Pregnancy and Gastric Cancer: A Narrative Review
Abstract
1. Introduction
2. Search Strategy
3. Epidemiology and Etiology
4. Diet and Eating Habits
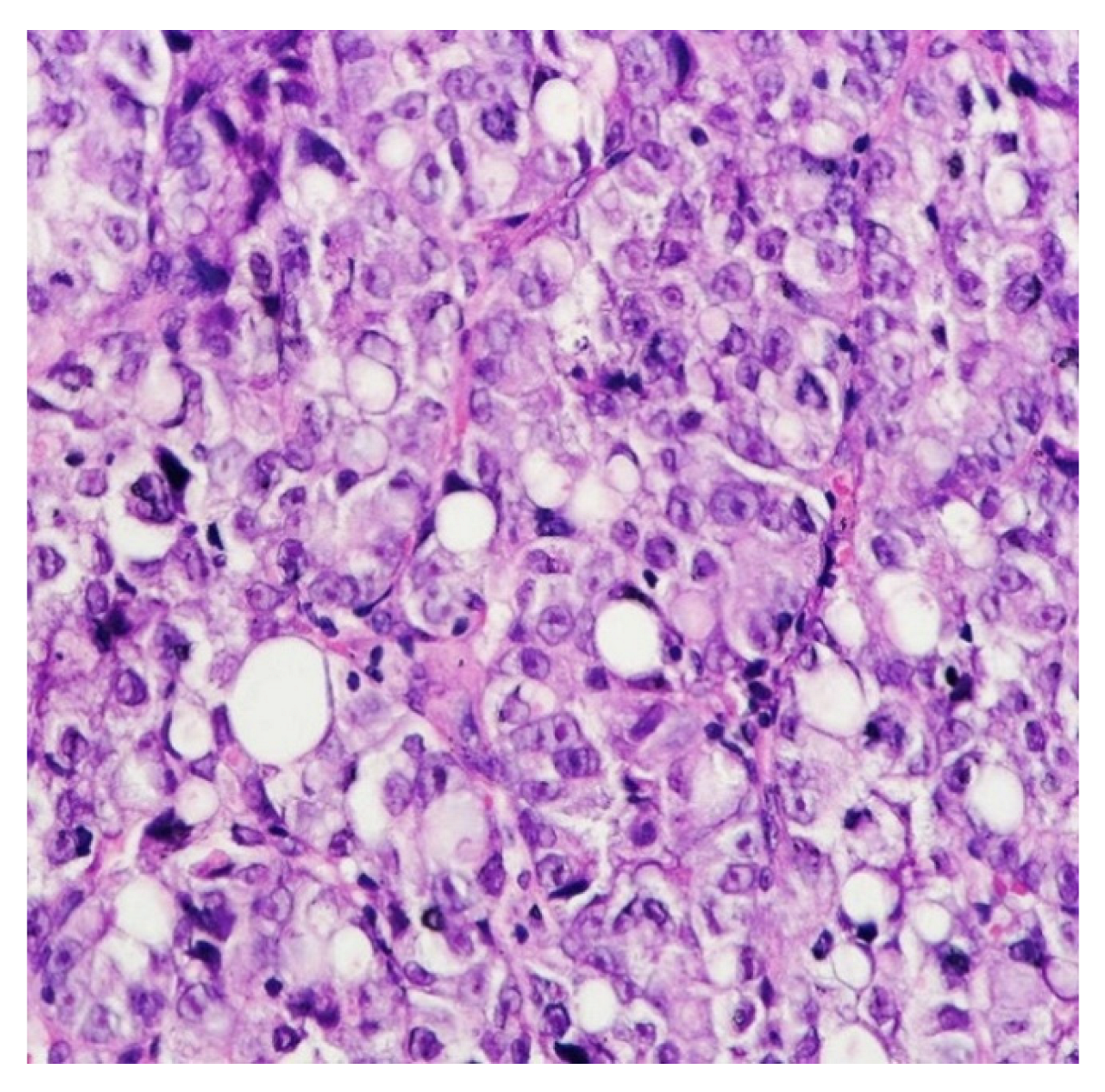
5. Histology
6. Diagnosis
6.1. Paraclinical Diagnosis
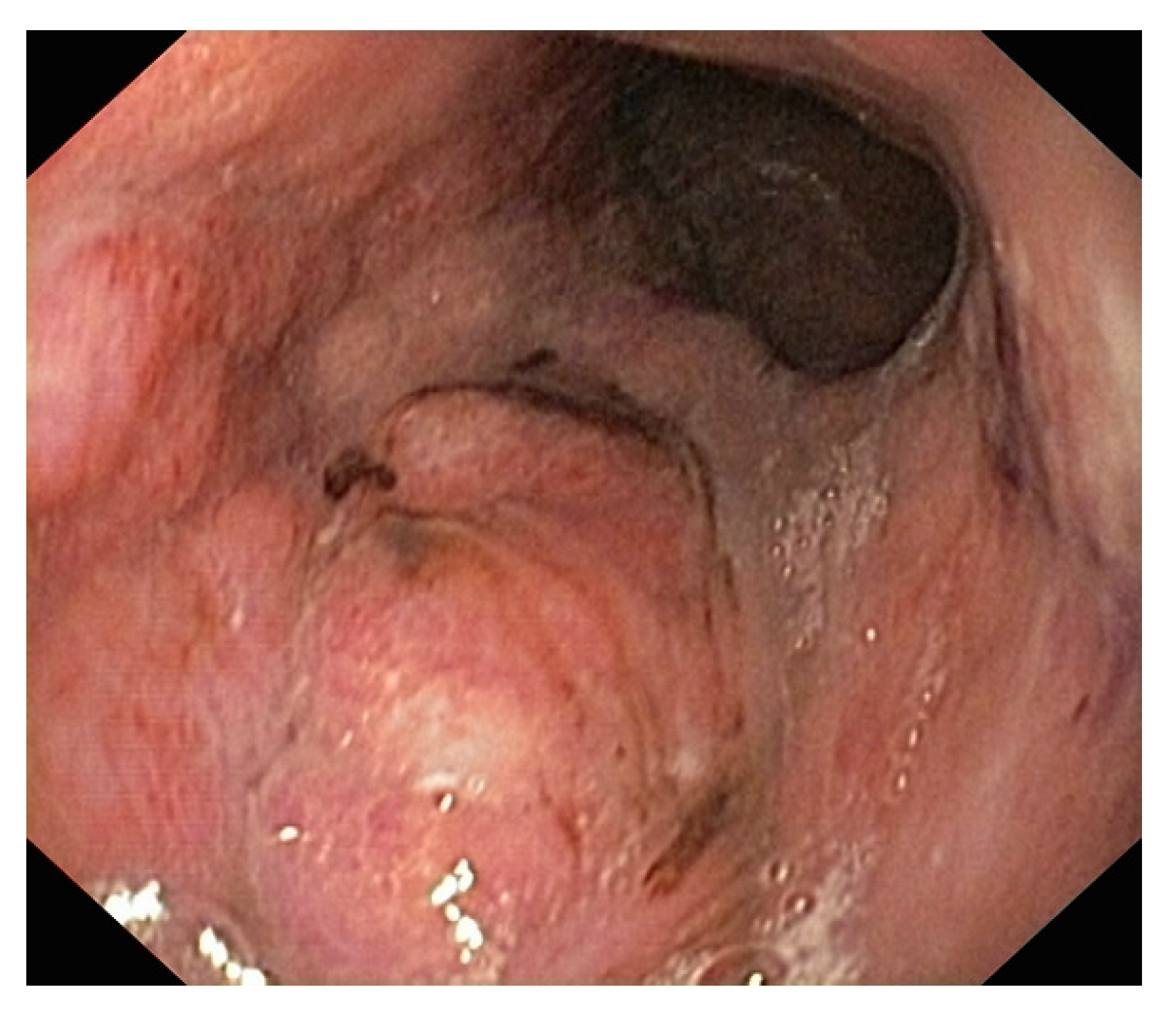
6.2. Upper Digestive Endoscopy
6.3. Abdominal Ultrasound (AU)
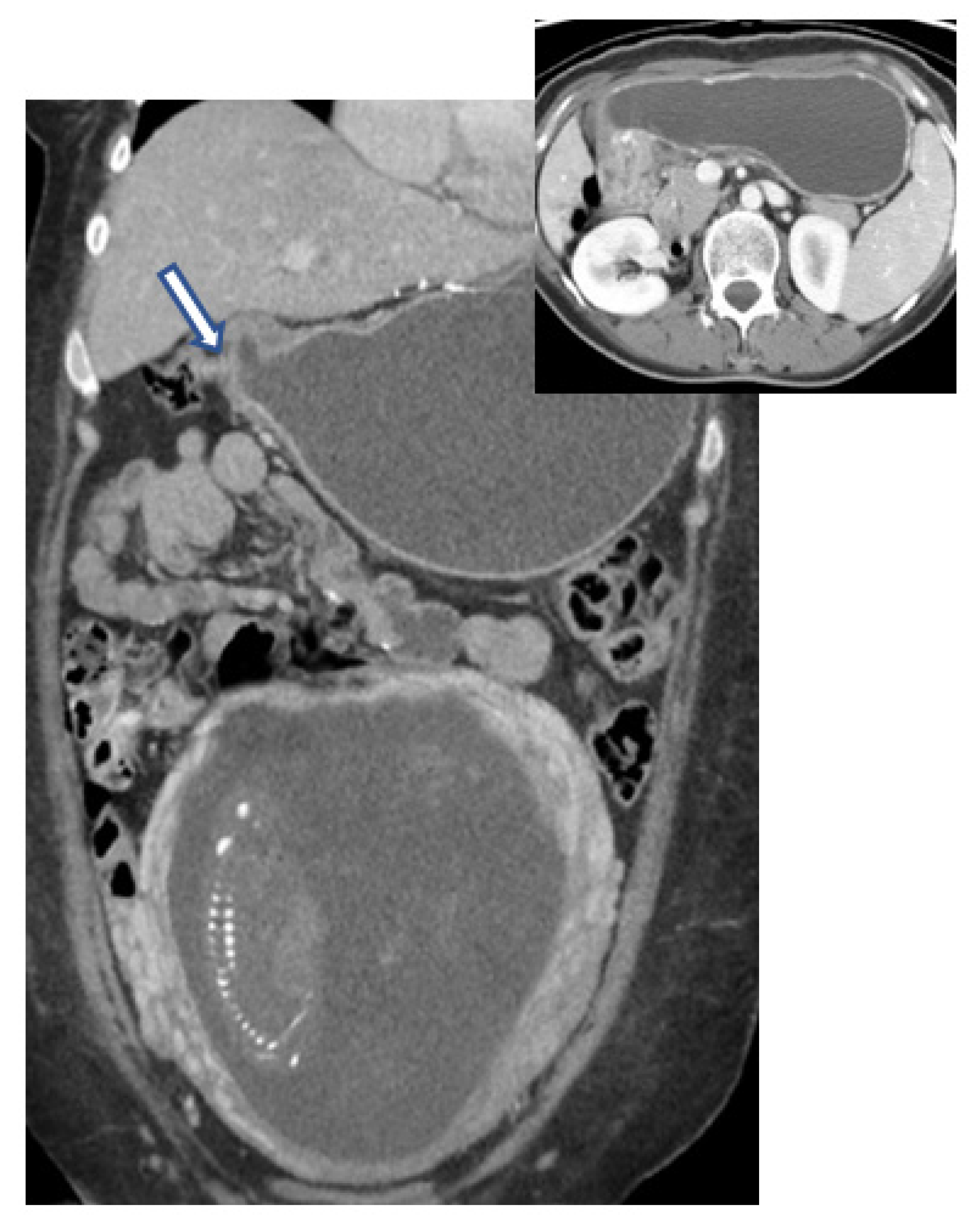
6.4. Computed Tomography (CT)
| Fetal Dose (mGys) | 0 | 0.001–0.1 | 0.1–1.0 | 1.0–10 | 10–50 |
|---|---|---|---|---|---|
| Imaging tests | US MRI | X-ray (head, chest, extremity) Mammography CT (head and neck) Cervical spine radiography | X-ray (abdomen, pelvis) Lumbar spine radiography CT (chest) | Abdominal CT Technetium-99 m bone scintigraphy | CT (pelvis) PET-CT FDG |
6.5. MRI
6.6. PET-CT
7. Treatment
7.1. Chemotherapy
7.2. Radiotherapy
7.3. Molecular Therapies
7.4. Anti-HER2 Therapy
7.5. Anti-EGFR Therapy
7.6. Anti-VEGF Therapy
7.7. Immunotherapy
7.8. Surgical Approach
8. Prognosis
Prognosis Markers in Pregnancy-Associated Gastric Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ten statistical highlights in global public health. In World Health Statistics; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.D.; Parkin, D. GLOBOCAN 2008: Cancer Incidence and Mortality Worldwide; IARC CancerBase no.10; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- Center, M.M.; Jemal, A.; Smith, R.A.; Ward, E. Worldwide variations in colorectal cancer. CA Cancer J. Clin. 2009, 59, 366–378. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Ward, E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1688–1694. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Kosary, C.L.; Krapcho, M.; Neyman, N. SEER Cancer Statistics Review, 1975–2007; National Cancer Institute: Bethesda, MD, USA, 2010. [Google Scholar]
- Tekesin, K.; Emin Gunes, M.; Tural, D.; Akar, E.; Zirtiloglu, A.; Karaca, M.; Selcukbiricik, F.; Bayrak, S.; Ozet, A. Clinicopathological characteristics, prognosis and survival outcome of gastric cancer in young patients: A large cohort retrospective study. J. BUON 2019, 24, 672–678. [Google Scholar]
- Parkin, D.M.; Bray, F.I.; Devesa, S.S. Cancer burden in the year 2000. The global picture. Eur. J. Cancer 2001, 37 (Suppl. S8), S4–S66. [Google Scholar] [CrossRef]
- Parkin, D.M. International variation. Oncogene 2004, 23, 6329–6340. [Google Scholar] [CrossRef]
- GLOBOCAN 2018 Data. Available online: http://gco.iarc.fr/ (accessed on 13 November 2018).
- Maggen, C.; Lok, C.A.; Cardonick, E.; van Gerwen, M.; Ottevanger, P.B.; Boere, I.A.; Koskas, M.; Halaska, M.J.; Fruscio, R.; Gziri, M.M.; et al. Gastric cancer during pregnancy: A report on 13 cases and review of the literature with focus on chemotherapy during pregnancy. Acta Obstet. Gynecol. Scand. 2020, 99, 79–88. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.; Sano, T. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- Yamamoto, S. Stomach cancer incidence in the world. Jpn. J. Clin. Oncol. 2001, 31, 471. [Google Scholar]
- Park, W.S. Molecular pathogenesis of gastric cancer. J. Korean Med. Assoc. 2010, 53, 270–282. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Fraumeni, J., Jr. (Eds.) Cancer Epidemiology and Prevention; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Buffart, T.E.; Carvalho, B.; Hopmans, E.; Brehm, V.; Kranenbarg, E.K.; Schaaij-Visser, T.B.; Eijk, P.P.; van Grieken, N.C.; Ylstra, B.; van de Velde, C.J.; et al. Gastric cancers in young and elderly patients show different genomic profiles. J. Pathol. 2007, 211, 45–51. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kanda, T.; Ohashi, M.; Kurabayashi, T.; Serikawa, T.; Matsunaga, M.; Hatakeyama, K. Management of patients with pregnancy-associated gastric cancer in Japan: A mini-review. Int. J. Clin. Oncol. 2009, 14, 392–396. [Google Scholar] [CrossRef]
- Jaspers, V.K.; Gillessen, A.; Quakernack, K. Gastric cancer in pregnancy: Do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 87, 13–22. [Google Scholar] [CrossRef]
- Zeng, H.; Zhou, X.; Xie, H.; Zhao, Y.; Fu, W. Gastric cancer in pregnancy in China: Case reports and a mini-review. J. Surg. 2015, 11, 165–168. [Google Scholar] [CrossRef]
- Pectasides, M.; Sekhar, A.; Dighe, M.K.; Schwartz, G.; Shah, S.N.; Mulcahy, M.F.; Horowitz, J.M. Gastrointestinal malignancies in pregnancy. Abdom. Radiol. 2023, 48, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J.; Park, Y.S.; Song, H.J.; Park, S.J.; Ahn, J.Y.; Choi, K.D.; Lee, G.H.; Jung, H.Y.; Yook, J.H.; Kim, B.S. Prognosis of Pregnancy-Associated Gastric Cancer: An Age-, Sex-, and Stage-Matched Case-Control Study. Gut Liver 2016, 10, 731–738. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- González, C.A.; Lujan-Barroso, L.; Bueno-De-Mesquita, H.B.; Jenab, M.; Duell, E.J.; Agudo, A.; Tjønneland, A.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Touillaud, M.; et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: A reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int. J. Cancer 2012, 131, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J.; Sala, N.; Travier, N.; Muñoz, X.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Barricarte, A.; Arriola, L.; Navarro, C.; Sánchez-Cantalejo, E.; et al. Genetic variation in alcohol dehydrogenase (ADH1A, ADH1B, ADH1C, ADH7) and aldehyde dehydrogenase (ALDH2), alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Carcinogenesis 2012, 33, 361–367. [Google Scholar] [CrossRef]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Correa, P. Helicobacter pylori and gastric cancer: State of the art. Cancer Epidemiol. Biomark. Prev. 1996, 5, 477–481. [Google Scholar]
- Wen, S.; Moss, S.F. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karner-Hanusch, J.; Mittlböck, M.; Fillipitsch, T.; Herbst, F. Family history as a marker of risk for colorectal cancer: Austrian experience. World J. Surg. 1997, 21, 205–209. [Google Scholar] [CrossRef]
- Watson, P.; Lynch, H.T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 1993, 71, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Tamura, G.; Sakata, K.; Nishizuka, S.; Maesawa, C.; Suzuki, Y.; Iwaya, T.; Terashima, M.; Saito, K.; Satodate, R. Inactivation of the E-cadherin gene in primary gastric carcinomas and gastric carcinoma cell lines. Jpn. J. Cancer Res. 1996, 87, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kim, W.H.; Kim, C.W.; Lee, J.B.; Lee, C.K.; Kim, Y.L. Detection of 17p loss in gastric carcinoma using polymerase chain reaction. Lab Investig. 1995, 72, 232–236. [Google Scholar] [PubMed]
- Polkowski, W.; van Sandick, J.W.; Offerhaus, G.J.; ten Kate, F.J.; Mulder, J.; Obertop, H.; van Lanschot, J.J. Prognostic value of Laurén classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann. Surg. Oncol. 1999, 6, 290–297. [Google Scholar] [CrossRef]
- Isobe, T.; Hashimoto, K.; Kizaki, J.; Miyagi, M.; Aoyagi, K.; Koufuji, K.; Shirouzu, K. Characteristics and prognosis of gastric cancer in young patients. Oncol. Rep. 2013, 30, 43–49. [Google Scholar] [CrossRef]
- Higashizono, K.; Nomura, S.; Yagi, K.; Aikou, S.; Nishida, M.; Yamashita, H.; Seto, Y. Pregnancy, delivery, and breastfeeding after total gastrectomy for gastric cancer: A case report. J. Surg. Oncol. 2018, 16, 229. [Google Scholar] [CrossRef]
- Caldas, C.; Carneiro, F.; Lynch, H.T.; Yokota, J.; Wiesner, G.L.; Powell, S.M.; Lewis, F.R.; Huntsman, D.G.; Pharoah, P.D.; Jankowski, J.A.; et al. Familial gastric cancer: Overview and guidelines for management. J. Med. Genet. 1999, 36, 873–880. [Google Scholar]
- Lauwers, G.Y.; Carneiro, F.; Graham, D.Y. Gastric carcinoma. In Classification of Tumours of the Digestive System; Bowman, F.T., Carneiro, F., Hruban, R.H., Eds.; IARC: Lyon, France, 2010; in press. [Google Scholar]
- Hamilton, R.; Aatonen, L.A. Tumors of Digestive System; IARC: Lyon, France, 2000; pp. 39–52. [Google Scholar]
- Marbun, V.M.G.; Putranto, A.S. Diagnosis and management of gastric cancer in pregnancy-An evidence-based case report. Int. J. Surg. Case Rep. 2020, 75, 338–344. [Google Scholar] [CrossRef]
- Lee, N.M.; Saha, S. Nausea and vomiting of pregnancy. Gastroenterol. Clin. N. Am. 2003, 40, 1–27. [Google Scholar] [CrossRef]
- Fell, D.B.; Dodds, L.; Joseph, K.S.; Allen, V.M.; Butler, B. Risk factors for hyperemesis gravidarum requiring hospital admission during pregnancy. Obstet. Gynecol. 2006, 107, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.K.; Kenny, L. Review on hyperemesis gravidarum. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 755–769. [Google Scholar] [CrossRef]
- Lagiou, P.; Tamimi, R.; Mucci, L.A.; Trichopoulos, D.; Adami, H.O.; Hsieh, C.C. Nausea and vomiting in pregnancy in relation to prolactin, estrogens, and progesterone: A prospective study. Obstet. Gynecol. 2003, 101, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Antonoff, M.; Jaramillo, S.; Sagebiel, T.; Murphy, M.B. Gastroesophageal Cancer During Pregnancy: A Case Report and Review of the Literature. J. Gastrointest. Cancer 2019, 50, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Cift, T.; Aydogan, B.; Akbaş, M.; Aydın, B.; Demirkiran, F.; Bakkaloglu, D.V.; Ilvan, S. Case report: Gastric carcinoma diagnosedat the second trimester of pregnancy, Case Rep. Obstet. Gynecol. 2011, 2011, 532854. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.F.; Sousa, M.; Lourenço, I.; Martins, D.; Torres, J. Gastrointestinal diseases during pregnancy: What does the gastroenterologist need to know? Ann. Gastroenterol. 2018, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.T.; Almashhrawi, A.A.; Rahman, R.N.; Hammoud, G.M.; Ibdah, J.A. Liver diseases in pregnancy: Diseases unique to pregnancy. World J. Gastroenterol. 2013, 19, 7639–7646. [Google Scholar] [CrossRef]
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802. [Google Scholar] [CrossRef]
- ASGE Standard of Practice Committee; Shergill, A.K.; Ben-Menachem, T.; Chandrasekhara, V.; Chathadi, K.; Decker, G.A.; Evans, J.A.; Early, D.S.; Fanelli, R.D.; Fisher, D.A.; et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest. Endosc. 2012, 76, 18–24, Erratum in Gastrointest. Endosc. 2013, 77, 833. [Google Scholar] [CrossRef]
- Cappell, M.S. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol. Clin. N. Am. 2003, 32, 123–179. [Google Scholar] [CrossRef] [PubMed]
- Debby, A.; Golan, A.; Sadan, O.; Glezerman, M.; Shirin, H. Clinical utility of esophagogastroduodenoscopy in the management of recurrent and intractable vomiting in pregnancy. J. Reprod. Med. 2008, 53, 347–351. [Google Scholar] [PubMed]
- Frank, B. Endoscopy in pregnancy. In Gastrointestinal Disorders during Pregnancy; Karlstadt, R.G., Surawicz, C.M., Croitoru, R., Eds.; American College of Gastroenterology: Arlington, VA, USA, 1994; pp. 24–29. [Google Scholar]
- Ueo, H.; Okudaira, Y.; Hirabayashi, M. Gastric cancer associated with pregnancy. A case report of early gastric cancer and review of 100 cases in Japan. J. Jpn. Soc. Clin. Surg. 1989, 50, 312–318. (In Japanese) [Google Scholar]
- Ohsawa, S.; Kondo, Y.; Shiroto, H. A case of an early gastric cancer associated with a pregnancy. Jpn. J. Cancer Clin. 1992, 38, 1269–1273. [Google Scholar]
- Serikawa, T.; Shichiri, K.; Sanada, H. Two cases of pregnancy associated with gastric cancer. Obstet. Gynecol. Ther. 1996, 73, 605–607. [Google Scholar]
- Cappell, M.S.; Colon, V.J.; Sidhom, O.A. A study of eight medical centers of the safety and clinical efficacy of esophagogastroduodenoscopy in 83 pregnant females with follow-up of fetal outcome with comparison control groups. Am. J. Gastroenterol. 1996, 91, 348–354. [Google Scholar]
- Kamani, L.; Achakzai, M.S.; Ismail, F.W.; Kayani, F. Safety of Endoscopy and Its Outcome in Pregnancy. Cureus 2019, 11, e6301. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Lebwohl, B.; Ekbom, A.; Kiran, R.P.; Green, P.H.; Höijer, J.; Stephansson, O. Outcomes of pregnancies for women undergoing endoscopy while they were pregnant: A nationwide cohort study. Gastroenterology 2017, 152, 554–563.e9. [Google Scholar] [CrossRef]
- Cappell, M.S. Improving the Safety of Endoscopy in Pregnancy: Approaching Gravidity with Gravitas. Dig. Dis. Sci. 2020, 65, 2745–2748. [Google Scholar] [CrossRef]
- Tang, S.J.; Mayo, M.J.; Rodriguez-Frias, E.; Armstrong, L.; Tang, L.; Sreenarasimhaiah, J.; Lara, L.F.; Rockey, D.C. Safety and utility of ERCP during pregnancy. Gastrointest. Endosc. 2009, 69, 453–461. [Google Scholar] [CrossRef]
- Flanagan, E.; Bell, S. Abdominal Imaging in pregnancy (maternal and foetal risks). Best Pract. Res. Clin. Gastroenterol. 2020, 44–45, 101664. [Google Scholar] [CrossRef] [PubMed]
- Perlas, A.; Chan, V.W.; Lupu, C.M.; Mitsakakis, N.; Hanbidge, A. Ultrasound assessment of gastric content and volume. Anesthesiology 2009, 111, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.N.; Shen, L.; Zhang, J. Meta-analysis of diagnostic value of gastric ultrasonography in gastric cancer. Chin. J. Med. Ultrasound 2021, 18, 344–354. [Google Scholar]
- Nan, M.; Ye, W.; Liu, Y.; Zhang, Z. Diagnostic accuracy of gastric filling ultrasonography in preoperative invasion depth (T stage) of gastric cancer: Meta-analysis. Medicine 2022, 101, e31066. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhang, L.; Lu, D.Y. Comparative analysis of diagnostic accuracy of transabdominal ultrasonography and gastroscopy for gastric cancer. J. Second Milit. Med. Univ. 2021, 42, 1189–1192. [Google Scholar]
- Yang, X.Y.; Li, C.J.; Guo, H. The diagnostic value of abdominal ultrasound combined with MSCT in preoperative TN staging of gastric cancer. Chin. PLA Med. J. 2019, 31, 20–24. [Google Scholar]
- Zhang, C.M.; Yang, X.; Xiong, M. Gastric window contrast-enhanced ultrasonography, MSCT and their combination in the diagnosis of preoperative TNM staging and postoperative pathological consistency of gastric cancer. Chin. J. CT MRI 2021, 19, 155–157. [Google Scholar]
- Thoeni, R.F. Colorectal cancer: Radiologic staging. Radiol. Clin. N. Am. 1997, 35, 457–485. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Bhattacharjya, T.; Baber, S.; Tibballs, J.M.; Watkinson, A.F.; Davidson, B.R. Prospective study of contrast-enhanced computed tomography, computed tomography during arterioportography, and magnetic resonance imaging for staging colorectal liver metastases for liver resection. Br. J. Surg. 2004, 91, 1361–1369. [Google Scholar] [CrossRef]
- Low, G.; Tho, L.M.; Leen, E.; Wiebe, E.; Kakumanu, S.; McDonald, A.C.; Poon, F.W. The role of imaging in the pre-operative staging and post-operative follow-up of rectal cancer. Surgeon 2008, 6, 222–231. [Google Scholar] [CrossRef]
- Goldberg-Stein, S.; Liu, B.; Hahn, P.F.; Lee, S.I. Body CT during pregnancy: Utilization trends, examination indications, and fetal radiation doses. Am. J. Roentgenol. 2011, 196, 146–151. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. ACR–SPR Practice Parameter for Imaging Pregnant or Potentially Pregnant Adolescents and Women with Ionizing Radiation. Resolution 39. Reston (VA): ACR. 2014. Available online: http://www.acr.org/~/media/9e2ed55531fc4b4fa53ef3b6d3b25df8.pdf (accessed on 27 October 2015).
- International Commission on Radiological Protection. ICRP publication 103: The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef]
- Gjelsteen, A.C.; Ching, B.H.; Meyermann, M.W.; Prager, D.A.; Murphy, T.F.; Berkey, B.D.; Mitchell, L.A. CT, MRI, PET, PET/CT, and ultrasound in the evaluation of obstetric and gynecologic patients. Surg. Clin. N. Am. 2008, 88, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Reede, D.L.; Katz, D.S.; Subramaniam, R.; Amorosa, J.K. Imaging the pregnant patient for nonobstetric conditions: Algorithms and radiation dose considerations. Radiographics 2007, 27, 1705–1722. [Google Scholar] [CrossRef]
- Tremblay, E.; Thérasse, E.; Thomassin-Naggara, I.; Trop, I. Quality initiatives: Guidelines for use of medical imaging during pregnancy and lactation. Radiographics 2012, 32, 897–911. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists(ACOG). Committee Opinion No. 723, Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet. Gynecol. 2017, 130, e210–e216, Erratum in Obstet. Gynecol. 2018, 132, 786. [Google Scholar] [CrossRef]
- Lowe, S. Diagnostic imaging in pregnancy: Making informed decisions. Obstet. Med. 2019, 12, 116–122. [Google Scholar] [CrossRef]
- American College of Radiology. ACR Manual on Contrast Media. American College of Radiology 2018. Version 10.3. Available online: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf (accessed on 7 October 2019).
- Webb, J.A.; Thomsen, H.S.; Morcos, S.K.; Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur. Radiol. 2005, 15, 1234–1240. [Google Scholar] [CrossRef]
- Atwell, T.D.; Lteif, A.N.; Brown, D.L.; McCann, M.; Townsend, J.E.; Leroy, A.J. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. Am. J. Roentgenol. 2008, 191, 268–271. [Google Scholar] [CrossRef]
- Hepner, A.; Negrini, D.; Hase, E.A.; Exman, P.; Testa, L.; Trinconi, A.F.; Filassi, J.R.; Francisco, R.P.V.; Zugaib, M.; O’Connor, T.L.; et al. Cancer During Pregnancy: The Oncologist Overview. World J. Oncol. 2019, 10, 28–34. [Google Scholar] [CrossRef]
- Zerhouni, E.A.; Rutter, C.; Hamilton, S.R.; Balfe, D.M.; Megibow, A.J.; Francis, I.R.; Moss, A.A.; Heiken, J.P.; Tempany, C.M.; Aisen, A.M.; et al. CT and MR imaging in the staging of colorectal carcinoma: Report of the Radiology Diagnostic Oncology Group II. Radiology 1996, 200, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.M.; Lee, J.M.; Lee, S.Y.; Ahn, B.Y.; Park, S.M.; Kim, K.M. Comparing MR imaging and CT in the staging of gastric carcinoma. Am. J. Roentgenol. 2000, 174, 1551–1557, Erratum in Am. J. Roentgenol. 2000, 175, 556. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.M.; Leung, J.L.; Cheng, C.S.; Lee, J.C.; Li, M.K.; Chung, C.C. Effect of endorectal coils on staging of rectal cancers by magnetic resonance imaging. Hong Kong Med. J. 2010, 16, 421–426. [Google Scholar] [PubMed]
- Heinrichs, W.L.; Fong, P.; Flannery, M.; Heinrichs, S.C.; Crooks, L.E.; Spindle, A.; Pedersen, R.A. Midgestational exposure of pregnant BALB/c mice to magnetic resonance imaging conditions. Magn. Reson. Imaging 1988, 6, 305–313. [Google Scholar] [CrossRef]
- Tyndall, D.A.; Sulik, K.K. Effects of magnetic resonance imaging on eye development in the C57BL/6J mouse. Teratology 1991, 43, 263–275. [Google Scholar] [CrossRef]
- Yip, Y.P.; Capriotti, C.; Talagala, S.L.; Yip, J.W. Effects of MR exposure at 1.5 T on early embryonic development of the chick. J. Magn. Reson. Imaging 1994, 4, 742–748. [Google Scholar] [CrossRef]
- National Radiological Protection Board. Principles for the Protection of Patients and Volunteers During Clinical Magnetic Resonance Diagnostic Procedures. In Documents of the NRPB.; HM Stationery Office: London, UK, 1991; Volume 2. [Google Scholar]
- Spencer, J.A.; Tomlinson, A.J.; Weston, M.J.; Lloyd, S.N. Early report: Comparison of breath-hold MR excretory urography, Doppler ultrasound and isotope renography in evaluation of symptomatic hydronephrosis in pregnancy. Clin. Radiol. 2000, 55, 446–453. [Google Scholar] [CrossRef]
- Chen, M.M.; Coakley, F.V.; Kaimal, A.; Laros, R.K., Jr. Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet. Gynecol. 2008, 112, 333–340. [Google Scholar] [CrossRef]
- Kanal, E.; Barkovich, A.J.; Bell, C.; Borgstede, J.P.; Bradley, W.G., Jr.; Froelich, J.W.; Gimbel, J.R.; Gosbee, J.W.; Kuhni-Kaminski, E.; Larson, P.A.; et al. Expert Panel on MR Safety; ACR guidance document on MR safe practices: 2013. J. Magn. Reason. Imaging 2013, 37, 501–530. [Google Scholar] [CrossRef]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef]
- Sachs, H.C.; Committee On Drugs; Frattarelli, D.A.; Galinkin, J.L.; Green, T.P.; Johnson, T.; Neville, K.; Paul, I.M.; Van den Anker, J. The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics 2013, 132, e796–e809. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Fregonara, P.; Jan, S.; Taieb, D.; Cammilleri, S.; Trébossen, R.; Hindié, E.; Mundler, O. Absorbed 18F-FDG dose to the fetus during early pregnancy. J. Nucl. Med. 2010, 51, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Takalkar, A.M.; Khandelwal, A.; Lokitz, S.; Lilien, D.L.; Stabin, M.G. 18F-FDG PET in pregnancy and fetal radiation dose estimates. J. Nucl. Med. 2011, 52, 1035–1040. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, Y.C.; Sun, S.S.; Chu, L.Y.; Yen, K.Y.; Kao, C.H. FDG PET/CT of a late-term pregnant woman with breast cancer. Clin. Nucl. Med. 2012, 37, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Umutlu, L.; Antoch, G.; Herrmann, K.; Grueneisen, J. PET/MR Imaging of the Female Pelvis. Semin. Nucl. Med. 2019, 49, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058, Erratum in J. Clin. Oncol. 2016, 34, 2562. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F.; ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v8–v30. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Fregonara, P.; Laforest, R.; Wallis, J.W. Fetal Radiation Dose from 18F-FDG in Pregnant Patients Imaged with PET, PET/CT, and PET/MR. J. Nucl. Med. 2015, 56, 1218–1222. [Google Scholar] [CrossRef]
- Xie, T.; Zanotti-Fregonara, P.; Edet-Sanson, A.; Zaidi, H. Patient-Specific Computational Model and Dosimetry Calculations for PET/CT of a Patient Pregnant with Twins. J. Nucl. Med. 2018, 59, 1451–1458. [Google Scholar] [CrossRef]
- Sawatzke, A.B.; Norris, A.W.; Spyropoulos, F.; Walsh, S.A.; Acevedo, M.R.; Hu, S.; Yao, J.; Wang, C.; Sunderland, J.J.; Boles Ponto, L.L. PET/CT imaging reveals unrivaled placental avidity for glucose compared to other tissues. Placenta 2015, 36, 115–120. [Google Scholar] [CrossRef]
- Gill, M.M.; Sia, W.; Hoskinson, M.; Niven, E.; Khurana, R. The use of PET/CT in pregnancy: A case report of malignant parathyroid carcinoma and a review of the literature. Obstet. Med. 2018, 11, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kal, H.B.; Struikmans, H. Radiotherapy during pregnancy: Fact and fiction. Lancet Oncol. 2005, 6, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Despierres, M.; Boudy, A.-S.; Selleret, L.; Gligorov, J.; Richard, S.; Thomassin, I.; Dabi, Y.; Zilberman, S.; Touboul, C.; Montravers, F.; et al. Feasibility, Safety and Impact of (18F)-FDG PET/CT in patients with pregnancy-associated cancer: Experience of the French CALG (Cancer Associé à La Grossesse) network. Acta Oncol. 2021, 61, 302–308. [Google Scholar] [CrossRef]
- Nishie, H.; Mizushima, T.; Suzuki, Y.; Fukusada, S.; Inoue, T.; Kachi, K.; Ozeki, T.; Anbe, K.; Iwasaki, H.; Okumura, F.; et al. Chemotherapy treatment of a pregnant woman with progressive gastric cancer. Intern. Med. 2015, 54, 1207–1212. [Google Scholar] [CrossRef]
- Cardonick, E.; Iacobucci, A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Carey, N.; Gagnon, R.; Maxwell, C.; Nulman, I.; Senikas, V. RETIRED: Cancer chemotherapy and pregnancy. J. Obstet. Gynaecol Can. 2013, 35, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.K.; Koil, C.; Rayburn, W.F. Chemotherapeutic drugs in pregnancy. Obstet. Gynecol. Clin. N. Am. 2005, 32, 627–640. [Google Scholar] [CrossRef]
- Karp, G.I.; von Oeyen, P.; Valone, F.; Khetarpal, V.K.; Israel, M.; Mayer, R.J.; Frigoletto, F.D.; Garnick, M.B. Doxorubicin in pregnancy: Possible transplacental passage. Cancer Treat. Rep. 1983, 67, 773–777. [Google Scholar]
- Germann, N.; Goffinet, F.; Goldwasser, F. Anthracyclines during pregnancy: Embryo-fetal outcome in 160 patients. Ann. Oncol. 2004, 15, 146–150. [Google Scholar] [CrossRef]
- Barni, S.; Ardizzoia, A.; Zanetta, G.; Strocchi, E.; Lissoni, P.; Tancini, G. Weekly doxorubicin chemotherapy for breast cancer in pregnancy. A case report. Tumori 1992, 78, 349–350. [Google Scholar] [CrossRef]
- Roboz, J.; Gleicher, N.; Wu, K.; Chahinian, P.; Kerenyi, T.; Holland, J. Does doxorubicin cross the placenta? Lancet 1979, 314, 1382–1383. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, K.; Verbesselt, R.; Ottevanger, N.; Halaska, M.; Heyns, L.; Van Bree, R.; de Bruijn, E.; Chai, D.; Delforge, M.; Noens, L.; et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: A preclinical and clinical study. Acta Obstet. Gynecol. Scand. 2010, 89, 1338–1345. [Google Scholar] [CrossRef]
- Doll, D.C.; Ringenberg, Q.S.; Yarbro, J.W. Antineoplastic agents and pregnancy. Semin. Oncol. 1989, 16, 337–346. [Google Scholar] [CrossRef]
- Silverstein, J.; Post, A.L.; Chien, A.J.; Olin, R.; Tsai, K.K.; Ngo, Z.; Van Loon, K. Multidisciplinary Management of Cancer During Pregnancy. JCO Oncol. Pract. 2020, 16, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Yamada, M.; Kasai, Y.; Miyauchi, A.; Andoh, K. Anticancer drugs during pregnancy. Jpn. J. Clin. Oncol. 2016, 46, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Ghosh, S. Pregnancy and perinatal outcomes following exposure to antineoplastic agents around pregnancy within the US FDA Adverse Event Reporting System. Future Oncol. 2022, 18, 2635–2642. [Google Scholar] [CrossRef]
- Van Calsteren, K.; Verbesselt, R.; Beijnen, J.; Devlieger, R.; De Catte, L.; Chai, D.C.; Van Bree, R.; Heyns, L.; de Hoon, J.; Amant, F. Transplacental transfer of anthracyclines, vinblastine, and 4-hydroxy-cyclophosphamide in a baboon model. Gynecol. Oncol. 2010, 119, 594–600. [Google Scholar] [CrossRef]
- Triarico, S.; Rivetti, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Ruggiero, A. Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy. Cancers 2022, 14, 3103. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Peccatori, F.A.; Pavlidis, N. Treatment of the pregnant mother with cancer: A systematic review on the use of cytotoxic, endocrine, targeted agents and immunotherapy during pregnancy. Part I: Solid tumors. Cancer Treat. Rev. 2010, 36, 101–109. [Google Scholar] [CrossRef]
- Schapira, D.V.; Chudley, A.E. Successful pregnancy following continuous treatment with combination chemotherapy before conception and throughout pregnancy. Cancer 1984, 54, 800–803. [Google Scholar] [CrossRef]
- Sweet, D.L., Jr.; Kinzie, J. Consequences of radiotherapy and antineoplastic therapy for the fetus. J. Reprod. Med. 1976, 17, 241–246. [Google Scholar] [PubMed]
- Brewer, M.; Kueck, A.; Runowicz, C.D. Chemotherapy in pregnancy. Clin. Obstet. Gynecol. 2011, 54, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, C.C.; Osborne, E.M.; Chihara, D.; Lai, P.; Zhou, S.; Ramirez, M.M.; Oki, Y.; Hagemeister, F.B.; Rodriguez, A.M.; Samaniego, F.; et al. Maternal and Fetal Outcomes After Therapy for Hodgkin or Non-Hodgkin Lymphoma Diagnosed During Pregnancy. JAMA Oncol. 2016, 2, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Cardonick, E.; Usmani, A.; Ghaffar, S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: Results of an international registry. Am. J. Clin. Oncol. 2010, 33, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Han, S.N.; Gziri, M.M.; Dekrem, J.; Van Calsteren, K. Chemotherapy during pregnancy. Curr Opin Oncol. 2012, 24, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Han, S.N.; von Minckwitz, G.; Bontenbal, M.; Ring, A.; Giermek, J.; Fehm, T.; Van Calsteren, K.; Linn, S.C.; Schlehe, B.; et al. Treatment of breast cancer during pregnancy: An observational study. Lancet Oncol. 2012, 13, 887–896. [Google Scholar] [CrossRef]
- Ellis, G.K.; Barlow, W.E.; Gralow, J.R.; Hortobagyi, G.N.; Russell, C.A.; Royce, M.E.; Perez, E.A.; Lew, D.; Livingston, R.B. Phase III comparison of standard doxorubicin and cyclophosphamide versus weekly doxorubicin and daily oral cyclophosphamide plus granulocyte colony-stimulating factor as neoadjuvant therapy for inflammatory and locally advanced breast cancer: SWOG 0012. J. Clin. Oncol. 2011, 29, 1014–1021. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Martino, S.; Jones, V.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; Wood, W.C.; Davidson, N.E. Weekly paclitaxel in the adjuvant treatment of breast cancer. N. Engl. J. Med. 2008, 358, 1663–1671, Erratum in N. Engl. J. Med. 2008, 359, 106; Erratum in N. Engl. J. Med. 2009, 360, 1685. [Google Scholar] [CrossRef]
- Kim, J.; Sun, C.L.; Mailey, B.; Prendergast, C.; Artinyan, A.; Bhatia, S.; Pigazzi, A.; Ellenhorn, J.D. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann. Oncol. 2010, 21, 152–160. [Google Scholar] [CrossRef]
- Matsui, M.; Kojima, O.; Uehara, Y.; Takahashi, T. Characterization of estrogen receptor in human gastric cancer. Cancer 1991, 68, 305–308. [Google Scholar] [CrossRef]
- Koullias, G.J.; Kouraklis, G.P.; Raftopoulos, I.S.; Davaris, P.S.; Papadopoulos, S.A.; Golematis, B.C. Increased estrogen receptor and epidermal growth factor receptor gene product co-expression in surgically resected gastric adenocarcinomas. J. Surg. Oncol. 1996, 63, 166–171. [Google Scholar] [CrossRef]
- Rogers, J.E.; Dasari, A.; Eng, C. The Treatment of Colorectal Cancer During Pregnancy: Cytotoxic Chemotherapy and Targeted Therapy Challenges. Oncologist 2016, 21, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Matei, L.; Necula, L.G.; Dragu, D.L.; Bleotu, C.; Diaconu, C.C. New therapeutic options opened by the molecular classification of gastric cancer. World J. Gastroenterol. 2018, 24, 1942–1961. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B., 3rd; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar]
- Grillo, F.; Fassan, M.; Sarocchi, F.; Fiocca, R.; Mastracci, L. HER2 heterogeneity in gastric/gastroesophageal cancers: Frombenchside to practice. World J. Gastroenterol. 2016, 22, 5879–5887. [Google Scholar] [CrossRef]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Rüschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; Van De Vijver, M.; Viale, G. HER2 testing in gastric cancer: A practical approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Maron, S.B.; Alpert, L.; Kwak, H.A.; Lomnicki, S.; Chase, L.; Xu, D.; O’Day, E.; Nagy, R.J.; Lanman, R.B.; Cecchi, F.; et al. Targeted therapies for targeted populations: Anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018, 8, 696–713. [Google Scholar] [CrossRef] [PubMed]
- De Vita, F.; Tirino, G.; Pompella, L.; Petrillo, A. Gastric Cancer: Advanced/Metastatic Disease. In Practical Medical Oncology Textbook; Russo, A., Peeters, M., Incorvaia, L., Rolfo, C., Eds.; UNIPA Springer Series; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Quintero Aldana, G.; Salgado, M.; Candamio, S.; Méndez, J.C.; Jorge, M.; Reboredo, M.; Vázquez Tuñas, L.; Romero, C.; Covela, M.; Fernández Montes, A.; et al. First-line panitumumab plus docetaxel and cisplatin in advanced gastric and gastro-oesophageal junction adenocarcinoma: Results of a phase II trial. Clin. Transl. Oncol. 2020, 22, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. Molecular targeted therapy for the treatment of gastric cancer. J. Exp. Clin. Cancer Res. 2016, 35, 1. [Google Scholar] [CrossRef]
- Grigore, D.; Simionescu, C.E.; Stepan, A.; Margaritescu, C.; Balasoiu, M.; Georgescu, C.C.; Cernea, D.; Dumitrescu, D. Assessment of CD105, alpha-SMA and VEGF expression in gastric carcinomas. Rom. J. Morphol. Embryol. 2013, 54 (Suppl. S3), 701–707. [Google Scholar] [PubMed]
- Jüttner, S.; Wiβmann, C.; Jöns, T.; Vieth, M.; Hertel, J.; Gretschel, S.; Schlag, P.M.; Kemmner, W.; Höcker, M. Vascular endothelial growth factor-D and its receptor VEGFR-3, two novel independent prognostic markers in gastric adenocarcinoma. J. Clin. Oncol. 2006, 24, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Ferrara, N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005, 65, 671–680. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Yoon, H.; Bendell, J.; Braiteh, F.; Firdaus, I.; Philip, P.; Cohn, A.; Lewis, N.; Anderson, D.M.; Arrowsmith, E.; Schwartz, J.D.; et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: A randomized, double-blind, multicenter phase II trial. Ann. Oncol. 2016, 27, 2196–2203. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 420–435. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Kojima, T.; Muro, K.; Francois, E.; Hsu, C.-H.; Moriwaki, T.; Kim, S.-B.; Lee, S.H.; Bennouna, J.; Kato, K.; Lin, S.; et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: Phase III KEYNOTE-181 study. J. Clin. Oncol. 2019, 37 (Suppl. S4), 2. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoyama, Y.; Narikawa, N. Krukenberg tumor metastasized from stomach cancer incidentally discovered at the caesarean section: A case report. Tokai J. Obstet. Gynecol. 2006, 43, 123–126. (In Japanese) [Google Scholar]
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013, 24 (Suppl. S6), vi160–vi170. [Google Scholar] [CrossRef]
- Upadya, M.; Saneesh, P.J. Anaesthesia for non-obstetric surgery during pregnancy. Indian J. Anaesth. 2016, 60, 234–241. [Google Scholar] [CrossRef]
- Cohen-Kerem, R.; Railton, C.; Oren, D.; Lishner, M.; Koren, G. Pregnancy outcome following non-obstetric surgical intervention. Am. J. Surg. 2005, 190, 467–473. [Google Scholar] [CrossRef]
- Okeagu, C.N.; Anandi, P.; Gennuso, S.; Hyatali, F.; Stark, C.W.; Prabhakar, A.; Cornett, E.M.; Urman, R.D.; Kaye, A.D. Clinical management of the pregnant patient undergoing non-obstetric surgery: Review of guidelines. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 269–281. [Google Scholar] [CrossRef]
- Yoshida, M. Successful prognosis of pregnancy with gastric cancer in the second trimester: A case report. J. Jpn. Soc. Perinat. Neonatal. Med. 2007, 43, 140–142. [Google Scholar]
- Maeda, S.; Takigawa, J.; Toyoyama, H. Two cases of gastric cancer during pregnancy. Surgery 2007, 69, 1077–1080. (In Japanese) [Google Scholar]
- Augustin, G. Acute Abdomen during Pregnancy. No. 14737; Springer International Publishing: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Association Japanese Gastric Cancer. Japanese Classifcation of Gastric Carcinoma, 15th ed.; Kanehara Shuppan: Tokyo, Japan, 2017. [Google Scholar]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classifcation of Malignant Tumours, 8th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Visser, B.C.; Glasgow, R.E.; Mulvihill, K.K.; Mulvihill, S.J. Safety and timing of nonobstetric abdominal surgery in pregnancy. Dig. Surg. 2001, 18, 409–417. [Google Scholar] [CrossRef]
- Fisher, S.C.; Siag, K.; Howley, M.M.; Van Zutphen, A.R.; Reefhuis, J.; Browne, M.L.; National Birth Defects Prevention Study. Maternal surgery and anesthesia during pregnancy and risk of birth defects in the National Birth Defects Prevention Study, 1997–2011. Birth Defects Res. 2020, 112, 162–174. [Google Scholar] [CrossRef]
- Auger, N.; Ayoub, A.; Piché, N. First trimester general anaesthesia and risk of central nervous system defects in offspring. Br. J. Anaesth. 2020, 124, E92–E94. [Google Scholar] [CrossRef]
- Shin, J. Anesthetic Management of the Pregnant Patient: Part 2. Anesth. Prog. 2021, 68, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Gandra, A.; Viveiros, F.; Cidade, C.; Maciel, J. Analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol. Oncol. Res. 2013, 19, 111–117. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.H.; Lim, B.J.; Kim, H.; Kim, H.; Park, J.J.; Youn, Y.H.; Park, H.; Noh, S.H.; Kim, J.W.; et al. Sex Disparity in Gastric Cancer: Female Sex is a Poor Prognostic Factor for Advanced Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 4344–4351, Erratum in Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 1062. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.A. Coexistence of pregnancy and malignancy. Oncologist 2002, 7, 279–287, Erratum in Oncologist 2002, 7, 585. [Google Scholar] [CrossRef]
- Altman, J.F.; Lowe, L.; Redman, B.; Esper, P.; Schwartz, J.L.; Johnson, T.M.; Haefner, H.K. Placental metastasis of maternal melanoma. J. Am. Acad. Dermatol. 2003, 49, 1150–1154. [Google Scholar] [CrossRef]
- Al-Adnani, M.; Kiho, L.; Scheimberg, I. Maternal pancreatic carcinoma metastatic to the placenta: A case report and literature review. Pediatr. Dev. Pathol. 2007, 10, 61–65. [Google Scholar] [CrossRef]
- Oga, S.; Hachisuga, M.; Hidaka, N.; Fujita, Y.; Tomonobe, H.; Yamamoto, H.; Kato, K. Gastric cancer during pregnancy with placental involvement: Case report and review of published works. Obstet. Gynecol. Sci. 2019, 62, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Hafiz, N.; Bienvenu, J.; Wellman, G.; Morris, J. A Rare Case of Placental Metastasis in Gastric Cancer. Am. J. Gastroenterol. 2018, 113, S1488–S1489. [Google Scholar] [CrossRef]
- Pihl, K.; Larsen, T.; Laursen, I.; Krebs, L.; Christiansen, M. Firsttrimester maternal serum pregnancy-specific beta-1-glycoprotein(SP1) as a marker of adverse pregnancy outcome. Prenat. Diagn. 2009, 29, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, F.A.; Warren, J.; Sulkowski, G.; Aparicio, M.; David, G.; Zudaire, E.; Dveksler, G.S. Pregnancy-specific glycoprotein 1 induces endothelial tubulogenesis through interaction with cell surface proteoglycans. J. Biol. Chem. 2011, 286, 7577–7586. [Google Scholar] [CrossRef]
- Ha, C.T.; Wu, J.A.; Irmak, S.; Lisboa, F.A.; Dizon, A.M.; Warren, J.W.; Ergun, S.; Dveksler, G.S. Human pregnancy specific beta-1- glycoprotein 1 (PSG1) has a potential role in placental vascular morphogenesis. Biol. Reprod. 2010, 83, 27–35. [Google Scholar] [CrossRef]
- Blois, S.M.; Sulkowski, G.; Tirado-González, I.; Warren, J.; Freitag, N.; Klapp, B.F.; Rifkin, D.; Fuss, I.; Strober, W.; Dveksler, G.S. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-β and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol. 2014, 7, 348–358. [Google Scholar] [CrossRef]
- Rodríguez-Esquivel, M.; Romero-Morelos, P.; Taniguchi-Ponciano, K.; Mendoza-Rodríguez, M.; Marrero-Rodríguez, D.; Bandera-Delgado, A.; Huerta-Padilla, V.; Serna-Reyna, L.; Gómez-Gutiérrez, G.; Gómez-Virgilio, L.; et al. Expression of pregnancy specific β-1 glycoprotein 1 in cervical cancer cells. Arch. Med. Res. 2020, 51, 504–514. [Google Scholar] [CrossRef]
- Kang, H.-G.; Kim, S.-J. High Expression of Pregnancy Specific Beta-1-glycoprotein 1 Is Associated with Poor Gastric Cancer Prognosis. In Vivo 2022, 36, 2700–2707. [Google Scholar] [CrossRef]

| Pregnancy Period | Implantation Period | Organogenesis Period | Fetal Development Period |
|---|---|---|---|
| Trimester | 1st trimester | 2nd trimester | 3rd trimester |
| Week | 1 2 3 4 5 6 7 8 9 10 11 12 16 24 32 40 | ||
| Chemotherapeutic agents | Major congenital malformations Fetal demise Impaired organ function Spontaneous abortion | No definite associations with significant teratogenic effects (limited data) | Minor associations with:
|
| Targeted agents | Relatively low risk | Stillbirth Lung disease Renal failure Respiratory distress syndrome Severe pulmonary hypoplasia Prematurity | Oligohydramnios/anhydramnios (most common adverse event) |
| Tyrosine kinase inhibitors TKIs | Avoid imatinib use in the first trimester:
| Teratogenic potential and major malformations: exencephaly, encephalopathies, and abnormalities in the skull bones | Severe maternal adverse effects |
| IgG4 antibodies | Spontaneous abortion Intrauterine growth restriction Congenital hypothyroidism | No increase in the rate of malformations | Miscarriage, stillbirth, prematurity, small birth weight, and infant mortality |
| Chemotherapeutic Agents | Studies on Humans | Studies on Animals | Pregnancy Category * |
|---|---|---|---|
| Bevacizumab | ND | T | C |
| Cetuximab | ND | T | C |
| Panitumumab | ND | T* | C |
| Sorafenib | ND | T | D |
| Imatinib | ND | T | D |
| TNM Stage | T1bN0 | T1N+ | T2-4N0 | T2-4N+M+ |
|---|---|---|---|---|
| Types of gastric surgery | Gastrectomy D1/D1+ | Gastrectomy D2 | Gastrectomy D2 | Palliative surgery Reduction surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, A.; Constantin, R.; Achim, F.; Socea, B.; Predescu, D. Pregnancy and Gastric Cancer: A Narrative Review. Diagnostics 2023, 13, 1909. https://doi.org/10.3390/diagnostics13111909
Constantin A, Constantin R, Achim F, Socea B, Predescu D. Pregnancy and Gastric Cancer: A Narrative Review. Diagnostics. 2023; 13(11):1909. https://doi.org/10.3390/diagnostics13111909
Chicago/Turabian StyleConstantin, Adrian, Roxana Constantin, Florin Achim, Bogdan Socea, and Dragos Predescu. 2023. "Pregnancy and Gastric Cancer: A Narrative Review" Diagnostics 13, no. 11: 1909. https://doi.org/10.3390/diagnostics13111909
APA StyleConstantin, A., Constantin, R., Achim, F., Socea, B., & Predescu, D. (2023). Pregnancy and Gastric Cancer: A Narrative Review. Diagnostics, 13(11), 1909. https://doi.org/10.3390/diagnostics13111909






