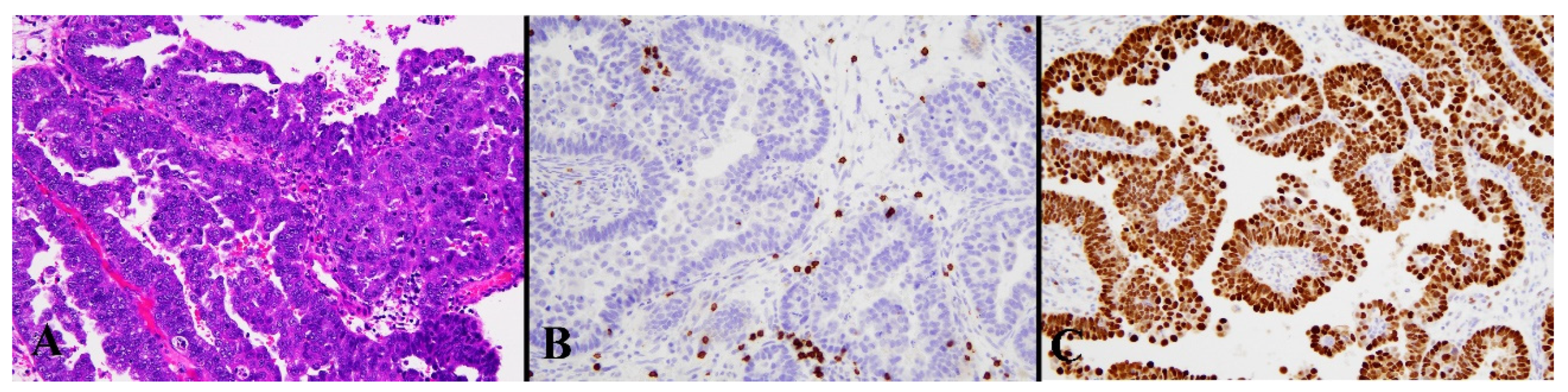
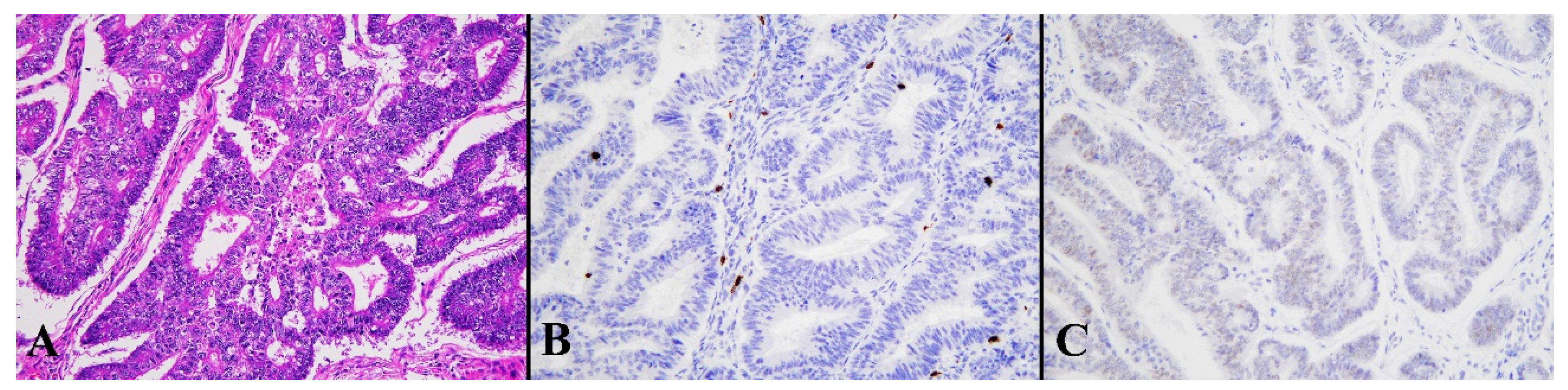

| Mean Age ± SD | 59.27 ± 12.0 years (range: 31–85 years) |
| Histology (n = 128) | |
| Endometrioid carcinoma (G1) | 73 (57%) |
| Endometrioid carcinoma (G2) | 32 (25%) |
| Endometrioid carcinoma (G3) | 17 (13.3%) |
| Serous carcinoma | 6 (4.7%) |
| Clinical stage (FIGO *) (n = 128) | |
| IA | 74 (57.8%) |
| IB | 22 (17.2%) |
| II | 5 (3.9%) |
| III | 23 (18.0%) |
| IV | 4 (3.1%) |
| LVSI ** | 15 (11.7%) |
| Lymph node metastasis (n = 112) | 22 (19.6%) |
| Follow up | 72.0 ± 34.5 months (range: 3–141 months) |
| Characteristics | Class | ||||
|---|---|---|---|---|---|
| HCD8 | MMR-D | LCD8 | p53 LCD8 | p | |
| Age (mean ± SD) | 59.7 ± 13.2 (range: 38–83) | 60.5 ± 7.8 (range: 43–77) | 56.5 ± 12.5 (range: 31–82) | 69.2 ± 9.3 (range: 53–85) | 0.001 |
| Clinical stage (FIGO) | |||||
| IA | 9 (56.25%) | 16 (59.26%) | 41 (61.19%) | 8 (59.1%) | NS ** |
| IB | 3 (18.75%) | 3 (11.11%) | 13 (19.4%) | 3 (16.67%) | |
| II | 1 (6.25%) | 1 (3.7%) | 3 (4.48%) | 0 (0%) | |
| III | 3 (18.75%) | 7 (25.93%) | 7 (10.45%) | 6 (33.33%) | |
| IV | 0 (0%) | 0 (0%) | 3 (4.48%) | 1 (5.56%) | |
| Histology (grade) | |||||
| Endometrioid (G1) | 6 (37.5%) | 12 (44.44%) | 52 (77.61%) | 3 (16.67%) | <0.0001 |
| Endometrioid (G2) | 6 (37.5%) | 10 (37.04%) | 12 (17.91%) | 4 (22.22%) | |
| Endometrioid (G3) | 4 (25.0%) | 5 (18.52%) | 3 (4.48%) | 5 (27.78%) | |
| Serous | 0 (0%) | 0 (0%) | 0 (0%) | 6 (33.33%) | |
| LVSI § | 1 (6.25%) | 6 (22.22%) | 5 (7.46%) | 3 (16.67%) | NS |
| Lymph node metastasis (n = 112) | 2/16 (12.5%) | 6/23 (26.09%) | 9/58 (15.52%) | 5/15 (33.33%) | NS |
| Total | 16 (12.5%) | 27 (21.09%) | 67 (52.34%) | 18 (14.06%) | |
| Characteristics | Class | ||||
|---|---|---|---|---|---|
| HCD8 | MMR-D | LCD8 | p53 LCD8 | p | |
| Intra-tumoral CD8 TCs ‡ | 833.1 (±544) | 413.7 (±609) | 112.1 (±85) | 90.4 (±78) | p < 0.0001 |
| Peri-tumoral CD8 TCs | 270.3 (±145) | 229.4 (±233) | 192 (±162) | 164.2 (±147) | NS § |
| OS ** | DFS § | |
|---|---|---|
| All cases (n = 128) | ||
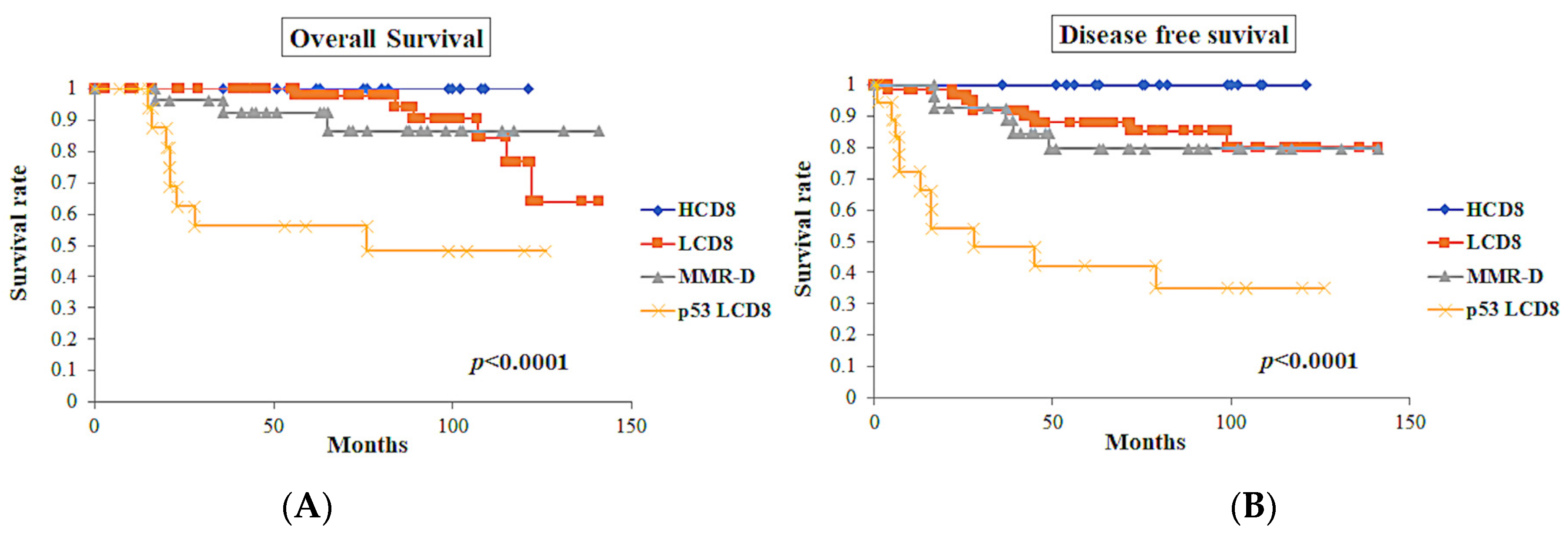
| ICEC | p < 0.0001 | p < 0.0001 |
| FIGO grade | p < 0.0001 | p < 0.0001 |
| FIGO stage | p < 0.0001 | p = 0.0002 |
| p53 status | p < 0.0001 | p < 0.0001 |
| Intra-tumoral CD8 TIL | p = 0.04 | p = 0.03 |
| Peri-tumoral CD8 TIL | p = 0.85 | p = 0.55 |
| LVSI ‡ | p = 0.01 | p = 0.001 |
| Lymph node metastasis (n = 112) | p = 0.0004 | p = 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munakata, S.; Ito, T.; Asano, T.; Yamashita, T. Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis. Diagnostics 2023, 13, 1985. https://doi.org/10.3390/diagnostics13121985
Munakata S, Ito T, Asano T, Yamashita T. Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis. Diagnostics. 2023; 13(12):1985. https://doi.org/10.3390/diagnostics13121985
Chicago/Turabian StyleMunakata, Satoru, Takahiro Ito, Takuya Asano, and Tsuyoshi Yamashita. 2023. "Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis" Diagnostics 13, no. 12: 1985. https://doi.org/10.3390/diagnostics13121985
APA StyleMunakata, S., Ito, T., Asano, T., & Yamashita, T. (2023). Tumor-Infiltrating CD8-Positive T-Cells Associated with MMR and p53 Protein Expression Can Stratify Endometrial Carcinoma for Prognosis. Diagnostics, 13(12), 1985. https://doi.org/10.3390/diagnostics13121985





