Ultrasound-Guided Percutaneous Bone Biopsy: Feasibility, Diagnostic Yield and Technical Notes
Abstract
1. Introduction
1.1. Percutaneous Bone Biopsy: General Indications
1.2. Imaging Guidance for Percutaneous Bone Biopsy
2. Technical Notes and Overview of Approaches
3. Success Rate and Feasibility
3.1. Type of Lesion
3.2. Type of Biopsy
3.2.1. Type of the Needle
3.2.2. Needle Approaches and Cores
3.2.3. Characteristics of the Lesion
3.2.4. Target Lesion Location and Hybrid Techniques
3.2.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rimondi, E.; Rossi, G.; Bartalena, T.; Ciminari, R.; Alberghini, M.; Ruggieri, P.; Errani, C.; Angelini, A.; Calabrò, T.; Abati, C.N.; et al. Percutaneous CT-guided biopsy of the musculoskeletal system: Results of 2027 cases. Eur. J. Radiol. 2011, 77, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Kattapuram, S.V. Musculoskeletal Neoplasms: Biopsy and Intervention. Radiol. Clin. N. Am. 2011, 49, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Traina, F.; Errani, C.; Toscano, A.; Pungetti, C.; Fabbri, D.; Mazzotti, A.; Donati, D.; Faldini, C. Current concepts in the biopsy of musculoskeletal tumors. J. Bone Jt. Surg. Am. 2015, 97, e7. [Google Scholar] [CrossRef] [PubMed]
- Epis, O.; Bruschi, E. Interventional ultrasound: A critical overview on ultrasound-guided injections and biopsies. Clin. Exp. Rheumatol. 2014, 32 (Suppl. 80), S78–S84. [Google Scholar]
- Garnon, J.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Chari, B.; Cazzato, R.L.; Gangi, A. Ultrasound-Guided Biopsies of Bone Lesions Without Cortical Disruption Using Fusion Imaging and Needle Tracking: Proof of Concept. Cardiovasc. Interv. Radiol. 2017, 40, 1267–1273. [Google Scholar] [CrossRef]
- Kovacevic, L.; Cavka, M.; Marusic, Z.; Kresic, E.; Stajduhar, A.; Grbanovic, L.; Dumic-Cule, I.; Prutki, M. Percutaneous CT-Guided Bone Lesion Biopsy for Confirmation of Bone Metastases in Patients with Breast Cancer. Diagnostics 2022, 12, 2094. [Google Scholar] [CrossRef]
- Criscitiello, C.; André, F.; Thompson, A.M.; De Laurentiis, M.; Esposito, A.; Gelao, L.; Fumagalli, L.; Locatelli, M.; Minchella, I.; Orsi, F.; et al. Biopsy confirmation of metastatic sites in breast cancer patients: Clinical impact and future perspectives. Breast Cancer Res. 2014, 16, 205. [Google Scholar] [CrossRef]
- Filippiadis, D.; Mazioti, A.; Kelekis, A. Percutaneous, Imaging-Guided Biopsy of Bone Metastases. Diagnostics 2018, 8, 25. [Google Scholar] [CrossRef]
- Spinnato, P.; Bazzocchi, A.; Facchini, G.; Filonzi, G.; Nanni, C.; Rambaldi, I.; Rimondi, E.; Fanti, S.; Albisinni, U. Vertebral Fractures of Unknown Origin: Role of Computed Tomography-Guided Biopsy. Int. J. Spine Surg. 2018, 12, 673–679. [Google Scholar] [CrossRef]
- Saifuddin, A.; Palloni, V.; du Preez, H.; Junaid, S.E. Review article: The current status of CT-guided needle biopsy of the spine. Skelet. Radiol. 2020, 50, 281–299. [Google Scholar] [CrossRef]
- Spinnato, P.; Patel, D.B.; Di Carlo, M.; Bartoloni, A.; Cevolani, L.; Matcuk, G.R.; Crombé, A. Imaging of Musculoskeletal Soft-Tissue Infections in Clinical Practice: A Comprehensive Updated Review. Microorganisms 2022, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Spinnato, P.; Tedeschi, S.; Zamparini, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Caldari, E.; Crombé, A.; Viale, P.; et al. Bone and Joint Infections: The Role of Imaging in Tailoring Diagnosis to Improve Patients’ Care. J. Pers. Med. 2021, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Nanni, P.; Landuzzi, L.; Manara, M.C.; Righi, A.; Nicoletti, G.; Cristalli, C.; Pasello, M.; Parra, A.; Carrabotta, M.; Ferracin, M.; et al. Bone sarcoma patient-derived xenografts are faithful and stable preclinical models for molecular and therapeutic investigations. Sci. Rep. 2019, 9, 12174. [Google Scholar] [CrossRef]
- Spinnato, P.; Rimondi, E.; Facchini, G. Percutaneous CT-Guided Biopsy of the Craniovertebral Junction: Safety, Diagnostic Yield, and Technical Notes. Diagnostics 2022, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- D’Ortenzio, R.M.; Tolhurst, S.; Harvey, M.; Ghag, R.; Heran, M.K. The CT guided transoral approach: A biopsy technique for a poorly differentiated chordoma in a 5 year old. J. Radiol. Case Rep. 2021, 15, 1–8. [Google Scholar] [CrossRef]
- Reddy, A.S.; Dinobile, D.; Orgeta, J.E.; Peri, N. Transoral approach to CT-guided C2 interventions. Pain Physician 2009, 12, 253–258. [Google Scholar]
- Singh, D.K.; Kumar, N.; Nayak, B.K.; Jaiswal, B.; Tomar, S.; Mittal, M.K.; Bajaj, S.K. Approach-based techniques of CT-guided percutaneous vertebral biopsy. Diagn. Interv. Radiol. 2020, 26, 143–146. [Google Scholar] [CrossRef]
- Rimondi, E.; Staals, E.L.; Errani, C.; Bianchi, G.; Casadei, R.; Alberghini, M.; Malaguti, M.C.; Rossi, G.; Durante, S.; Mercuri, M. Percutaneous CT-guided biopsy of the spine: Results of 430 biopsies. Eur. Spine J. 2008, 17, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Hillen, T.; Jennings, J. Percutaneous CT-Guided Skull Biopsy: Feasibility, Safety, and Diagnostic Yield. Am. J. Neuroradiol. 2019, 40, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.H.; Cox, M.; Sedora-Roman, N.; Ranganathan, S.; Hurst, R.; Pukenas, B. Image-Guided Percutaneous Calvarial Biopsy with Low-Dose CT-Fluoroscopy: Technique, Safety, and Utility in 12 Patients. Cardiovasc. Interv. Radiol. 2021, 45, 134–136. [Google Scholar] [CrossRef]
- Chira, R.I.; Chira, A.; Manzat-Saplacan, R.M.; Nagy, G.; Bintintan, A.; Mircea, P.A.; Valean, S. Ultrasound-guided bone lesions biopsies—A systematic review. Med. Ultrason. 2017, 19, 302–309. [Google Scholar] [CrossRef]
- Chira, R.I.; Chira, A.; Calauz, A.; Saplacan, R.M.M.; Nagy, G.; Mircea, P.A.; Valean, S. Ultrasound-guided biopsy of osteolytic metastasis—Could be less than three cores enough? Med. Ultrason. 2018, 1, 50. [Google Scholar] [CrossRef]
- Bazzocchi, M.; Gozzi, G.; Zuiani, C.; Mucelli, R.S.P. Ultrasonic-guided fine-needle biopsy of osteolytic lesions. Radiol. Med. 1988, 76, 23–27. [Google Scholar]
- Hsu, W.H.; Chiang, C.D.; Hsu, J.Y.; Huang, W.L. Impalpable thoracic bony lesions diagnosed by sonographically guided needle aspiration biopsy. J. Ultrasound Med. 1992, 11, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, S.; Tandon, P.; Chhabra, D.K. Ultrasound-guided needle biopsy of lytic lesions of the cervical spine. J. Clin. Ultrasound 1993, 21, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Targhetta, R.; Balmes, P.; Marty-Double, C.; Mauboussin, J.-M.; Bourgeois, J.-M.; Pourcelot, L. Ultrasonically Guided Aspiration Biopsy in Osteolytic Bone Lesions of the Chest Wall. Chest 1993, 103, 1403–1408. [Google Scholar] [CrossRef]
- Vogel, B. Ultrasonographic Detection and Guided Biopsy of Thoracic Osteolysis. Chest 1993, 104, 1003–1005. [Google Scholar] [CrossRef]
- Civardi, G.; Livraghi, T.; Colombo, P.; Fornari, F.; Cavanna, L.; Buscarini, L. Lytic bone lesions suspected for metastasis: Ultrasonically guided fine-needle aspiration biopsy. J. Clin. Ultrasound 1994, 22, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Astrom, K.G.; Sundstrom, J.C.; Lindgren, P.G.; Ahlstrom, K.H. Automatic biopsy instruments used through a coaxial bone biopsy system with an eccentric drill tip. Acta Radiol. 1995, 36, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Konermann, W.; Wuisman, P.; Hillmann, A.; Rossner, A.; Blasius, S. Value of sonographically guided biopsy in the histological diagnosis of benign and malignant soft-tissue and bone tumors. Z. Orthop. Ihre Grenzgeb. 1995, 133, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Rubens, D.J.; Fultz, P.J.; Gottlieb, R.H.; Rubin, S.J. Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J. Ultrasound Med. 1997, 16, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, A.; Burnett, S.; Mitchell, R. Pictorial review: Ultrasonography of primary bone tumours. Clin. Radiol. 1998, 53, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takhtani, D.; Gulati, M.; Khandelwal, N.; Gupta, D.; Rajwanshi, A.; Gupta, S.; Suri, S. Sonographically guided fine-needle aspiration biopsy of lytic lesions of the spine: Technique and indications. J. Clin. Ultrasound 1999, 27, 123–129. [Google Scholar] [CrossRef]
- Konermann, W.; Wuisman, P.; Ellermann, A.; Gruber, G. Ultrasonographically guided needle biopsy of benign and malignant soft tissue and bone tumors. J. Ultrasound Med. 2000, 19, 465–471. [Google Scholar] [CrossRef]
- Saifuddin, A.; Mitchell, R.; Burnett, S.J.; Sandison, A.; Pringle, J.A. Ultrasound-guided needle biopsy of primary bone tumours. J. Bone Jt. Surg. Br. 2000, 82, 50–54. [Google Scholar] [CrossRef]
- Gil-Sánchez, S.; Marco-Doménech, S.F.; Irurzun-López, J.; Fernández-García, P.; de La Iglesia-Cardeña, P.; Ambit-Capdevila, S. Ultrasound-guided skeletal biopsies. Skelet. Radiol. 2001, 30, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Torriani, M.; Etchebehere, M.; Amstalden, E.M.I. Sonographically Guided Core Needle Biopsy of Bone and Soft Tissue Tumors. J. Ultrasound Med. 2002, 21, 275–281. [Google Scholar] [CrossRef]
- Ahrar, K.; Himmerich, J.U.; Herzog, C.E.; Raymond, A.K.; Wallace, M.J.; Gupta, S.; Madoff, D.C.; Morello, F.A.; Murthy, R.; McRae, S.E.; et al. Percutaneous Ultrasound-guided Biopsy in the Definitive Diagnosis of Osteosarcoma. J. Vasc. Interv. Radiol. 2004, 15, 1329–1333. [Google Scholar] [CrossRef]
- Lopez, J.I.; Del Cura, J.L.; Zabala, R.; Bilbao, F.J. Usefulness and limitations of ultrasound-guided core biopsy in the diagnosis of musculoskeletal tumours. Apmis 2005, 113, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Wang, W.C.; Liu, M.H.; Zhou, Q.C.; Chen, H.P. Application of ultrasound-guided percutaneous biopsy in the preoperative diagnosis and treatment of bone tumors. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2005, 30, 694–696. [Google Scholar] [PubMed]
- Wu, J.S.; Goldsmith, J.D.; Horwich, P.J.; Shetty, S.K.; Hochman, M.G. Bone and soft-tissue lesions: What factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008, 248, 962–970. [Google Scholar] [CrossRef]
- Datir, A.; Pechon, P.; Saifuddin, A. Imaging-Guided Percutaneous Biopsy of Pathologic Fractures: A Retrospective Analysis of 129 Cases. Am. J. Roentgenol. 2009, 193, 504–508. [Google Scholar] [CrossRef]
- Jakanani, G.C.; Saifuddin, A. Percutaneous image-guided needle biopsy of rib lesions: A retrospective study of diagnostic outcome in 51 cases. Skelet. Radiol. 2012, 42, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pressney, I.; Saifuddin, A. Percutaneous image-guided needle biopsy of clavicle lesions: A retrospective study of diagnostic yield with description of safe biopsy routes in 55 cases. Skelet. Radiol. 2014, 44, 497–503. [Google Scholar] [CrossRef]
- Azrumelashvili, T.; Mizandari, M.; Magalashvili, D.; Dundua, T. Imaging Guided Percutaneal Core Biopsy of Thoracic Bone and Soft Tissue Lesions—Technique and Complications. Georgian Med. News 2016, 250, 17–24. [Google Scholar]
- Huang, J.; Souza, C.; Jibri, Z.; Rakhra, K. Ultrasound-guided percutaneous rib biopsy: A safe procedure with high diagnostic yield. Clin. Radiol. 2019, 74, 650.e1–650.e6. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Gitto, S.; Pescatori, L.C.; Albano, D.; Messina, C.; Sconfienza, L.M. Technical Feasibility of Electromagnetic US/CT Fusion Imaging and Virtual Navigation in the Guidance of Spine Biopsies. Ultraschall Med. 2022, 43, 387–392. [Google Scholar] [CrossRef]
- Welker, J.A.; Henshaw, R.M.; Jelinek, J.; Shmookler, B.M.; Malawer, M.M. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer 2000, 89, 2677–2686. [Google Scholar] [CrossRef]
- Yeow, K.M.; Tan, C.F.; Chen, J.S.; Hsueh, C. Diagnostic sensitivity of ultrasound-guided needle biopsy in soft tissue masses about superficial bone lesions. J. Ultrasound Med. 2000, 19, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Papalexis, N.; Peta, G.; Ponti, F.; Tuzzato, G.; Colangeli, M.; Facchini, G.; Spinnato, P. CT-Guided Radiofrequency Thermal Ablation for the Treatment of Atypical, Early-Onset Osteoid Osteoma in Children Younger than 4 Years Old: Single-Institution Experience and Literature Review. Diagnostics 2022, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Khalil, J.G.; Mott, M.P.; Parsons, T.W.; Banka, T.R.; van Holsbeeck, M. 2011 Mid-America Orthopaedic Association Dallas B. Phemister Physician in Training Award: Can Musculoskeletal Tumors be Diagnosed with Ultrasound Fusion-Guided Biopsy? Clin. Orthop. Relat. Res. 2012, 470, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Loizides, A.; Widmann, G.; Freuis, T.; Peer, S.; Gruber, H. Optimizing Ultrasound-Guided Biopsy of Musculoskeletal Masses by Application of an Ultrasound Contrast Agent. Ultraschall Med. 2010, 32, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Sahlani, L.; Thompson, L.; Vira, A.; Panchal, A.R. Bedside ultrasound procedures: Musculoskeletal and non-musculoskeletal. Eur. J. Trauma Emerg. Surg. 2015, 42, 127–138. [Google Scholar] [CrossRef] [PubMed]
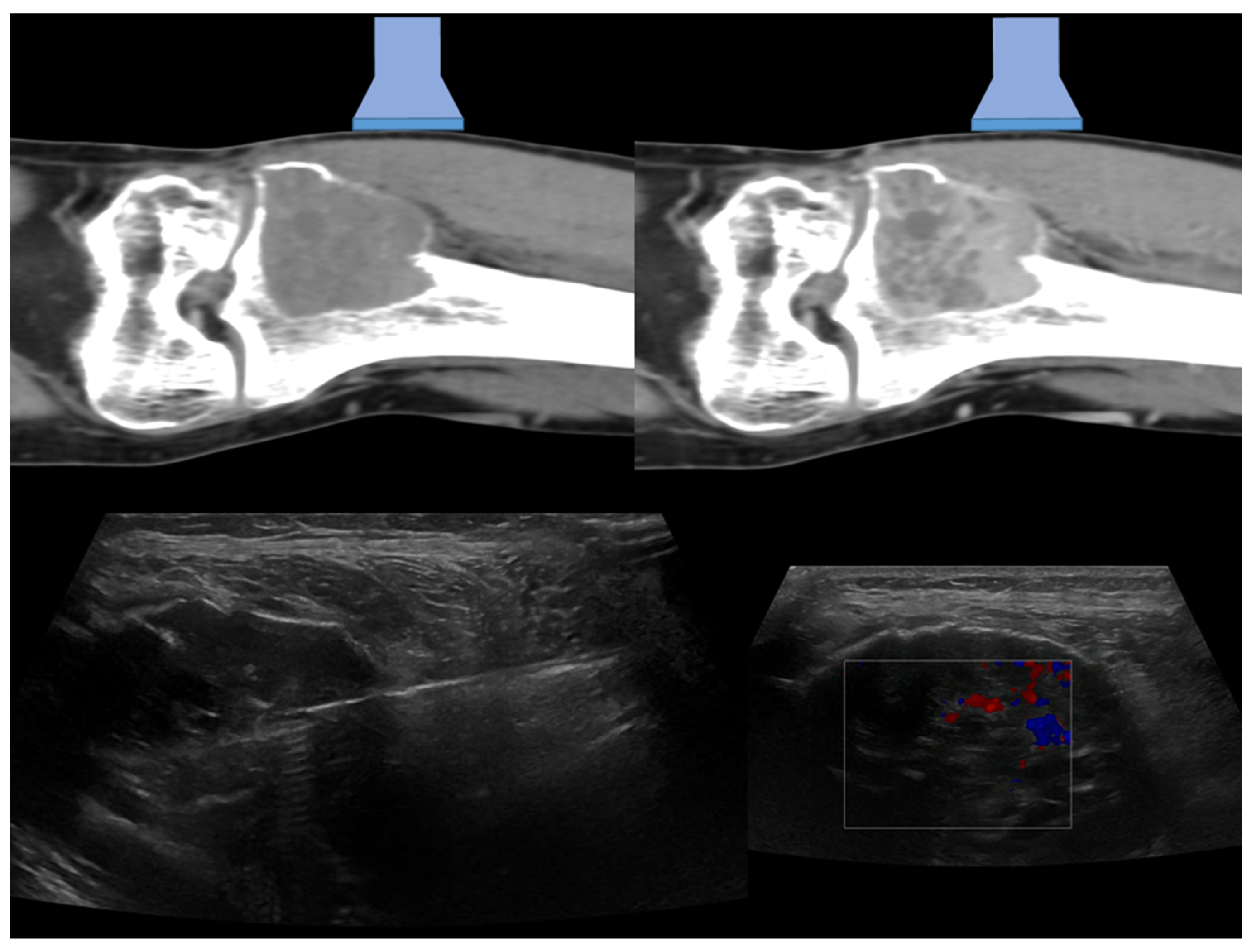

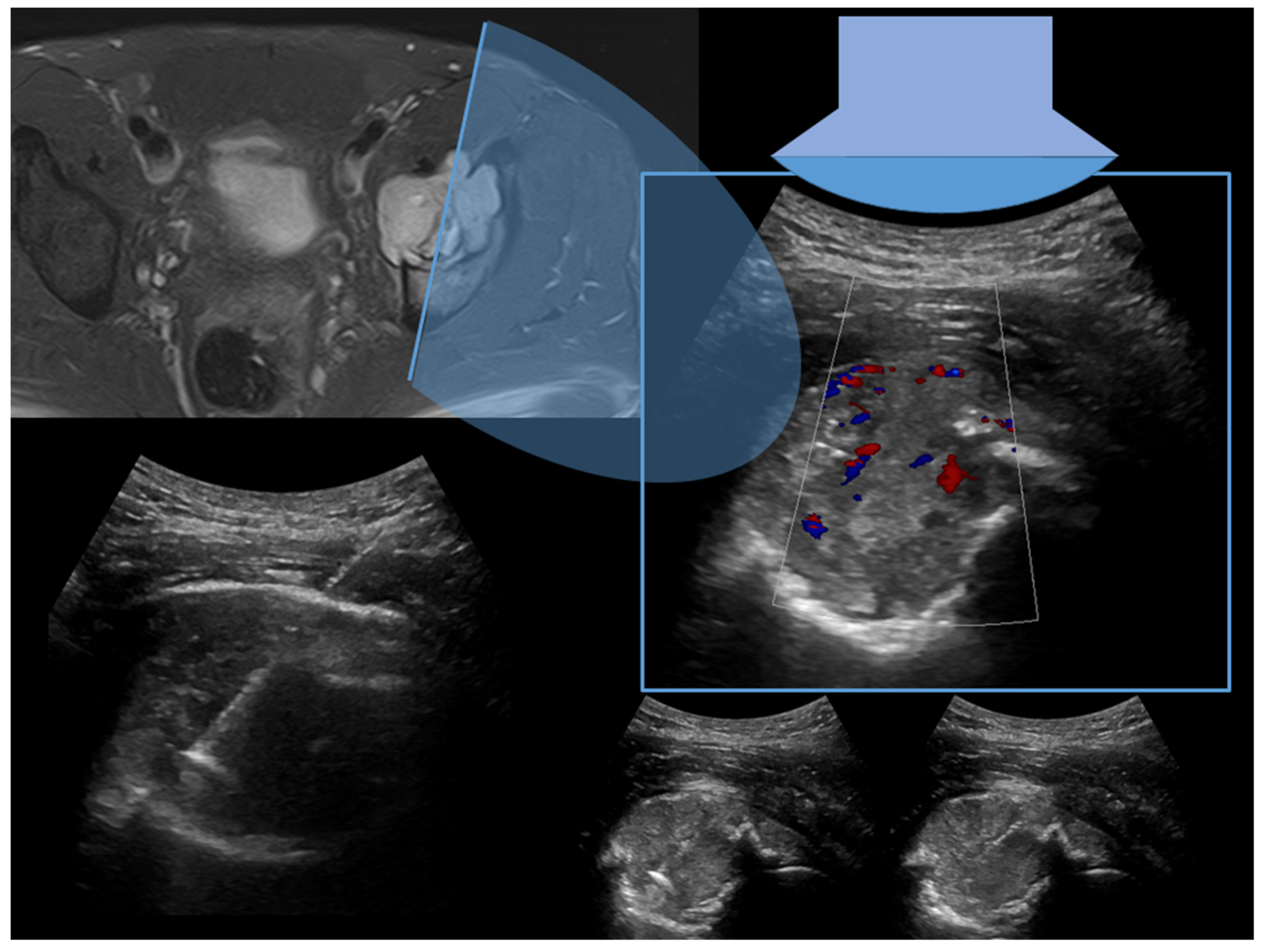
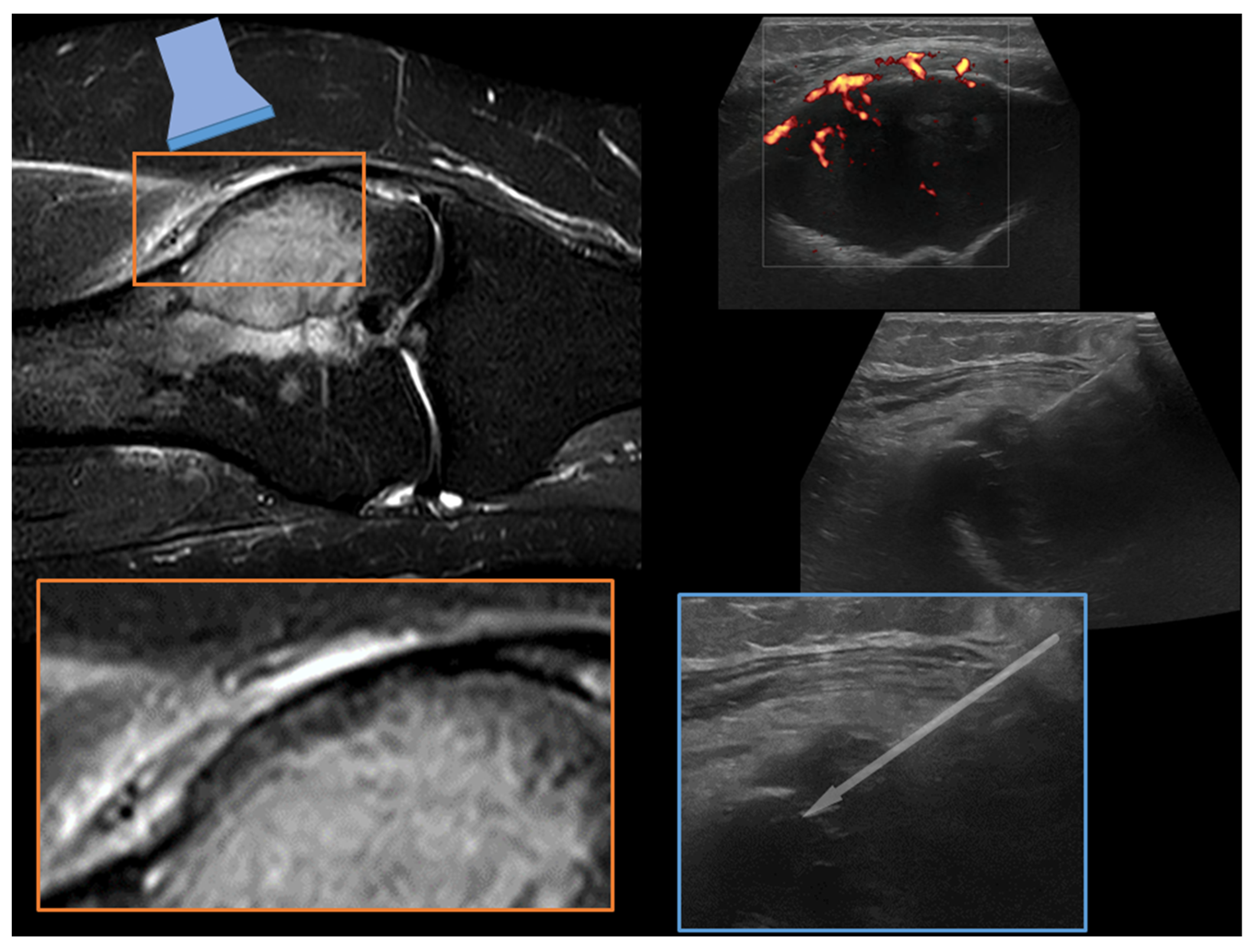
| Affected Skeletal Region | Radiologic Pattern | Ultrasound Guidance |
|---|---|---|
| Appendicular skeleton | Sclerotic pattern, osteolytic or mixed pattern without cortical disruption or intramedullary only involvement | +/− |
| Appendicular Skeleton | Osteolytic or mixed pattern with cortical disruption or cortical thinning | ++ |
| Pelvic bones | Sclerotic lesions without large extra-skeletal soft-tissue involvement | +/− |
| Pelvic bones | Osteolytic or mixed with large soft-tissue extra-skeletal involvement | + |
| Spine | All patterns (intraosseous lesions) | − |
| Spine | Large lesions of vertebral body (even if with extra-osseous component) | − |
| Spine | Lesions with posterior large extra-skeletal component | ++ |
| Ribs | Osteolytic or mixed pattern with cortical disruption or cortical thinning | ++ |
| Ribs | Sclerotic pattern, osteolytic or mixed pattern without cortical disruption or intramedullary only involvement | +/− |
| Sternum | All | +/− |
| Sternum | Anterior extra-skeletal soft-tissue involvement | ++ |
| Skull and Craniovertebral junction | All | − |
| Advantages of US-Guided Bone Biopsy | Disadvantages of US-Guided Bone Biopsy |
|---|---|
| Fast Acquisition-Time | Absence of visualization of intramedullary bone if cortex is preserved |
| Less expansive compared to CT-guided ones | Not safely applicable in all skeletal sites (e.g., deep one, vertebral body) |
| Real-time needle evaluation | Operator experience dependent |
| Evaluation of vascular structures (extra- and intra-lesion) | |
| Precision of target areas of lesions (avoiding necrosis or fluid collection) | |
| Absence of ionizing radiation exposure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponti, F.; Arioli, A.; Longo, C.; Miceli, M.; Colangeli, M.; Papalexis, N.; Spinnato, P. Ultrasound-Guided Percutaneous Bone Biopsy: Feasibility, Diagnostic Yield and Technical Notes. Diagnostics 2023, 13, 1773. https://doi.org/10.3390/diagnostics13101773
Ponti F, Arioli A, Longo C, Miceli M, Colangeli M, Papalexis N, Spinnato P. Ultrasound-Guided Percutaneous Bone Biopsy: Feasibility, Diagnostic Yield and Technical Notes. Diagnostics. 2023; 13(10):1773. https://doi.org/10.3390/diagnostics13101773
Chicago/Turabian StylePonti, Federico, Alessio Arioli, Chiara Longo, Marco Miceli, Marco Colangeli, Nicolas Papalexis, and Paolo Spinnato. 2023. "Ultrasound-Guided Percutaneous Bone Biopsy: Feasibility, Diagnostic Yield and Technical Notes" Diagnostics 13, no. 10: 1773. https://doi.org/10.3390/diagnostics13101773
APA StylePonti, F., Arioli, A., Longo, C., Miceli, M., Colangeli, M., Papalexis, N., & Spinnato, P. (2023). Ultrasound-Guided Percutaneous Bone Biopsy: Feasibility, Diagnostic Yield and Technical Notes. Diagnostics, 13(10), 1773. https://doi.org/10.3390/diagnostics13101773








