Abstract
Cognitive dysfunctions represent a core feature of schizophrenia-spectrum disorders due to their presence throughout different illness stages and their impact on functioning. Abnormalities in electrophysiology (EEG) measures are highly related to these impairments, but the use of EEG indices in clinical practice is still limited. A systematic review of articles using Pubmed, Scopus and PsychINFO was undertaken in November 2021 to provide an overview of the relationships between EEG indices and cognitive impairment in schizophrenia-spectrum disorders. Out of 2433 screened records, 135 studies were included in a qualitative review. Although the results were heterogeneous, some significant correlations were identified. In particular, abnormalities in alpha, theta and gamma activity, as well as in MMN and P300, were associated with impairments in cognitive domains such as attention, working memory, visual and verbal learning and executive functioning during at-risk mental states, early and chronic stages of schizophrenia-spectrum disorders. The review suggests that machine learning approaches together with a careful selection of validated EEG and cognitive indices and characterization of clinical phenotypes might contribute to increase the use of EEG-based measures in clinical settings.
1. Introduction
Cognitive dysfunctions are now widely recognized as pivotal features of schizophrenia-spectrum disorders. In recent decades, the interest in the study of cognitive deficits has grown, since they impact the daily functioning of subjects with schizophrenia more than other psychopathological dimensions and do not respond satisfactorily to current available treatments [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26].
These deficits are present since the first manifestations of the disease when the first episode of psychosis (FEP) or of schizophrenia (FES) occurs [6,12,27], in subjects at clinical high risk of psychosis [28,29,30,31,32], as well as, in an attenuated form, in non-affected relatives of subjects with schizophrenia [17].
Most subjects with schizophrenia experience a broad range of cognitive deficits in different neurocognitive domains, such as working memory, attention/vigilance, verbal/visual learning, reasoning/problem solving and executive functioning [3,14,33,34,35,36]. In addition, subjects with schizophrenia often show impairments in social cognition, a cognitive domain defined as a range of abilities guiding the interpretation of other’s emotions or intentions, leading to informed conclusions and behaviours [1,2,4,16,25,37,38,39,40,41,42,43].
Throughout the years, several test batteries and assessment scales, such as the MATRICS Consensus Cognitive Battery (MCCB) [35,44,45,46] and the Brief Assessment of Cognition (BACS) [47], have been developed to provide a standardized evaluation of cognitive deficits in schizophrenia [48]. In addition to neuropsychological batteries and tests, neuroimaging studies have investigated these deficits and tried to untangle the associations between neuronal abnormalities and cognitive impairments in schizophrenia-spectrum disorders. For instance, studies employing functional magnetic resonance imaging (fMRI) have highlighted that neuronal abnormalities localized in temporal and frontal brain regions (temporal gyrus and the dorsolateral prefrontal cortex), as well as dysfunctions within broad neuronal circuits, such as frontal, striatal, parietal and thalamic circuits, could be at the core of the cognitive deficits observed in schizophrenia-spectrum disorders [49,50,51,52,53,54,55].
Numerous studies have also shown the presence of abnormalities in electroencephalographic (EEG) indices when subjects with schizophrenia-spectrum disorders and healthy controls were compared [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. EEG recordings, due to their high temporal resolution, have been vastly employed to characterize the complex cascade of neuronal signalling underlying cognitive processing and to detect which steps of this processing might be impaired in subjects with severe mental health disorders [57]. Two main approaches have been used to investigate the electrophysiological correlates of cognition: the analysis of activity or connectivity in different EEG frequency bands and the analysis of event-related potentials (ERPs).
Frequency band analysis dissects EEG activity in its subcomponent frequencies, recorded either while subjects are at rest or while they perform a task. Different indices can be employed, such as the spectral power of spontaneous or evoked activity or parameters evaluating brain connectivity, which refers to the synchronization of neuronal signals across different regions of the cerebral cortex [73].
ERPs are wave deflections, time-locked to the occurrence of specific events of interest, such as the onset of visual or auditory stimuli or subjects’ responses during behavioural tasks [74]. Therefore, ERPs have been vastly employed to dissect the steps of the neural processing cascade supporting cognitive functions [75]. Finally, in addition to these two main types of EEG analysis approaches (frequency bands and ERPs), the accumulating evidence of sleep’s involvement in cognition has propelled the use of sleep-related measures, such as sleep spindles and K-complexes, for research purposes in neuroscience [76,77].
Nevertheless, although the use of EEG-based measures has allowed significant advances in understanding the neurobiology of cognitive functions, it has not yet led to the identification of reliable biomarkers for schizophrenia. Previous reviews, aimed to summarize results from electrophysiological studies, have focused mainly on the differences in EEG indices between patients and healthy controls, but have not provided an overview on the associations between these measures and cognitive dysfunctions in schizophrenia-spectrum disorders [56,58,78,79,80].
Enhancing knowledge on the neuronal bases of dysfunctions in different cognitive domains represents an important step, since it could help in the development of new effective treatments.
Therefore, this systematic review aims to provide a detailed report on the available evidence related to the associations between EEG indices and cognitive dysfunctions in schizophrenia-spectrum disorders.
2. Methods
2.1. Aim and Design of the Review
The present manuscript aims to provide a systematic review of studies focusing on correlations between EEG-based measures and cognitive domains, in order to discuss which electrophysiological indices might be used in clinical and research practice as potential biomarker of cognitive dysfunctions. The current systematic review search was performed in line with the PRISMA-Statement [81]. The studies selected included recordings during resting states or sensory and cognitive tasks in subjects with clinical and ultra-high risk of psychosis (CHR and UHR), first-episode psychosis (FEP) and first-episode schizophrenia (FES) or in subjects with a diagnosis of schizophrenia-spectrum disorders (SCZ). The choice of including subjects at-risk or who experienced a first episode of psychosis is motivated by the vast evidence of studies reporting cognitive deficits already during at-risk, prodromal and early phases of the illness [6,27,30].
2.2. Search Strategy
A systematic literature search was performed on 2 November 2021 with no time limit using the following databases: PubMed, Scopus and PsychInfo (Table 1). The keywords selected had to be included either in the title or in the abstract of the articles. In addition, reference lists were hand-searched to identify additional publications missed by the search strategy.

Table 1.
Systematic search strategy.
2.3. Selection Process and Eligibility Criteria
Any duplicate from the combination of the three databases was excluded. The remaining articles were included in the systematic review only if they met the following criteria:
Inclusion criteria
- Meta-analyses, reviews, cohort and case–control articles published in English language and including human subjects;
- Studies had to include data relevant to at least one EEG index, measured in subjects with at risk mental states, first episode psychosis or schizophrenia, or with a schizophrenia-spectrum disorder according to validated diagnostic criteria;
- Studies had to include measurements of at least one cognitive domain using standardized tests or test batteries, or interviews.
- Studies had to report at least one statistical analysis of correlation (Pearson’s or Spearman’s correlation) between one EEG index and a cognitive domain or a regression model in which an EEG index was used as a predictor of a cognitive domain.
Exclusion criteria
- Book chapters, comments, editorials, case reports/case series, theses, proceedings, letters, short surveys, notes;
- Studies irrelevant to the topic;
- Full text unavailable.
Two researchers (A.P. and G.M.G.) independently screened for eligibility all the articles by titles and abstracts and then proceeded to read the full text. Discrepancies in the selection of the eligible articles have been discussed in advance with the whole group and were resolved by discussion and consensus.
2.4. Data Extraction
The following information was extracted onto customized sheets from included articles: authors and year of publication; domains of cognition considered; tests or scales employed to assess cognitive domains; EEG-based measures analysed in correlation with cognitive domains; comparison of the EEG indices between patients/at-risk samples and healthy controls; and outcomes of the analysis correlating electrophysiological data and cognitive scores. Three tables were generated according to the type of EEG index considered for the associations: frequency bands, ERPs and sleep EEG. Given the heterogeneity of the experimental paradigms, of the EEG indices and of the cognitive domains used in the eligible studies, we did not plan to carry out a meta-analysis.
3. Results
3.1. Characteristics of the Included Studies
The combined outcome of the three databases results (Pubmed: 1733; SCOPUS: 460; PsycINFO: 1357) included 3550 records (Figure 1). In addition, 17 studies were included by hand search, yielding a total of 3567 studies. A total of 1134 studies were excluded because they were duplicates. After reading the titles and abstracts, 1871 were excluded since they did not meet the inclusion criteria (i.e., animal studies, no English text available, sample population that did not match the inclusion criteria) or were not relevant to the topic of the review. Four hundred and twenty-seven studies were eliminated because no data on associations between EEG indices and cognitive domains were found in the full text. Therefore, the final number of studies included was 135 (Figure 1).
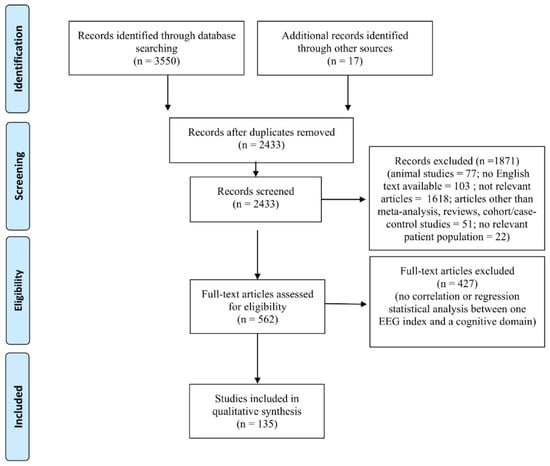
Figure 1.
PRISMA flow chart of included studies. The PRISMA diagram details the search and selection process applied during our systematic literature search and review.
3.2. EEG Frequency Bands Indices
Neuronal oscillations can be grouped in five main frequency bands, delta (0.5–4.0 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz) and gamma (30–100 Hz) bands [57,82,83]. These data can be recorded either during a resting-state condition or during sensory stimulation and task performance. In addition to spectral power measures, other indices have been employed to investigate connectivity, synchronization and the level of neuronal activity integration across distributed cerebral networks.
The studies included in the following subsections are reported in Table 2.

Table 2.
Frequency bands activity studies.
3.2.1. Delta and Theta Activity
Low frequency activity can be subdivided in delta (0.5–4.0 Hz) and theta (4–8 Hz) bands. Both bands appear to be involved in the orchestration of several cognitive processes such as working memory, detection of novelties, learning and allocation of attentive resources [124,125]. It has been reported that, in these two frequencies, subjects with schizophrenia present different abnormalities, often characterised by an increase in activity compared to physiological conditions [56,57,66,68,69,126,127,128].
Studies focusing on the relationship between delta activity and cognitive functions in schizophrenia-spectrum disorders are very few. In subjects with schizophrenia, delta studies reported an association between delta activity during resting-state conditions and emotion recognition [85], but found no association with attention, working memory, speed of processing, verbal and visual learning, reasoning or problem solving [86].
In task-related conditions, evoked delta activity in SCZ was positively (decreased EEG activity—worse cognitive performance) correlated with visual learning, attention, speed of processing [88] and social perception [87] and was negatively (increased EEG activity—worse cognitive performance) correlated with working memory [89]. Furthermore, delta activity coherence, as measured by intertrial coherence (ITC), showed a significant positive correlation with speed of processing [91]. However, other task-related studies [84,91] did not report an association between evoked delta activity and cognitive functions both in SCZ and CHR. Furthermore, Qu et al. [90] employed machine learning methods to develop a model combining clinical and electrophysiological variables (evoked activity in delta, theta and alpha bands and MMN amplitude) in order to predict cognitive impairments. In this study, the authors found that analysis of frequency band activity, including delta, did not contribute significantly to the model while only MMN did (as explained in Section 3.3.4).
A high number of studies focused on the association between theta activity and cognitive deficits. In SCZ three studies found significant negative correlations (increased EEG activity—worse cognitive performance) between theta power recorded at rest with cognitive functions, such as visuospatial memory [107], working memory [86], verbal learning [86,107], executive functioning [107] and emotion recognition [85]. In addition, theta band connectivity during resting state was a significant predictor of deficits in lexical processing [101] and verbal memory [92] in FES and CHR subjects and was also associated with deficits in the ability to initiate a consistent and coherent cognitive activity during a verbal fluency test in FES [102]. Finally, in FEP subjects, a positive correlation was found between executive functioning and verbal memory on the one hand and theta and gamma activity coupling at rest in the brain regions of the default-mode network on the other hand [103].
Many studies have also investigated task-related theta activity. In particular, in SCZ theta power evoked during working memory or visual tasks was correlated with working memory performance [89,98,99], attention [84], speed of processing [84,88,89], visual learning [88] and social perception [87]. Using a machine learning approach, Johannesen et al. [99], found that the machine learning model, combining the evoked activity of theta, alpha, beta and gamma bands, successfully predicted working memory performance. However, other studies did not find any significant correlation between theta power evoked during working memory or visual tasks with working memory [104,106], attention [106], and global cognitive scores derived from the BACS [106] or the MCCB [90] in SCZ and FEP.
Studies that analysed theta activity in SCZ during auditory paradigms found a significant correlation between social cognition and the theta band evoked power and phase locking values [96], and between verbal memory (but not working memory or conceptual flexibility) and theta amplitude [100]. In addition, no correlations were found between evoked theta power during an auditory oddball task and cognitive domains assessed with MCCB in FES and SCZ [108]. Interestingly, two studies focused on the effects of auditory-based targeted cognitive training (TCT) in schizophrenia and showed that higher baseline values of theta evoked power and phase-locking values could be used to predict greater improvements in attention, working memory and general cognitive abilities after the completion of the intervention [93,97]. Finally, theta connectivity during an auditory task was associated with processing speed, verbal fluency, verbal memory [95] and general cognitive abilities assessed with the BACS in SCZ subjects [94].
3.2.2. Alpha Activity
Alpha activity is characterised by a frequency spectrum ranging from 8 to 13 Hz. Neuronal oscillations within this frequency band play a pivotal role in orchestrating cognitive functions, such as attention, working memory and cognitive control [129,130,131,132,133]. In subjects with SCZ most studies reported a decrease in absolute power during resting-state recordings [57,66,68,69] and disruptions in temporal coherence of evoked activity during sensory stimulation and cognitive tasks [134].
Studies focusing on associations between resting-state alpha features and cognition in SCZ have reported mixed results. Indeed, while some studies found a positive correlation (decreased EEG activity—worse cognitive performance) between alpha power and measures of working, visual and verbal memory [109] and emotion recognition [85], other studies did not report any significant correlation between this EEG measure and the six neurocognitive domains assessed through the MCCB [66], or verbal learning [86], working memory [86] and metacognitive functions [122]. Moreover, one study reported a positive correlation between individual alpha peak frequency (IAPF) and measures of speed of processing, perceptual reasoning, working memory, verbal reasoning and attention in SCZ [113]. Interestingly, one study investigating the effects of cognitive remediation found no significant correlation at baseline (pre-treatment) between IAPF and a global score of cognition but found that higher values in IAPF at baseline predicted higher responsiveness to the intervention and greater improvements on cognitive skills after the completion of the therapy sessions [111]. Finally, a decreased alpha band connectivity in the prefrontal cortex reported in FES as compared to controls was found to be correlated with cognitive processing impairments [101].
As to the evoked alpha activity, in SCZ a relationship between evoked alpha power and speed of processing [89] and working memory [99] has been detected. Furthermore, deficits in EEG signal coherence between cortical, temporal and occipital areas in the alpha band were related to worse vigilance skills in SCZ [112]. However, a lack of correlation between event-related alpha activity and different cognitive domains such as speed of processing, memory and learning [90,110], or between evoked alpha desynchronization and social cognition skills [87] have also been reported.
3.2.3. Beta and Gamma
Beta (12–30 Hz) and gamma (30–100 Hz) frequency bands occupy the highest part of the neuronal activity spectrum. These bands have been found to be crucial for learning, top-down control, executive functions and formation of memories [135,136,137,138]. Dysfunctions in the activity and synchronization of beta and gamma bands have been vastly reported in schizophrenia [66,68,69,82,139,140]. However, clear-cut associations between beta activity and cognitive domains are not supported by the relevant literature. Two studies that used resting-state conditions in SCZ found that beta power was positively correlated (decreased in EEG activity—worse cognitive performance) with different cognitive functions, such as emotion recognition [85], vigilance, working memory, visual and verbal memory [109]. Furthermore, two studies investigating task-related activity reported a positive association of evoked beta bursts [114] and evoked beta activity [99] with working memory. However, also a lack of associations between beta power and verbal learning [86], working memory [86] and metacognitive functions [122] has been reported.
Very few resting-state studies investigated the associations between gamma band and cognition and reported discrepant results. Resting-state gamma power has been associated with the ability to consider multiple aspects of a situation (decentration) [122], with verbal learning [121] and working memory [121], but also a lack of association of this EEG index with working memory and verbal learning has been reported in SCZ [86]. In a study conducted in subjects with at risk mental state (ARMS) resting-state gamma activity was increased, as compared to HCs, and higher values were associated with better abstract reasoning [119].
Most of the studies focusing on gamma activity employed tasks or stimuli presentation. Two studies, using a memory task, found a negative correlation between gamma power and the performance on a working memory task in SCZ [98,99]. Another study, using an emotion perception task, showed that higher gamma synchrony elicited by facial expression stimuli was associated with higher scores of social cognition abilities (emotion identification, negativity bias and emotional intelligence) [123]. Studies employing an auditory task observed an association of gamma features with reasoning and problem solving, and with working memory skills in SCZ [117,120], as well as with working memory in FES [116]. Interestingly, one study focused on the effects of auditory-based targeted cognitive training (TCT) in schizophrenia, showing that higher baseline values of gamma evoked power could be used to predict greater improvements in general neurocognitive skills after the completion of the intervention [118]. In the context of studies relevant to gamma activity, recordings of auditory steady-state response (ASSR), an oscillatory brain response generated by the presentation of periodic auditory stimuli, have often been employed. One ASSR study found a positive correlation between gamma-band intertrial coherence (ITC) and event-related spectral perturbation (ERSP) indices and working memory skills in a large sample of SCZ [115]. However, other articles on ASSR data reported no significant associations between gamma activity and verbal memory [100,116,117], working memory [100], mental flexibility [100,117], attention [116,120], speed of processing [120], verbal [115,120] and visual learning [120] in SCZ and FES.
3.3. ERPs
The millisecond-level temporal resolution of ERPs has been precious to investigate the neuronal activity associated with processing of sounds and images and more complex cognitive processes such as the allocation of attentive resources and decision-making. ERPs represent an important tool for exploring the neurobiological bases of cognitive impairments in different disorders, such as ADHD [141], schizophrenia [56,142] and Alzheimer’s disease [143]. The studies and results reported in the following sections are described in Table 3.

Table 3.
Event-related potential studies.
3.3.1. P50
The P50 is an early positive component of the auditory sensory responses, recorded 50 ms after a sound is presented. Generally, when two clicks are presented in a very brief interval, a reduction in P50 in response to the second click (S2) is observed, compared to the one produced after the first click (S1). This physiological process is known as sensory gating and can be analysed through the calculation of the P50 amplitude ratio (P50-S2/P50-S1) [58,144,147,148,149,150,151,152]. In subjects with SCZ, an increase in the P50 ratio is often reported, suggesting an impairment in sensory gating [58].
Studies evaluating the association between P50 ratio and cognitive functions in SCZ have reported inconsistent results, since some studies found a significant correlation between this EEG index and impairments in different cognitive domains such as attention [144,149,150], working memory [145,149], speed of processing [145], visual learning [146] and executive functions [150], while other studies found no significant correlations between P50 ratio and cognitive domains evaluated with the MCCB [151], verbal learning [144,145,147,148,150,152], memory [144,147,148,150,152], attention [145,147,148,152], processing speed [147,148] and executive functions [147,148].
3.3.2. N100
The N100 is a large, negative-going evoked potential elicited by any unpredicted stimulus and related to the early stage of sensory processing [62]. Numerous studies assessing both N100 amplitude and sensory gating characteristics have found deficits in SCZ, which are thought to reflect an impairment in perceptual and attentional processing [62,228,229].
Deficits in N100 sensory gating were correlated to worse problem-solving abilities [154], attention [149] and working memory [149] in SCZ. However, the association between N100 amplitude and cognitive functions in SCZ is not clear since some studies reported a correlation between N100 amplitude and different cognitive abilities, such as verbal and visual memory [159,163], executive functioning [153], attention [201] and general cognition [153], while other studies did not find a significant correlation between N100 amplitude and attention [147,155,156], visual learning and memory [146,147,157,162], verbal learning and memory [160,161], executive functioning [147,161,162,197], working memory [164] or attention to stimuli related to social scenarios [158].
3.3.3. P100
The P100 component is a positive potential, which peaks between 80 and 120 ms after stimulus onset. It primarily indexes perceptual stages of cortical visual processing, and its amplitude is influenced by selective attention [230,231,232]. In subjects with SCZ, compared with healthy subjects, reductions in P100 amplitude have frequently been reported in response to facial and non-facial stimuli [232,233].
Few studies have investigated the relationship between P100 and cognition, reporting inconsistent results: a positive correlation with attention deficits, suggesting an interference in effective sensory processing [155], or a negative correlation with the performance during a phonological task [165], or even a lack of association with the performance during a visuospatial attention [156] or a working memory task [164] have been reported in SCZ.
3.3.4. MMN
The mismatch negativity (MMN) is a negative-going ERP component, which generally appears 150–250 ms after the presentation of unexpected stimuli [234,235,236,237]. The MMN reflects pre-attentive processing and is commonly regarded as an index of sensory memory [235,236]. This ERP is elicited through the auditory oddball paradigm and appears in response to the deviant tones, which are played rarely in a sequence of standard and frequent tones. The deviant tones can differ from the standard stimuli for different characteristics of the sound, such as frequency (eliciting, the pitch MMN, the pMMN), duration (eliciting dMMN), intensity, or for spatial location where the sound is played [238]. Abnormalities of both pMMN and dMMN have consistently been reported in patients with SCZ [79,239,240].
pMMN
In subjects with chronic SCZ, FES and CHR, deficits in pMMN amplitude have been associated with impairments in a variety of cognitive functions, such as verbal fluency [166], vigilance [192], verbal [174] and working memory [166,171], verbal learning [169], speed of processing [169], MCCB neurocognitive composite score [108,177] and emotion recognition [170,173]. However, some studies did not find associations between pMMN and cognitive composite scores [175] in SCZ, attention [197] in FEP subjects and verbal memory, verbal executive functions, spatial working memory, sustained attention and executive functioning in subjects with a prodromal syndrome of psychosis [168].
dMMN
Discrepant findings have also been reported for associations between dMMN amplitude and cognitive functions in SCZ, FEP and CHR. Most studies reported significant correlations between lower dMMN amplitude and impairments in several cognitive domains, such as reasoning [184], problem solving [184], verbal fluency [166,182], verbal learning [115,169,186,187], vigilance [192], visual attention [180,186,188], attentional switching [186], executive functioning [194], contextual processing [178], working memory [96,115,166,179,191,193], speed of processing [169,180,189], non-verbal memory [96], social perception [195], social cognition [96,181], abstraction and thought flexibility [96], emotion affective prosody [183,195], as well as composite cognitive scores [167,190]. An innovative longitudinal machine learning study [90] carried out in FEP subjects, used a combination of clinical, functional, cognitive and several EEG indices (delta, theta and alpha event-related activity and dMMN amplitude values recorded at multiple electrodes). The model showed that one group of subjects that presented an increase in dMMN amplitude at 6-month follow-up visit also had better cognitive functioning, as compared to baseline values [90]. Conversely, the other group did not present an improvement in either dMMN amplitude or cognitive functions. Interestingly, one study using cognitive training in SCZ, showed that changes in dMMN (i.e., a decrease in latency), upon completion of just one hour of training, predicted improvement in verbal learning after a full cycle of treatment [172]. Furthermore, an additional study that focused on pre versus post cognitive training found that improvements in reasoning and problem-solving domains were associated with an increase in dMMN amplitude values [184].
However, other studies found no association of dMMN amplitude with memory [185], working memory [168,176], visual memory [185], semantic memory, decision making, cognitive control, learning capacities [185], executive functioning [168], attention [168,176,185], processing speed [193], facial emotion identification [183] or the capacity of making social inferences [195], as well as with general assessment of cognitive abilities [108,175,177] in SCZ, FEP and FES. A lack of association between dMMN and cognition was also reported by a study that did not find a predictive power of baseline dMMN amplitude for improvements in cognition upon completion of cognitive training therapy sessions [93].
Other Types of MMN Deviants
In addition to pitch and duration deviants, other studies employed deviant stimuli that were characterized, as compared to standard tones, by a different sound location (ear to which sounds were played to) or intensity. One study found that MMN amplitude elicited by deviant stimuli that were played from a different earphone as compared to standard ones was negatively correlated to speed of processing, verbal learning, visual learning and working memory in SCZ, while no significant correlations were observed in CHR subjects [177]. As to MMN amplitude elicited by stimuli deviant for intensity, negative correlations were reported with speed of processing, verbal learning and cognitive composite score (as assessed with the MCCB) [169,177].
3.3.5. P200
The P200 component is a positive deflection with a typical peak latency of approximately 150–250 ms elicited by auditory, somatosensory and visual stimuli [241,242].
Most studies did not find a relationship between P200 and different cognitive functions, such as working memory [147,164], episodic memory [160], attention [147], executive functioning [147,196], speed of processing [196] and verbal and visual memory [147,196] in SCZ. However, a study reported a correlation between P200 amplitude and executive functioning in SCZ [162], and another one carried out in FEP subjects found a correlation with processing speed (Morales-Muñoz et al., 2017).
3.3.6. N200
The N200 component is a negative-going component peaking between 200 and 350 ms after stimulus onset, which is often elicited in paradigms focused on visual attention and language processing [243].
Some studies did not find significant associations between N200 and cognitive domains as assessed through the MCCB [67,198,199,202], while other studies found that blunted N200 peaks were correlated to worse performance in memory [157,200], visual working memory [159] and attention [201] tasks in SCZ and FES. Furthermore, one study found that a less prominent N200 response was associated with better visuospatial attention in SCZ patients [156].
3.3.7. P300
P300 is a positive peak that can be observed 300 ms after the presentation of a deviant or rare stimulus [244,245]. This ERP has been considered as an index of cognitive processing and attention shifts to changes in the environment and consistent deficits have been detected in its amplitude and latency in different stages of schizophrenia-spectrum disorders [56,246,247,248,249].
Most studies using different paradigms (visual, visuospatial memory and phonological tasks) reported a positive correlation of P300 amplitude reduction with an impairment in different cognitive abilities, such as visual attention [156,203,250], working memory [204,206], self-referential memory [207], language processing [206] in SCZ and with executive functioning in both SCZ and at-risk subjects [205]. Interestingly, a study that focused on the predictive value of ERPs in the efficacy of the combination of two cognitive remediation programs showed that higher baseline P300 amplitude values were associated with greater improvements in attention, memory and speed of processing [93]. However, two studies found no significant association of P300 amplitude with memory [164] or attention in social scenarios [158].
Several studies investigated P300 during auditory tasks and distinguished two different P300 values that present a distinct scalp topography and peak latency, suggesting different neural generators and association to different functions. In particular, the P3a component (230–350 ms after the stimulus onset), localized mainly in frontal cerebral regions, is elicited by presenting rare non-target stimuli and can be observed even under passive conditions and is related to novelty detection and attentional shifts [244]; the P3b component (275–600 ms after the stimulus onset), localized in temporo-parietal areas, is elicited by rare target stimuli when subjects are asked to perform a stimuli-related task, which reflects working memory updating and deficits in this component have been associated with difficulties in maintaining goal-directed behaviour [244,251].
P3a
Discrepant findings have been reported on associations between P3a amplitude and cognitive functions in SCZ, FEP and FES subjects. In particular, most studies reported a positive correlation between P3a amplitude and different cognitive domains, such as attention [180,192,197,208], verbal learning [115,180], working memory [96,115,180], non-verbal memory [96], executive functioning, abstraction and flexibility capacities [96] and social cognition [96,183,210]. Interestingly, one study testing the effects of cognitive training showed that the changes in P3a features (an amplitude increase and a latency decrease) upon completion of just one hour of training were significantly associated with improvements in verbal learning abilities after a full treatment cycle in SCZ subjects [172]. In addition, one study using low-resolution electromagnetic tomography analysis (LORETA) showed that the increase in P3a activity in the left superior temporal gyrus was associated with improvement in verbal learning after a six-month olanzapine treatment in SCZ subjects [209]. However, in SCZ, FES and FEP a lack of correlations between P3a amplitude and several cognitive domains were also reported [105,176,183,186,190,221].
P3b
Discrepancies are also found in the literature relevant to correlation between P3b and cognitive functions in SCZ, FES and high-risk subjects. Indeed, most studies found a positive correlation between P3b amplitude and several cognitive domains [91,157,162,163,197,200,201,204,208,211,212,214,215,216,217,218,219,220,221], while some studies found no significant correlation [161,210,213,220].
3.3.8. N400
N400 is a negative deflection associated with language, faces, memory and visual processing [252]. Abnormalities in this ERP are present both during chronic [253] and early or prodromal stages of the illness [224]. Discrepant findings have been reported on correlations between N400 amplitude and cognitive functions. Indeed, some authors reported significant negative correlations of N400 amplitude with language comprehension [222], and the MCCB cognitive composite score [224] in SCZ, as well as with verbal learning and memory [223] in CHR. Other studies reported no significant correlations between N400 and episodic memory [160], executive functioning [196], speed of processing [196], verbal and visual memory [196] in SCZ.
3.3.9. ERN and Pe
Error-related negativity (ERN) and error positivity (Pe) are two ERPs that have been associated with error detection, learning and conflict monitoring [254]. Error-related negativity (ERN) is a negative deflection ERP, occurring approximately 50–100 ms after an erroneous response and might be directly linked to the awareness of the error made [255]. The Pe reflects conscious error processing and is visualised as a positive-going component occurring from 200 to 500 ms following the erroneous response [256].
Studies involving subjects with SCZ [205,227] or those with genetic risk of developing schizophrenia [205] revealed that blunted ERN was associated with impaired executive [205,227], attention [227] and general cognitive functions [227]. However, also a lack of association between ERN amplitude and attention or cognitive control has been reported in SCZ [225]. As regards to Pe, in SCZ [205,227], CHR [205], or in subjects with a history of psychosis [226] blunted Pe was associated with impaired executive functioning [205,227], attention [227] and self-appraisal of task performance [226].
3.4. Sleep EEG Activity
Studies on electrophysiological activity during sleep in schizophrenia suggested that abnormalities during non-REM sleep represent indices of disturbances in cognitive functioning [257]. Physiologically, during non-REM sleep stages the frequency of EEG activity slows down. In addition, throughout non-REM stage 2 of sleep recurring phasic electrical events, such as sleep spindles and K-complexes have been reported [258,259]. Results of sleep studies are summarized in Table 4.

Table 4.
Sleep Studies.
Sleep spindles, traditionally defined as waveforms between 12 and 14 Hz lasting up to 3 s in duration, are cortical signatures of the patterned thalamocortical and/or hippocampal-cortical network activity that supports the role of NREM sleep in overnight memory consolidation [269,270]. A reduction in the density of sleep spindles in SCZ was correlated to worse performance in tasks assessing memory skills after sleep [264,268], due to the role of sleep spindles in memory consolidation. Furthermore, another study highlighted that SCZ, as compared to healthy controls, presented abnormalities in the slow wave synchronization and density of sleep spindles [261]. Both indices predicted memory consolidation in healthy controls, but not in SCZ. Associations between sleep spindle characteristics and cognitive functions other than memory consolidation have also been observed [262,266]; however, a lack of associations has also been reported [260,263].
Furthermore, in a sample of subjects with SCZ, a study focusing on K-complexes (large electrical sharp waves in the EEG recordings) showed that a low number of K-complexes was associated with poor executive functioning and problem-solving skills [267]. A long duration of slow wave sleep was related to better problem solving [267], and a study focusing on nonlinear EEG complexity during sleep in FES reported that a low nonlinear complexity was associated with poor memory and deficits in executive functions [265].
4. Discussion
The examination of the alterations of EEG indices in subjects with schizophrenia-spectrum disorders may advance our understanding of the neural mechanisms underpinning cognitive impairments. However, discrepancies in the findings are numerous, possibly due to the heterogeneity in EEG parameters and cognitive measures employed across different studies, thus hindering the use of these measures in routine clinical practice.
4.1. Frequency Bands Activity
Studies that focused on frequency band analysis in subjects with schizophrenia suggest that aberrant cortical neural activity and failures in the synchronization of cerebral activity might underlie the impairments in different cognitive functions, such as memory, attention and cognitive control [82,271].
The studies included in the present review addressing delta activity at rest recorded not very robust findings (association with emotion recognition but not with attention, working memory, speed of processing, verbal and visual learning, reasoning or problem solving) [85,86], while those focussing on task-related delta activity, more consistently reported an association between reduced activity in this frequency band and impairments in attention and speed of processing [88,89,91].
Within the slow activity range, the band most robustly associated with cognitive deficits was theta, probably due to its role in the coordination of neural activity of the default-mode network, which is highly involved in automated processing of information [89,272], effortful cognitive processing and efficient allocation of attentive resources [98,102,107]. Furthermore, relationships between alterations in connectivity and measures of verbal memory and speed of processing suggest a possible role of disturbed communication across cortical areas and aberrant temporal synchronization of neuronal oscillators in the genesis of cognitive dysfunctions in schizophrenia [92,94,95,101,102].
Studies on resting state and evoked alpha power produced heterogeneous results [66,111,113,122], and it is therefore difficult to draw conclusions. Most of the studies, however, used standard alpha frequency instead of individual alpha peak frequency (IAPF) which may cause heterogeneity in the results of studies investigating the association of alpha frequency and cognitive functions. In fact, IAPF has been shown to be much more reliable and reproducible across sessions and cognitive tasks than standard alpha frequency measurements [113,273,274]. Studies investigating IAPF have reported a reduction in subjects with schizophrenia and some studies have hypothesized that dysfunctional timing of alpha waves could be at the core of defective cognitive processing [111,113,275]. Thus, the vast inter-subject’s variability in the standard frequency alpha range in both physiological and pathological conditions places some restrains in its use as assessment tools of cognitive impairments. Therefore, the hypothesis that defects in the coordination of alpha activity across brain networks could be responsible for inappropriate allocation of neuronal resources according to the cognitive load demand deserves further investigation using the IAPF and the most accurate method of assessment of this biomarker [273].
Finally, the high-frequency bands abnormalities in gamma activity have consistently been related to the impairment in various cognitive domains [82,98,116,117,119,120,121,122]. Deficits in synchronization of neural activity within the gamma frequency band might lead to cognitive dysfunctions by hindering efficient higher-order cognitive processes [82,98,116,117,119,120,121,122].
Overall, the results of the studies addressing frequency bands activity seem to suggest that deficits in the elicitation of balanced and synchronous oscillatory activity are often present in schizophrenia, and lead to difficulties in preserving both accurate and fast performance in the context of automated and effortful cognitive processing.
However, although frequency band analysis seems to offer manifold possibilities in evaluating cognitive dysfunctions, the main challenge in translating these measures into clinical tools remains the establishment of cut-off values that would flag the presence of cognitive impairments and the translation of results obtained at group level into application at the individual level. In the authors opinion, further studies, employing standardized paradigms and frequency ranges, using both power spectrum analysis and connectivity measures together with the implementation of machine learning approaches, might help to overcome the limitations of current findings.
4.2. ERPs
Investigations on correlations between cognitive functions and ERPs related to early sensory processing, such as P50, N100 and P100 led to discrepant results. However, most studies showed an association between these ERPs and several cognitive functions, such as attention, memory, learning, problem solving and executive functions [144,145,146,149,150,153,154,155,159,163,165,201]. It has been hypothesized that dysfunctions in auditory and visual sensory processing, as flagged by blunted amplitude of these EPRs, could derive from abnormalities in frontal and temporal regions and might contribute to impairments in higher-order cognitive functions [144,145,146,159]. Conversely, some studies found no association between P50, N100, P100 with cognitive skills [146,147,148,151,155,156,157,158,160,161,162,164].
A large body of research has been devoted to the relationship between cognitive deficits and abnormalities in MMN. Indeed, reductions in MMN amplitude, probably driven by abnormalities in frontal and temporal lobes [191], have consistently been linked to cognitive deficits and psychosocial functioning impairment in subjects with schizophrenia [179,276], independently from the clinical state [277]. Although the number of MMN studies included in the current review is high and results are more consistent as compared to other ERPs studies, drawing conclusions remains difficult. This might be due to the heterogeneity of sample sizes, of electrophysiological paradigms and of the assessment instruments used across different studies.
According to data reviewed in the present contribution, MMN recordings in schizophrenia seem to relate to deficits in encoding sensory information and storing information, as highlighted by the association between lower amplitude values and worse performance on memory tests [166]. Furthermore, since MMN is also a measure of salience of unexpected stimuli, the relationship between MMN measures and attention is probably due to a dysfunction in efficient filtering of relevant environmental stimuli in schizophrenia. Thus, according to current reviewed literature, both dMMN and pMMN could be considered as possible markers in the evaluation of memory and attentional deficits in schizophrenia. Interestingly, some studies on the efficacy of cognitive remediation therapies (CRTs) in subjects with schizophrenia suggested the potential role of MMN indices in monitoring and/or predicting the efficacy of these intervention in ameliorating cognitive dysfunctions [167,172,184].
The available literature on N200 and P200 is very heterogeneous, probably due to the use of different paradigms (e.g., reward-based, semantic, visual or acoustic tasks).
The P300 component, which is associated with the detection of novel stimuli, updating of working memory, inhibitory control, and selective attention processes [243], may be highly sensitive to deficits in cognition. As such, P300 has been one of the most studied ERP components in this context [278]. Associations between amplitude reductions in P300 [156,203] or its two subtypes, P3a [180,192,208] and P3b [201,208,211,215,216,218,219,220], with deficits in attention is one of the most consistent EEG finding in schizophrenia [156,163,192,201,203,208,215,218], first-episode psychosis [180,197,211] and clinical high-risk [216,219] subjects. Associations of P300 amplitude reduction with verbal learning and memory deficits have also been reported [96,115,157,162,180,200,206,217,218,220] and suggest that impairments in identifying and responding to stimuli that are either salient or novel might also influence higher-order steps of cognitive processing relevant to goal directed behaviour. Furthermore, studies involving CRTs have showed that P300 values could be used to predict patients’ responses to these interventions [93,172]. It was hypothesized that participants that presented larger reductions in P300 amplitude might benefit more from CRT, thanks to neuroplastic changes restoring cognitive processing.
Finally, more studies are required for other ERPs, such as N400, ERN and Pe to obtain a clearer insight on their associations with cognitive deficits.
In conclusion, ERPs studies provide an interesting perspective on the neuronal correlates of cognitive impairments due to their high temporal resolution. However, in order to obtain a clearer view on the results, we also need to consider that several factors might have influenced or biased the correlations reported. Few studies, in fact, have considered the possible confounding effects of illness duration, antipsychotic doses and severity of psychopathological aspects on the outcomes [63,79]. Furthermore, physiological processes such as ageing have also often been associated with a progressing deterioration of P300 and MMN elicitation [279,280].
Therefore, conducting studies on early stages of the illness, which are less biased by the effects of illness duration and medication usage, and the synergistic use of multiple ERPs might help to disentangle the complex biological mechanisms underlying cognitive deficits in schizophrenia.
4.3. Sleep EEG Activity
In the last two decades, studies focusing on sleep abnormalities measured through EEG recordings have suggested that sleep-related measures may improve our insight into the neurophysiological alterations leading to cognitive dysfunctions, and, especially, to memory impairments [281].
Most of the EEG sleep studies focused on the examination of sleep spindle density and characteristics of K-complexes that might be related to the disruption in memory consolidation and executive functions. Disturbances in sleep organization have mainly been attributed to disruption in the neural signalling of the thalamocortical network [261,263], which could lead to deficits in memory integration, information processing and consolidation of novel information [282,283]. However, so far, few studies have focused on associations between EEG sleep disturbances and cognitive dysfunctions in schizophrenia and results are not consistent [260,261,262,263,264,265,266,267,268]. Therefore, more studies are required to broaden the evidence currently available.
4.4. The Limits and Obstacles of EEG-Based Measures in Clinical Settings and Possible Strategies to Overcome Them
The results presented in the current review highlight how electrophysiological recordings might contribute to the evaluation of cognitive impairments in subjects with schizophrenia-spectrum disorders. However, limitations related to the use of this technique should be addressed.
Important limitations are the paucity of clinically relevant studies, the heterogeneity of employed methods and the frequent discrepancies in reported results. As a matter of fact, cognitive domains were evaluated using different scales or tasks; studies often reported the association with a total cognitive score and not with individual cognitive domains; a variety of EEG measurements/parameters is investigated: for instance a study focuses on alpha power, while another on alpha coherence; different experimental paradigms are used to record the same EEG index [165]; the samples included in different studies varied in terms of size, age range and illness-related variables, such as severity of negative and positive symptoms, or dosage of antipsychotic drugs [100,109,145,151]. This might be also due to the presence of flexible inclusion criteria in the current systematic review, which aimed at providing a broad and inclusive assessment of the available literature on the topic, with no exclusion criteria for duration of illness, type of EEG indices or preprocessing methodology. In addition, most studies reported data on one single EEG index or, if multiple EEG indices were recorded, statistical analyses were performed separately for each of them. In addition, few studies that investigated the potential of EEG indices in predicting the efficacy of rehabilitation interventions showed promising results. However, the paucity of data and the heterogeneity of methods used across studies, do not allow conclusions.
Therefore, we suggest some possible improvements and approaches for future studies to overcome these limitations.
Standardized assessment scales, such as the MCCB, which is considered the state-of-the-art instrument for cognitive evaluation in schizophrenia, should be used as the primary tool to evaluate the impairment in different cognitive domains.
Large research networks are needed to collect data by applying a common protocol in large patient cohorts, possibly with a longitudinal study design to investigate patterns of associations between changes in EEG parameters and cognitive performance over time. In addition, combining recordings of multiple EEG indices, rather than relying on a single electrophysiological measure, would definitely provide a more effective strategy. Machine learning methods have been increasingly adopted in psychiatry to optimize the use of neurobiological indices combined with other factors, such as clinical, behavioural, genetic and environmental data translation [284,285,286,287]. Some studies used the machine learning approach, also incorporating EEG measures to build classifiers able to discriminate patients from healthy controls or to predict disease trajectories [284,288,289,290,291,292], but very few EEG machine learning studies were conducted to investigate associations between cognitive impairments and EEG-based measures [90,99], and support the hypothesis that an objective and precise characterization of patient’s cognitive phenotypes.
EEG-based measures have a potential for clinical translation; however, limitations, as discussed above, need to be carefully addressed. Large cross-sectional and longitudinal studies based on in depth clinical characterization of the studied population, standardized methods, and adopting state of the art tools and indices, together with the implementation of machine learning approaches, might contribute to increase the use of EEG-based measures in clinical settings (Figure 2).
Figure 2.
Future directions for the implementation of EEG-based measures in clinical settings. Large studies based on in depth characterization of the studied population, standardized methods, and adopting state of the art tools and indices, together with the implementation of machine learning approaches, might contribute to increase the use of EEG-based measures in clinical settings.
Author Contributions
A.P., G.M.G. and S.G. contributed to the conceptualization and supervision of the manuscript. A.P., G.M.G. and S.G. contributed to the establishment of the methodology and the literature research. All authors (A.P., G.M.G., F.B., L.G., P.P., A.M. and S.G.) contributed to writing, critically revising and editing the content of the manuscript, and approved the final manuscript for submission to Diagnostics. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in the current review are all available within the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014, 13, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Rucci, P.; Kirkpatrick, B.; Mucci, A.; Gibertoni, D.; Rocca, P.; Rossi, A.; Bertolino, A.; Strauss, G.P.; Aguglia, E.; et al. Interplay Among Psychopathologic Variables, Personal Resources, Context-Related Factors, and Real-life Functioning in Individuals with Schizophrenia: A Network Analysis. JAMA Psychiatry 2018, 75, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Lepage, M.; Bodnar, M.; Bowie, C.R. Neurocognition: Clinical and Functional Outcomes in Schizophrenia. Can. J. Psychiatry 2014, 59, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Galderisi, S.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Biondi, M.; et al. Factors Associated with Real-Life Functioning in Persons with Schizophrenia in a 4-Year Follow-up Study of the Italian Network for Research on Psychoses. JAMA Psychiatry 2021, 78, 550. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Murray, R.M. Meta-analysis of Cognitive Deficits in Ultra-high Risk to Psychosis and First-Episode Psychosis: Do the Cognitive Deficits Progress Over, or After, the Onset of Psychosis? Schizophr. Bull. 2014, 40, 744–755. [Google Scholar] [CrossRef]
- Rund, B.R.; Barder, H.E.; Evensen, J.; Haahr, U.; ten Velden Hegelstad, W.; Joa, I.; Johannessen, J.O.; Langeveld, J.; Larsen, T.K.; Melle, I.; et al. Neurocognition and Duration of Psychosis: A 10-year Follow-up of First-Episode Patients. Schizophr. Bull. 2016, 42, 87–95. [Google Scholar] [CrossRef]
- Martínez, A.L.; Brea, J.; Rico, S.; de Los Frailes, M.T.; Loza, M.I. Cognitive Deficit in Schizophrenia: From Etiology to Novel Treatments. Int. J. Mol. Sci. 2021, 22, 9905. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Wong, A.H.C. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef]
- Baldez, D.P.; Biazus, T.B.; Rabelo-Da-Ponte, F.D.; Nogaro, G.P.; Martins, D.S.; Kunz, M.; Czepielewski, L.S. The effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: Network meta-analyses of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 126, 265–275. [Google Scholar] [CrossRef]
- Menon, V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry 2020, 19, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Hu, Y.; Zhu, Y.; Zhang, T.; Wang, J.; Ma, K.; Shi, C.; Yu, X.; Li, C. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen. Psychiatry 2019, 32, e100043. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A.; Velthorst, E.; Davidson, M. Cognitive impairment and psychosis in schizophrenia: Independent or linked conditions? World Psychiatry 2019, 18, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A. The assessment of neuropsychological functioning in schizophrenia. Dialog- Clin. Neurosci. 2010, 12, 383–392. [Google Scholar] [CrossRef]
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. Pathways to functional outcome in subjects with schizophrenia living in the community and their unaffected first-degree relatives. Schizophr. Res. 2016, 175, 154–160. [Google Scholar] [CrossRef]
- Galderisi, S.; Rucci, P.; Mucci, A.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Bozzatello, P.; et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: Stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry 2020, 19, 81–91. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Green, M.F.; Nuechterlein, K.; Rucci, P.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Bucci, P.; et al. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol. Med. 2018, 48, 1359–1366. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, M.; Parle, M.; Dhingra, S.; Dhull, D.K. Potential drug targets and treatment of schizophrenia. Inflammopharmacology 2017, 25, 277–292. [Google Scholar] [CrossRef]
- Kotov, R.; Jonas, K.G.; Carpenter, W.T.; Dretsch, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Hobbs, K.; Reininghaus, U.; Slade, T.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 2020, 19, 151–172. [Google Scholar] [CrossRef]
- Krueger, R.F.; Hobbs, K.A.; Conway, C.C.; Dick, D.M.; Dretsch, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Keyes, K.M.; Latzman, R.D.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): II. Externalizing superspectrum. World Psychiatry 2021, 20, 171–193. [Google Scholar] [CrossRef]
- Sanislow, C.A. RDoC at 10: Changing the discourse for psychopathology. World Psychiatry 2020, 19, 311–312. [Google Scholar] [CrossRef]
- Maj, M.; Van Os, J.; De Hert, M.; Gaebel, W.; Galderisi, S.; Green, M.F.; Guloksuz, S.; Harvey, P.D.; Jones, P.B.; Malaspina, D.; et al. The clinical characterization of the patient with primary psychosis aimed at personalization of management. World Psychiatry 2021, 20, 4–33. [Google Scholar] [CrossRef]
- Lahey, B.B.; Moore, T.M.; Kaczkurkin, A.N.; Zald, D.H. Hierarchical models of psychopathology: Empirical support, implications, and remaining issues. World Psychiatry 2021, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Gaebel, W.; Maj, M.; Stein, D.J.; Kogan, C.S.; Saunders, J.B.; Poznyak, V.B.; Gureje, O.; Lewis-Fernández, R.; Maercker, A.; et al. An organization- and category-level comparison of diagnostic requirements for mental disorders in ICD-11 and DSM-5. World Psychiatry 2021, 20, 34–51. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S.; Deste, G.; Rocca, P.; Rossi, A.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Biondi, M.; et al. The influence of autistic symptoms on social and non-social cognition and on real-life functioning in people with schizophrenia: Evidence from the Italian Network for Research on Psychoses multicenter study. Eur. Psychiatry 2020, 63, e98. [Google Scholar] [CrossRef]
- Rampino, A.; Di Carlo, P.; Fazio, L.; Ursini, G.; Pergola, G.; De Virgilio, C.; Gadaleta, G.; Giordano, G.M.; Bertolino, A.; Blasi, G. Association of functional genetic variation in PP2A with prefrontal working memory processing. Behav. Brain Res. 2017, 316, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Santesteban-Echarri, O.; Paino, M.; Rice, S.; González-Blanch, C.; McGorry, P.; Gleeson, J.; Alvarez-Jimenez, M. Predictors of functional recovery in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Clin. Psychol. Rev. 2017, 58, 59–75. [Google Scholar] [CrossRef]
- Seidman, L.J.; Mirsky, A.F. Evolving Notions of Schizophrenia as a Developmental Neurocognitive Disorder. J. Int. Neuropsychol. Soc. 2017, 23, 881–892. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Deste, G.; Smieskova, R.; Barlati, S.; Yung, A.R.; Howes, O.; Stieglitz, R.-D.; Vita, A.; McGuire, P.; Borgwardt, S. Cognitive Functioning in Prodromal Psychosis: A Meta-analysis. Arch. Gen. Psychiatry 2012, 69, 562–571. [Google Scholar] [CrossRef]
- Kahn, R.S.; Keefe, R.S.E. Schizophrenia Is a Cognitive Illness: Time for a change in focus. JAMA Psychiatry 2013, 70, 1107–1112. [Google Scholar] [CrossRef]
- Catalan, A.; de Pablo, G.S.; Aymerich, C.; Damiani, S.; Sordi, V.; Radua, J.; Oliver, D.; McGuire, P.; Giuliano, A.J.; Stone, W.S.; et al. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 859. [Google Scholar] [CrossRef] [PubMed]
- Glenthøj, L.B.; Mariegaard, L.S.; Fagerlund, B.; Jepsen, J.R.M.; Kristensen, T.D.; Wenneberg, C.; Krakauer, K.; Medalia, A.; Roberts, D.L.; Hjorthøj, C.; et al. Effectiveness of cognitive remediation in the ultra-high risk state for psychosis. World Psychiatry 2020, 19, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Akdede, B.B.; Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychol. Med. 2017, 47, 2401–2413. [Google Scholar] [CrossRef] [PubMed]
- Kharawala, S.; Hastedt, C.; Podhorna, J.; Shukla, H.; Kappelhoff, B.; Harvey, P.D. The relationship between cognition and functioning in schizophrenia: A semi-systematic review. Schizophr. Res. Cogn. 2021, 27, 100217. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Nuechterlein, K.H.; Gold, J.M.; Barch, D.M.; Cohen, J.; Essock, S.; Fenton, W.S.; Frese, F.; Goldberg, T.E.; Heaton, R.K.; et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 2004, 56, 301–307. [Google Scholar] [CrossRef]
- Lysaker, P.H.; Hasson-Ohayon, I. Metacognition in psychosis: A renewed path to understanding of core disturbances and recovery-oriented treatment. World Psychiatry 2021, 20, 359–361. [Google Scholar] [CrossRef]
- Silberstein, J.; Harvey, P.D. Cognition, social cognition, and Self-assessment in schizophrenia: Prediction of different elements of everyday functional outcomes. CNS Spectr. 2019, 24, 88–93. [Google Scholar] [CrossRef]
- Giuliani, L.; Giordano, G.M.; Bucci, P.; Pezzella, P.; Brando, F.; Galderisi, S. Improving Knowledge on Pathways to Functional Outcome in Schizophrenia: Main Results from the Italian Network for Research on Psychoses. Front. Psychiatry 2021, 12, 791117. [Google Scholar] [CrossRef]
- Rocca, P.; Galderisi, S.; Rossi, A.; Bertolino, A.; Rucci, P.; Gibertoni, D.; Montemagni, C.; Sigaudo, M.; Mucci, A.; Bucci, P.; et al. Social cognition in people with schizophrenia: A cluster-analytic approach. Psychol. Med. 2016, 46, 2717–2729. [Google Scholar] [CrossRef]
- Gennarelli, M.; Monteleone, P.; Minelli, A.; Monteleone, A.M.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellino, S.; et al. Genome-wide association study detected novel susceptibility genes for social cognition impairment in people with schizophrenia. World J. Biol. Psychiatry 2022, 23, 46–54. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Lee, J.; Wynn, J.K. Experimental approaches to social disconnection in the general community: Can we learn from schizophrenia research? World Psychiatry 2020, 19, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Pinkham, A.E.; Penn, D.L.; Green, M.F.; Buck, B.; Healey, K.; Harvey, P.D. The Social Cognition Psychometric Evaluation Study: Results of the Expert Survey and RAND Panel. Schizophr. Bull. 2014, 40, 813–823. [Google Scholar] [CrossRef]
- Keefe, R.S.E.; Fox, K.H.; Harvey, P.D.; Cucchiaro, J.; Siu, C.; Loebel, A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr. Res. 2011, 125, 161–168. [Google Scholar] [CrossRef]
- Kern, R.S.; Gold, J.M.; Dickinson, D.; Green, M.F.; Nuechterlein, K.H.; Baade, L.E.; Keefe, R.S.E.; Mesholam-Gately, R.I.; Seidman, L.J.; Lee, C.; et al. The MCCB impairment profile for schizophrenia outpatients: Results from the MATRICS psychometric and standardization study. Schizophr. Res. 2011, 126, 124–131. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J., 3rd; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef]
- Keefe, R.S.E.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2003, 68, 283–297. [Google Scholar] [CrossRef]
- Schulz, S.C.; Murray, A. Assessing Cognitive Impairment in Patients with Schizophrenia. J. Clin. Psychiatry 2016, 77 (Suppl. 2), 3–7. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Laird, A.R.; Thelen, S.; Carter, C.S.; Glahn, D.C. Meta-analysis of 41 Functional Neuroimaging Studies of Executive Function in Schizophrenia. Arch. Gen. Psychiatry 2009, 66, 811–822. [Google Scholar] [CrossRef]
- Achim, A.M.; Lepage, M. Episodic memory-related activation in schizophrenia: Meta-analysis. Br. J. Psychiatry 2005, 187, 500–509. [Google Scholar] [CrossRef]
- Barch, D.M.; Ceaser, A. Cognition in schizophrenia: Core psychological and neural mechanisms. Trends Cogn. Sci. 2012, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.E.; Gur, R.C. Functional magnetic resonance imaging in schizophrenia. Dialogues-Clin. Neurosci. 2010, 12, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Aleksandrowicz, A.; Dąbkowska, M.; Gawęda, Ł. Neural Correlates of Aberrant Salience and Source Monitoring in Schizophrenia and At-Risk Mental States—A Systematic Review of fMRI Studies. J. Clin. Med. 2021, 10, 4126. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Barch, D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016, 61, 108–120. [Google Scholar] [CrossRef]
- Smucny, J.; Dienel, S.J.; Lewis, D.A.; Carter, C.S. Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia. Neuropsychopharmacology 2022, 47, 292–308. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Volpe, U.; Boutros, N. Evidence-Based Medicine and Electrophysiology in Schizophrenia. Clin. EEG Neurosci. 2009, 40, 62–77. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2019, 12, 521. [Google Scholar] [CrossRef]
- Atagun, M.I.; Drukker, M.; Hall, M.H.; Altun, I.K.; Tatli, S.Z.; Guloksuz, S.; van Os, J.; van Amelsvoort, T. Meta-analysis of auditory P50 sensory gating in schizophrenia and bipolar disorder. Psychiatry Res. Neuroimaging 2020, 300, 111078. [Google Scholar] [CrossRef]
- Kambeitz, J.; Kambeitz-Ilankovic, L.; Leucht, S.; Wood, S.; Davatzikos, C.; Malchow, B.; Falkai, P.; Koutsouleris, N. Detecting Neuroimaging Biomarkers for Schizophrenia: A Meta-Analysis of Multivariate Pattern Recognition Studies. Neuropsychopharmacology 2015, 40, 1742–1751. [Google Scholar] [CrossRef]
- Perrottelli, A.; Giordano, G.M.; Brando, F.; Giuliani, L.; Mucci, A. EEG-Based Measures in At-Risk Mental State and Early Stages of Schizophrenia: A Systematic Review. Front. Psychiatry 2021, 12, 653642. [Google Scholar] [CrossRef]
- Urban, A.; Kremlácek, J.; Libiger, J. Mismatch Negativity in Patients with Schizophrenia. Acta Medica 2007, 50, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Rosburg, T. Auditory N100 gating in patients with schizophrenia: A systematic meta-analysis. Clin. Neurophysiol. 2018, 129, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Perrottelli, A.; Mucci, A.; Di Lorenzo, G.; Altamura, M.; Bellomo, A.; Brugnoli, R.; Corrivetti, G.; Girardi, P.; Monteleone, P.; et al. Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia. Brain Sci. 2021, 11, 1632. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Giuliani, L.; Perrottelli, A.; Bucci, P.; Di Lorenzo, G.; Siracusano, A.; Brando, F.; Pezzella, P.; Fabrazzo, M.; Altamura, M.; et al. Mismatch Negativity and P3a Impairment through Different Phases of Schizophrenia and Their Association with Real-Life Functioning. J. Clin. Med. 2021, 10, 5838. [Google Scholar] [CrossRef]
- Giordano, G.M.; Koenig, T.; Mucci, A.; Vignapiano, A.; Amodio, A.; Di Lorenzo, G.; Siracusano, A.; Bellomo, A.; Altamura, M.; Monteleone, P.; et al. Neurophysiological correlates of Avolition-apathy in schizophrenia: A resting-EEG microstates study. NeuroImage: Clin. 2018, 20, 627–636. [Google Scholar] [CrossRef]
- Vignapiano, A.; Koenig, T.; Mucci, A.; Giordano, G.M.; Amodio, A.; Altamura, M.; Bellomo, A.; Brugnoli, R.; Corrivetti, G.; Di Lorenzo, G.; et al. Disorganization and cognitive impairment in schizophrenia: New insights from electrophysiological findings. Int. J. Psychophysiol. 2019, 145, 99–108. [Google Scholar] [CrossRef]
- Vignapiano, A.; Mucci, A.; Merlotti, E.; Giordano, G.M.; Amodio, A.; Palumbo, D.; Galderisi, S. Impact of Reward and Loss Anticipation on Cognitive Control: An Event-Related Potential Study in Subjects with Schizophrenia and Healthy Controls. Clin. EEG Neurosci. 2018, 49, 46–54. [Google Scholar] [CrossRef]
- Boutros, N.N.; Mucci, A.; Vignapiano, A.; Galderisi, S. Electrophysiological Aberrations Associated with Negative Symptoms in Schizophrenia. In Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology; Kumari, V., Bob, P., Boutros, N.N., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 129–156. [Google Scholar]
- Galderisi, S.; Vignapiano, A.; Mucci, A.; Boutros, N.N. Physiological Correlates of Positive Symptoms in Schizophrenia. In Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology; Kumari, V., Bob, P., Boutros, N.N., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 103–128. [Google Scholar]
- Qiu, Y.-Q.; Tang, Y.-X.; Chan, R.C.K.; Sun, X.-Y.; He, J. P300 Aberration in First-Episode Schizophrenia Patients: A Meta-Analysis. PLoS ONE 2014, 9, e97794. [Google Scholar] [CrossRef]
- Maran, M.; Grent-‘T-Jong, T.; Uhlhaas, P.J. Electrophysiological insights into connectivity anomalies in schizophrenia: A systematic review. Neuropsychiatr. Electrophysiol. 2016, 2, 6. [Google Scholar] [CrossRef]
- Tada, M.; Kirihara, K.; Mizutani, S.; Uka, T.; Kunii, N.; Koshiyama, D.; Fujioka, M.; Usui, K.; Nagai, T.; Araki, T.; et al. Mismatch negativity (MMN) as a tool for translational investigations into early psychosis: A review. Int. J. Psychophysiol. 2019, 145, 5–14. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroğlu, C.; Güntekin, B.; Yener, G.G. Chapter 2—Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: Proposal for biomarker strategies. In Supplements to Clinical Neurophysiology; Başar, E., Başar-Eroĝlu, C., Özerdem, A., Rossini, P.M., Yener, G.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 19–54. [Google Scholar]
- Sur, S.; Sinha, V.K. Event-related potential: An overview. Ind. Psychiatry J. 2009, 18, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Rissling, A.J.; Light, G.A. Neurophysiological Measures of Sensory Registration, Stimulus Discrimination, and Selection in Schizophrenia Patients. In Behavioral Neurobiology of Schizophrenia and Its Treatment; Swerdlow, N.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 283–309. [Google Scholar]
- Cox, R.; Fell, J. Analyzing human sleep EEG: A methodological primer with code implementation. Sleep Med. Rev. 2020, 54, 101353. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.M. The role of sleep in cognitive processing: Focusing on memory consolidation. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, e1433. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-S.; Chung, K.-F.; Yung, K.-P.; Yeung, W.-F. Sleep in schizophrenia: A systematic review and meta-analysis of polysomnographic findings in case-control studies. Sleep Med. Rev. 2017, 32, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Haigh, S.M.; Coffman, B.A.; Salisbury, D.F. Mismatch Negativity in First-Episode Schizophrenia: A Meta-Analysis. Clin. EEG Neurosci. 2016, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients with Schizophrenia: A Meta-analysis. JAMA Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Reilly, T.J.; Nottage, J.F.; Studerus, E.; Rutigliano, G.; De Micheli, A.I.; Fusar-Poli, P.; McGuire, P. Gamma band oscillations in the early phase of psychosis: A systematic review. Neurosci. Biobehav. Rev. 2018, 90, 381–399. [Google Scholar] [CrossRef]
- Babiloni, C.; Barry, R.J.; Başar, E.; Blinowska, K.J.; Cichocki, A.; Drinkenburg, W.H.; Klimesch, W.; Knight, R.T.; da Silva, F.L.; Nunez, P.; et al. International Federation of Clinical Neurophysiology (IFCN)—EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin. Neurophysiol. 2020, 131, 285–307. [Google Scholar] [CrossRef]
- Dias, E.C.; Van Voorhis, A.C.; Braga, F.; Todd, J.; Lopez-Calderon, J.; Martinez, A.; Javitt, D.C. Impaired Fixation-Related Theta Modulation Predicts Reduced Visual Span and Guided Search Deficits in Schizophrenia. Cereb. Cortex 2020, 30, 2823–2833. [Google Scholar] [CrossRef]
- Gica, S.; Poyraz, B.C.; Gulec, H. Are emotion recognition deficits in patients with schizophrenia states or traits? A 6-month follow-up study. Indian J. Psychiatry 2019, 61, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, D.; Miyakoshi, M.; Tanaka-Koshiyama, K.; Joshi, Y.B.; Sprock, J.; Braff, D.L.; Light, G.A. Abnormal phase discontinuity of alpha- and theta-frequency oscillations in schizophrenia. Schizophr. Res. 2021, 231, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Tobe, R.; Dias, E.C.; Ardekani, B.A.; Veenstra-VanderWeele, J.; Patel, G.; Breland, M.; Lieval, A.; Silipo, G.; Javitt, D.C. Differential Patterns of Visual Sensory Alteration Underlying Face Emotion Recognition Impairment and Motion Perception Deficits in Schizophrenia and Autism Spectrum Disorder. Biol. Psychiatry 2019, 86, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Gaspar, P.A.; Hillyard, S.A.; Andersen, S.K.; Lopez-Calderon, J.; Corcoran, C.M.; Javitt, D.C. Impaired Motion Processing in Schizophrenia and the Attenuated Psychosis Syndrome: Etiological and Clinical Implications. Am. J. Psychiatry 2018, 175, 1243–1254. [Google Scholar] [CrossRef]
- Prieto, M.; Iglesias-Parro, S.; Soriano, M.F.; Ibáñez-Molina, A. Local Oscillatory Brain Dynamics of Mind Wandering in Schizophrenia. Brain Sci. 2021, 11, 910. [Google Scholar] [CrossRef]
- Qu, X.; Liukasemsarn, S.; Tu, J.; Higgins, A.; Hickey, T.J.; Hall, M.-H. Identifying Clinically and Functionally Distinct Groups Among Healthy Controls and First Episode Psychosis Patients by Clustering on EEG Patterns. Front. Psychiatry 2020, 11, 541659. [Google Scholar] [CrossRef]
- Wu, G.; Tang, X.; Gan, R.; Zeng, J.; Hu, Y.; Xu, L.; Wei, Y.; Tang, Y.; Chen, T.; Li, C.; et al. Temporal and time–frequency features of auditory oddball response in distinct subtypes of patients at clinical high risk for psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 272, 449–459. [Google Scholar] [CrossRef]
- Andreou, C.; Leicht, G.; Nolte, G.; Polomac, N.; Moritz, S.; Karow, A.; Hanganu-Opatz, I.L.; Engel, A.K.; Mulert, C. Resting-state theta-band connectivity and verbal memory in schizophrenia and in the high-risk state. Schizophr. Res. 2015, 161, 299–307. [Google Scholar] [CrossRef]
- Best, M.W.; Milanovic, M.; Shamblaw, A.L.; Muere, A.; Lambe, L.J.; Hong, I.K.; Haque, M.K.; Bowie, C.R. An examination of the moderating effects of neurophysiology on treatment outcomes from cognitive training in schizophrenia-spectrum disorders. Int. J. Psychophysiol. 2020, 154, 59–66. [Google Scholar] [CrossRef]
- Cea-Cañas, B.; Gomez-Pilar, J.; Núñez, P.; Rodríguez-Vázquez, E.; de Uribe, N.; Díez, Á.; Pérez-Escudero, A.; Molina, V. Connectivity strength of the EEG functional network in schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109801. [Google Scholar] [CrossRef]
- Gomez-Pilar, J.; Poza, J.; Gómez, C.; Northoff, G.; Lubeiro, A.; Cea-Cañas, B.B.; Molina, V.; Hornero, R. Altered predictive capability of the brain network EEG model in schizophrenia during cognition. Schizophr. Res. 2018, 201, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hochberger, W.C.; Joshi, Y.B.; Zhang, W.; Thomas, M.L.; Consortium of Genomics in Schizophrenia; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Decomposing the constituent oscillatory dynamics underlying mismatch negativity generation in schizophrenia: Distinct relationships to clinical and cognitive functioning. Int. J. Psychophysiol. 2019, 145, 23–29. [Google Scholar] [CrossRef]
- Hochberger, W.C.; Thomas, M.L.; Joshi, Y.B.; Molina, J.; Treichler, E.B.H.; Nungaray, J.; Cardoso, L.; Sprock, J.; Swerdlow, N.; Light, G.A. Oscillatory biomarkers of early auditory information processing predict cognitive gains following targeted cognitive training in schizophrenia patients. Schizophr. Res. 2020, 215, 97–104. [Google Scholar] [CrossRef]
- Hoy, K.E.; Coyle, H.; Gainsford, K.; Hill, A.T.; Bailey, N.W.; Fitzgerald, P.B. Investigating neurophysiological markers of impaired cognition in schizophrenia. Schizophr. Res. 2021, 233, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, J.K.; Bi, J.; Jiang, R.; Kenney, J.G.; Chen, C.-M.A. Machine learning identification of EEG features predicting working memory performance in schizophrenia and healthy adults. Neuropsychiatr. Electrophysiol. 2016, 2, 3. [Google Scholar] [CrossRef]
- Kirihara, K.; Rissling, A.J.; Swerdlow, N.R.; Braff, D.L.; Light, G.A. Hierarchical Organization of Gamma and Theta Oscillatory Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Krukow, P.; Jonak, K.; Karakula-Juchnowicz, H.; Podkowiński, A.; Jonak, K.; Borys, M.; Harciarek, M. Disturbed functional connectivity within the left prefrontal cortex and sensorimotor areas predicts impaired cognitive speed in patients with first-episode schizophrenia. Psychiatry Res. Neuroimaging 2018, 275, 28–35. [Google Scholar] [CrossRef]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wojcik, M.; Karakula-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, M.; Hwang, W.J.; Kim, T.; Kwak, Y.B.; Kwon, J.S. Relationship between resting-state theta phase-gamma amplitude coupling and neurocognitive functioning in patients with first-episode psychosis. Schizophr. Res. 2020, 216, 154–160. [Google Scholar] [CrossRef]
- Liu, X.L.; Ranganath, C.; Hsieh, L.-T.; Hurtado, M.; Niendam, T.A.; Lesh, T.A.; Carter, C.S.; Ragland, J.D. Task-specific Disruptions in Theta Oscillations during Working Memory for Temporal Order in People with Schizophrenia. J. Cogn. Neurosci. 2020, 32, 2117–2130. [Google Scholar] [CrossRef]
- Solís-Vivanco, R.; Mondragón-Maya, A.; Reyes-Madrigal, F.; de la Fuente-Sandoval, C. Impairment of novelty-related theta oscillations and P3a in never medicated first-episode psychosis patients. NPJ Schizophr. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.B.; Lasagna, C.A.; Martínez-Cancino, R.; Pamidighantam, P.; Deldin, P.J.; Tso, I.F. Neural Oscillatory Abnormalities During Gaze Processing in Schizophrenia: Evidence of Reduced Theta Phase Consistency and Inter-areal Theta-Gamma Coupling. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 6, 370–379. [Google Scholar] [CrossRef]
- Wichniak, A.; Okruszek, Ł.; Linke, M.; Jarkiewicz, M.; Jędrasik-Styła, M.; Ciołkiewicz, A.; Wierzbicka, A.; Jernajczyk, W.; Jarema, M. Electroencephalographic theta activity and cognition in schizophrenia: Preliminary results. World J. Biol. Psychiatry 2015, 16, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-B.; Bo, Q.-J.; Wang, C.-M.; Tian, Q.; Liu, Y.; Wang, C.-Y. Differential of Frequency and Duration Mismatch Negativity and Theta Power Deficits in First-Episode and Chronic Schizophrenia. Front. Behav. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Adler, G.; Grieshaber, S.; Faude, V.; Thebaldi, B.; Dressing, H. Clozapine in Patients with Chronic Schizophrenia: Serum Level, EEG and Memory Performance. Pharmacopsychiatry 2002, 35, 190–194. [Google Scholar] [CrossRef]
- Billeke, P.; Armijo, A.; Castillo, D.; López, T.; Zamorano, F.; Cosmelli, D.; Aboitiz, F. Paradoxical Expectation: Oscillatory Brain Activity Reveals Social Interaction Impairment in Schizophrenia. Biol. Psychiatry 2015, 78, 421–431. [Google Scholar] [CrossRef]
- Castelluccio, B.C.; Kenney, J.G.; Johannesen, J.K. Individual Alpha Peak Frequency Moderates Transfer of Learning in Cognitive Remediation of Schizophrenia. J. Int. Neuropsychol. Soc. 2020, 26, 19–30. [Google Scholar] [CrossRef]
- Hoffman, R.E.; Buchsbaum, M.S.; Escobar, M.D.; Makuch, R.W.; Nuechterlein, K.H.; Guich, S.M. EEG coherence of prefrontal areas in normal and schizophrenic males during perceptual activation. J. Neuropsychiatry Clin. Neurosci. 1991, 3, 169–175. [Google Scholar] [CrossRef]
- Ramsay, I.S.; Lynn, P.A.; Schermitzler, B.; Sponheim, S.R. Individual alpha peak frequency is slower in schizophrenia and related to deficits in visual perception and cognition. Sci. Rep. 2021, 11, 17852. [Google Scholar] [CrossRef]
- Briley, P.M.; Liddle, E.B.; Simmonite, M.; Jansen, M.; White, T.P.; Balain, V.; Palaniyappan, L.; Bowtell, R.; Mullinger, K.J.; Liddle, P.F. Regional Brain Correlates of Beta Bursts in Health and Psychosis: A Concurrent Electroencephalography and Functional Magnetic Resonance Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 1145–1156. [Google Scholar] [CrossRef]
- Koshiyama, D.; Thomas, M.L.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr. Bull. 2021, 47, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Leicht, G.; Andreou, C.; Polomac, N.; Lanig, C.; Schöttle, D.; Lambert, M.; Mulert, C. Reduced auditory evoked gamma band response and cognitive processing deficits in first episode schizophrenia. World J. Biol. Psychiatry 2015, 16, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Hsu, J.L.; Hsieh, M.H.; Meyer-Gomes, K.; Sprock, J.; Swerdlow, N.R.; Braff, D.L. Gamma Band Oscillations Reveal Neural Network Cortical Coherence Dysfunction in Schizophrenia Patients. Biol. Psychiatry 2006, 60, 1231–1240. [Google Scholar] [CrossRef]
- Molina, J.L.; Thomas, M.L.; Joshi, Y.B.; Hochberger, W.C.; Koshiyama, D.; Nungaray, J.A.; Cardoso, L.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; et al. Gamma oscillations predict pro-cognitive and clinical response to auditory-based cognitive training in schizophrenia. Transl. Psychiatry 2020, 10, 405. [Google Scholar] [CrossRef]
- Ramyead, A.; Kometer, M.; Studerus, E.; Koranyi, S.; Ittig, S.; Gschwandtner, U.; Fuhr, P.; Riecher-Rössler, A. Aberrant Current Source-Density and Lagged Phase Synchronization of Neural Oscillations as Markers for Emerging Psychosis. Schizophr. Bull. 2015, 41, 919–929. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, P.; Wang, C.; Fan, Y.; Tian, Q.; Dong, F.; Zhou, F.; Wang, C. Defects of Gamma Oscillations in Auditory Steady-State Evoked Potential of Schizophrenia. Shanghai Arch. Psychiatry 2018, 30, 27–38. [Google Scholar] [CrossRef]
- Tanaka-Koshiyama, K.; Koshiyama, D.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Sprock, J.; Braff, D.L.; Light, G.A. Abnormal Spontaneous Gamma Power Is Associated with Verbal Learning and Memory Dysfunction in Schizophrenia. Front. Psychiatry 2020, 11, 832. [Google Scholar] [CrossRef]
- Vohs, J.L.; Leonhardt, B.L.; Francis, M.M.; Westfall, D.; Howell, J.; Bolbecker, A.R.; O’Donnell, B.F.; Hetrick, W.P.; Lysaker, P.H. A Preliminary Study of the Association Among Metacognition and Resting State EEG in Schizophrenia. J. Psychophysiol. 2015, 30, 47–54. [Google Scholar] [CrossRef]
- Williams, L.M.; Whitford, T.J.; Nagy, M.; Flynn, G.; Harris, A.W.F.; Silverstein, S.M.; Gordon, E. Emotion-elicited gamma synchrony in patients with first-episode schizophrenia: A neural correlate of social cognition outcomes. J. Psychiatry Neurosci. 2009, 34, 303–313. [Google Scholar]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Review of evoked and event-related delta responses in the human brain. Int. J. Psychophysiol. 2016, 103, 43–52. [Google Scholar] [CrossRef]
- Hunt, M.J.; Kopell, N.J.; Traub, R.D.; Whittington, M.A. Aberrant Network Activity in Schizophrenia. Trends Neurosci. 2017, 40, 371–382. [Google Scholar] [CrossRef]
- Adams, R.A.; Bush, D.; Zheng, F.; Meyer, S.S.; Kaplan, R.; Orfanos, S.; Marques, T.R.; Howes, O.D.; Burgess, N. Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia. Brain 2020, 143, 1261–1277. [Google Scholar] [CrossRef]
- Galderisi, S.; Maj, M.; Mucci, A.; Bucci, P.; Kemali, D. QEEG alpha1 changes after a single dose of high-potency neuroleptics as a predictor of short-term response to treatment in schizophrenic patients. Biol. Psychiatry 1994, 35, 367–374. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Clayton, M.S.; Yeung, N.; Kadosh, R.C. The many characters of visual alpha oscillations. Eur. J. Neurosci. 2018, 48, 2498–2508. [Google Scholar] [CrossRef]
- Bazanova, O.M.; Vernon, D. Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 2014, 44, 94–110. [Google Scholar] [CrossRef]
- Van Ede, F. Mnemonic and attentional roles for states of attenuated alpha oscillations in perceptual working memory: A review. Eur. J. Neurosci. 2018, 48, 2509–2515. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Kleinschmidt, A. Brain Networks and α-Oscillations: Structural and Functional Foundations of Cognitive Control. Trends Cogn. Sci. 2016, 20, 805–817. [Google Scholar] [CrossRef]
- Başar, E.; Güntekin, B. Chapter 19—Review of delta, theta, alpha, beta, and gamma response oscillations in neuropsychiatric disorders. In Supplements to Clinical neurophysiology; Başar, E., Başar-Eroĝlu, C., Özerdem, A., Rossini, P.M., Yener, G.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 303–341. [Google Scholar]
- Marco-Pallarés, J.; Münte, T.F.; Rodriguez-Fornells, A. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci. Biobehav. Rev. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009, 32, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Herman, P.; Warden, M.R.; Brincat, S.L.; Miller, E.K. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat. Commun. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues- Clin. Neurosci. 2013, 15, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Parciauskaite, V.; Bjekic, J.; Griskova-Bulanova, I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sci. 2021, 11, 217. [Google Scholar] [CrossRef]
- Bucci, P.; Mucci, A.; Merlotti, E.; Volpe, U.; Galderisi, S. Induced Gamma Activity and Event-Related Coherence in Schizophrenia. Clin. EEG Neurosci. 2007, 38, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Aggensteiner, P.-M.; Baumeister, S.; Holz, N.E.; Banaschewski, T.; Brandeis, D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 117–134. [Google Scholar] [CrossRef]
- Rissling, A.J.; Makeig, S.; Braff, D.L.; Light, G.A. Neurophysiologic Markers of Abnormal Brain Activity in Schizophrenia. Curr. Psychiatry Rep. 2010, 12, 572–578. [Google Scholar] [CrossRef]
- Morrison, C.; Rabipour, S.; Knoefel, F.; Sheppard, C.; Taler, V. Auditory Event-related Potentials in Mild Cognitive Impairment and Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 702–715. [Google Scholar] [CrossRef]
- Cullum, C.M.; Harris, J.G.; Waldo, M.C.; Smernoff, E.; Madison, A.; Nagamoto, H.T.; Griffith, J.; Adler, L.E.; Freedman, R. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr. Res. 1993, 10, 131–141. [Google Scholar] [CrossRef]
- Hamilton, H.K.; Williams, T.J.; Ventura, J.; Jasperse, L.J.; Owens, E.M.; Miller, G.A.; Subotnik, K.L.; Nuechterlein, K.H.; Yee, C.M. Clinical and Cognitive Significance of Auditory Sensory Processing Deficits in Schizophrenia. Am. J. Psychiatry 2018, 175, 275–283. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Liu, K.; Liu, S.-K.; Chiu, M.-J.; Hwu, H.-G.; Chen, A.C.N. Memory impairment and auditory evoked potential gating deficit in schizophrenia. Psychiatry Res. Neuroimaging 2004, 130, 161–169. [Google Scholar] [CrossRef]
- Şahin, D.; Hever, F.; Bossert, M.; Herwig, K.; Aschenbrenner, S.; Weisbrod, M.; Sharma, A. Early and middle latency auditory event-related potentials do not explain differences in neuropsychological performance between schizophrenia spectrum patients and matched healthy controls. Psychiatry Res. 2021, 304, 114162. [Google Scholar] [CrossRef]
- Sánchez-Morla, E.M.; Santos, J.L.; Aparicio, A.; García-Jiménez, M.; Soria, C.; Arango, C. Neuropsychological correlates of P50 sensory gating in patients with schizophrenia. Schizophr. Res. 2013, 143, 102–106. [Google Scholar] [CrossRef]
- Smith, A.K.; Edgar, J.C.; Huang, M.; Lu, B.Y.; Thoma, R.J.; Hanlon, F.M.; McHaffie, G.; Jones, A.P.; Paz, R.D.; Miller, G.A.; et al. Cognitive Abilities and 50- and 100-msec Paired-Click Processes in Schizophrenia. Am. J. Psychiatry 2010, 167, 1264–1275. [Google Scholar] [CrossRef]
- Toyomaki, A.; Hashimoto, N.; Kako, Y.; Tomimatsu, Y.; Koyama, T.; Kusumi, I. Different P50 sensory gating measures reflect different cognitive dysfunctions in schizophrenia. Schizophr. Res. Cogn. 2015, 2, 166–169. [Google Scholar] [CrossRef]
- Xia, L.; Yuan, L.; Du, X.-D.; Wang, D.; Wang, J.; Xu, H.; Huo, L.; Tian, Y.; Dai, Q.; Wei, S.; et al. P50 inhibition deficit in patients with chronic schizophrenia: Relationship with cognitive impairment of MATRICS consensus cognitive battery. Schizophr. Res. 2020, 215, 105–112. [Google Scholar] [CrossRef]
- Xia, L.; Wang, D.; Wang, J.; Xu, H.; Huo, L.; Tian, Y.; Dai, Q.; Wei, S.; Wang, W.; Zhang, G.; et al. Association of cognitive and P50 suppression deficits in chronic patients with schizophrenia. Clin. Neurophysiol. 2020, 131, 725–733. [Google Scholar] [CrossRef]
- Arjona-Valladares, A.; Fondevila-Estévez, S.; Fernández-Linsenbarth, I.; Díez, Á.; Ruiz-Sanz, F.J.; Rodríguez-Lorenzana, A.; Molina, V. Event-related potentials associated to N-back test performance in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110347. [Google Scholar] [CrossRef] [PubMed]
- Boutros, N.N.; Brockhaus-Dumke, A.; Gjini, K.; Vedeniapin, A.; Elfakhani, M.; Burroughs, S.; Keshavan, M. Sensory-gating deficit of the N100 mid-latency auditory evoked potential in medicated schizophrenia patients. Schizophr. Res. 2009, 113, 339–346. [Google Scholar] [CrossRef]
- Brodeur, M.B.; Kiang, M.; Christensen, B.K. The beneficial influence of inattention on visual interference in schizophrenia. Neuropsychology 2016, 30, 664–672. [Google Scholar] [CrossRef]
- Bruder, G.; Kayser, J.; Tenke, C.; Rabinowicz, E.; Friedman, M.; Amador, X.; Sharif, Z.; Gorman, J. The time course of visuospatial processing deficits in schizophrenia: An event-related brain potential study. J. Abnorm. Psychol. 1998, 107, 399–411. [Google Scholar] [CrossRef]
- Bruder, G.E.; Kayser, J.; Tenke, C.E.; Friedman, M.; Malaspina, D.; Gorman, J.M. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: Relations to diagnostic subtype, symptom features and verbal memory. Biol. Psychiatry 2001, 50, 447–452. [Google Scholar] [CrossRef]
- Catalano, L.T.; Wynn, J.K.; Lee, J.; Green, M.F. A comparison of stages of attention for social and nonsocial stimuli in schizophrenia: An ERP study. Schizophr. Res. 2021, 238, 128–136. [Google Scholar] [CrossRef]
- Dias, E.C.; Butler, P.D.; Hoptman, M.J.; Javitt, D.C. Early Sensory Contributions to Contextual Encoding Deficits in Schizophrenia. Arch. Gen. Psychiatry 2011, 68, 654–664. [Google Scholar] [CrossRef]
- Green, A.E.; Fitzgerald, P.B.; Johnston, P.J.; Nathan, P.J.; Kulkarni, J.; Croft, R.J. Evidence for a differential contribution of early perceptual and late cognitive processes during encoding to episodic memory impairment in schizophrenia. World J. Biol. Psychiatry 2016, 18, 369–381. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, S.-S.; Youn, T.; Kang, D.-H.; Kim, J.-J.; Kwon, J.S. Neuropsychological correlates of P300 abnormalities in patients with schizophrenia and obsessive–compulsive disorder. Psychiatry Res. Neuroimaging 2003, 123, 109–123. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kamiya, T.; Kawasaki, Y.; Higashima, M.; Urata, K.; Sakai, N.; Koshino, Y. The relationship between auditory ERP and neuropsychological assessments in schizophrenia. Int. J. Psychophysiol. 1999, 34, 267–274. [Google Scholar] [CrossRef]
- Sumich, A.; Kumari, V.; Dodd, P.; Ettinger, U.; Hughes, C.; Zachariah, E.; Sharma, T. N100 and P300 amplitude to Go and No–Go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophr. Res. 2008, 98, 265–277. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Tan, S.P.; Yang, F.D.; Wang, L.L.; Feng, W.F.; Chan, R.C.K.; Gao, X.; Zhou, D.F.; Li, B.B.; Song, C.S.; et al. Dysfunction in different phases of working memory in schizophrenia: Evidence from ERP recordings. Schizophr. Res. 2011, 133, 112–119. [Google Scholar] [CrossRef]
- Spironelli, C.; Romeo, Z.; Maffei, A.; Angrilli, A. Comparison of automatic visual attention in schizophrenia, bipolar disorder, and major depression: Evidence from P1 event-related component. Psychiatry Clin. Neurosci. 2019, 73, 331–339. [Google Scholar] [CrossRef]
- Baldeweg, T.; Hirsch, S.R. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: A comparison with bipolar disorder and Alzheimer’s disease. Int. J. Psychophysiol. 2015, 95, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Biagianti, B.; Roach, B.J.; Fisher, M.; Loewy, R.; Ford, J.M.; Vinogradov, S.; Mathalon, D.H. Trait aspects of auditory mismatch negativity predict response to auditory training in individuals with early illness schizophrenia. Neuropsychiatr. Electrophysiol. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Brockhaus-Dumke, A.; Tendolkar, I.; Pukrop, R.; Schultze-Lutter, F.; Klosterkötter, J.; Ruhrmann, S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr. Res. 2005, 73, 297–310. [Google Scholar] [CrossRef]
- Carrión, R.E.; Cornblatt, B.A.; McLaughlin, D.; Chang, J.; Auther, A.M.; Olsen, R.H.; Javitt, D.C. Contributions of early cortical processing and reading ability to functional status in individuals at clinical high risk for psychosis. Schizophr. Res. 2015, 164, 1–7. [Google Scholar] [CrossRef]
- Csukly, G.; Stefanics, G.; Komlósi, S.; Czigler, I.; Czobor, P. Emotion-Related Visual Mismatch Responses in Schizophrenia: Impairments and Correlations with Emotion Recognition. PLoS ONE 2013, 8, e75444. [Google Scholar] [CrossRef]
- Haigh, S.M.; Coffman, B.A.; Murphy, T.K.; Butera, C.D.; Salisbury, D.F. Abnormal auditory pattern perception in schizophrenia. Schizophr. Res. 2016, 176, 473–479. [Google Scholar] [CrossRef][Green Version]
- Hochberger, W.C.; Joshi, Y.B.; Thomas, M.L.; Zhang, W.; Bismark, A.W.; Treichler, E.B.H.; Tarasenko, M.; Nungaray, J.; Sprock, J.; Cardoso, L.; et al. Neurophysiologic measures of target engagement predict response to auditory-based cognitive training in treatment refractory schizophrenia. Neuropsychopharmacology 2019, 44, 606–612. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Hoptman, M.J.; Leitman, D.I.; Moreno-Ortega, M.; Lehrfeld, J.M.; Dias, E.; Sehatpour, P.; Laukka, P.; Silipo, G.; Javitt, D.C. Neural Substrates of Auditory Emotion Recognition Deficits in Schizophrenia. J. Neurosci. 2015, 35, 14909–14921. [Google Scholar] [CrossRef]
- Kärgel, C.; Sartory, G.; Kariofillis, D.; Wiltfang, J.; Müller, B.W. Mismatch Negativity Latency and Cognitive Function in Schizophrenia. PLoS ONE 2014, 9, e84536. [Google Scholar] [CrossRef]
- McCleery, A.; Mathalon, D.H.; Wynn, J.K.; Roach, B.J.; Hellemann, G.S.; Marder, S.R.; Green, M.F. Parsing components of auditory predictive coding in schizophrenia using a roving standard mismatch negativity paradigm. Psychol. Med. 2019, 49, 1195–1206. [Google Scholar] [CrossRef]
- Randau, M.; Oranje, B.; Miyakoshi, M.; Makeig, S.; Fagerlund, B.; Glenthøj, B.; Bak, N. Attenuated mismatch negativity in patients with first-episode antipsychotic-naive schizophrenia using a source-resolved method. NeuroImage: Clin. 2019, 22, 101760. [Google Scholar] [CrossRef] [PubMed]
- Sehatpour, P.; Avissar, M.; Kantrowitz, J.T.; Corcoran, C.M.; De Baun, H.M.; Patel, G.H.; Girgis, R.R.; Brucato, G.; Lopez-Calderon, J.; Silipo, G.; et al. Deficits in Pre-attentive Processing of Spatial Location and Negative Symptoms in Subjects at Clinical High Risk for Schizophrenia. Front. Psychiatry 2021, 11, 629144. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Whitson, L.; Smith, E.; Michie, P.T.; Schall, U.; Ward, P.B. What’s intact and what’s not within the mismatch negativity system in schizophrenia. Psychophysiology 2014, 51, 337–347. [Google Scholar] [CrossRef]
- Baldeweg, T.; Klugman, A.; Gruzelier, J.; Hirsch, S.R. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr. Res. 2004, 69, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Hermens, D.F.; Ward, P.B.; Hodge, M.A.R.; Kaur, M.; Naismith, S.L.; Hickie, I.B. Impaired MMN/P3a complex in first-episode psychosis: Cognitive and psychosocial associations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 822–829. [Google Scholar] [CrossRef]
- Higgins, A.; Lewandowski, K.E.; Liukasemsarn, S.; Hall, M.-H. Longitudinal relationships between mismatch negativity, cognitive performance, and real-world functioning in early psychosis. Schizophr. Res. 2021, 228, 385–393. [Google Scholar] [CrossRef]
- Higuchi, Y.; Sumiyoshi, T.; Seo, T.; Miyanishi, T.; Kawasaki, Y.; Suzuki, M. Mismatch Negativity and Cognitive Performance for the Prediction of Psychosis in Subjects with At-Risk Mental State. PLoS ONE 2013, 8, e54080. [Google Scholar] [CrossRef]
- Jahshan, C.; Wynn, J.K.; Green, M.F. Relationship between auditory processing and affective prosody in schizophrenia. Schizophr. Res. 2013, 143, 348–353. [Google Scholar] [CrossRef]
- Jahshan, C.; Vinogradov, S.; Wynn, J.K.; Hellemann, G.; Green, M.F. A randomized controlled trial comparing a “bottom-up” and “top-down” approach to cognitive training in schizophrenia. J. Psychiatr. Res. 2019, 109, 118–125. [Google Scholar] [CrossRef]
- Kaser, M.; Soltesz, F.; Lawrence, P.; Miller, S.; Dodds, C.; Croft, R.; Dudas, R.B.; Zaman, R.; Fernandez-Egea, E.; Muller, U.; et al. Oscillatory Underpinnings of Mismatch Negativity and Their Relationship with Cognitive Function in Patients with Schizophrenia. PLoS ONE 2013, 8, e83255. [Google Scholar] [CrossRef]
- Kaur, M.; Battisti, R.A.; Ward, P.B.; Ahmed, A.; Hickie, I.B.; Hermens, D.F. MMN/P3a deficits in first episode psychosis: Comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr. Res. 2011, 130, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, Y.; Kasai, K.; Kudo, N.; Rogers, M.A.; Nakagome, K.; Itoh, K.; Kato, N. Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. NeuroReport 2006, 17, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Sung, K.; Lee, K.-S.; Moon, E.; Kim, C.-G. Mismatch negativity is a stronger indicator of functional outcomes than neurocognition or theory of mind in patients with schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 213–219. [Google Scholar] [CrossRef]
- Lho, S.K.; Kim, M.; Park, J.; Hwang, W.J.; Moon, S.-Y.; Oh, S.; Kwon, J.S. Progressive Impairment of Mismatch Negativity Is Reflective of Underlying Pathophysiological Changes in Patients with First-Episode Psychosis. Front. Psychiatry 2020, 11, 587. [Google Scholar] [CrossRef]
- Light, G.A.; Swerdlow, N.R.; Thomas, M.L.; Calkins, M.E.; Green, M.F.; Greenwood, T.A.; Gur, R.E.; Gur, R.C.; Lazzeroni, L.C.; Nuechterlein, K.H.; et al. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res. 2015, 163, 63–72. [Google Scholar] [CrossRef]
- Miyanishi, T.; Sumiyoshi, T.; Higuchi, Y.; Seo, T.; Suzuki, M. LORETA Current Source Density for Duration Mismatch Negativity and Neuropsychological Assessment in Early Schizophrenia. PLoS ONE 2013, 8, e61152. [Google Scholar] [CrossRef]
- Rissling, A.J.; Park, S.-H.; Young, J.W.; Rissling, M.B.; Sugar, C.A.; Sprock, J.; Mathias, D.J.; Pela, M.; Sharp, R.F.; Braff, D.L.; et al. Demand and modality of directed attention modulate “pre-attentive” sensory processes in schizophrenia patients and nonpsychiatric controls. Schizophr. Res. 2013, 146, 326–335. [Google Scholar] [CrossRef][Green Version]
- Rowland, L.M.; Summerfelt, A.; Wijtenburg, S.A.; Du, X.; Chiappelli, J.J.; Krishna, N.; West, J.; Muellerklein, F.; Kochunov, P.; Hong, L.E. Frontal Glutamate and γ-Aminobutyric Acid Levels and Their Associations with Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry 2016, 73, 166–174. [Google Scholar] [CrossRef]
- Toyomaki, A.; Kusumi, I.; Matsuyama, T.; Kako, Y.; Ito, K.; Koyama, T. Tone duration mismatch negativity deficits predict impairment of executive function in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 95–99. [Google Scholar] [CrossRef]
- Wynn, J.K.; Sugar, C.; Horan, W.P.; Kern, R.; Green, M.F. Mismatch Negativity, Social Cognition, and Functioning in Schizophrenia Patients. Biol. Psychiatry 2010, 67, 940–947. [Google Scholar] [CrossRef]
- Favre, G.; Horat, S.K.; Herrmann, F.R.; Gothuey, I.; Ventura, J.; Merlo, M.C.G.; Missonnier, P. False memory production in schizophrenia: A neurophysiological investigation. Schizophr. Res. Cogn. 2020, 20, 100174. [Google Scholar] [CrossRef]
- Morales-Muñoz, I.; Jurado-Barba, R.; Fernández-Guinea, S.; Álvarez-Alonso, M.J.; Rodríguez-Jiménez, R.; Jiménez-Arriero, M.A.; Rubio, G. Cognitive impairments in patients with first episode psychosis: The relationship between neurophysiological and neuropsychological assessments. J. Clin. Neurosci. 2017, 36, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Coffman, B.A.; Haigh, S.M.; Murphy, T.K.; Salisbury, D.F. Event-related potentials demonstrate deficits in acoustic segmentation in schizophrenia. Schizophr. Res. 2016, 173, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Coffman, B.A.; Haigh, S.M.; Murphy, T.K.; Leiter-Mcbeth, J.; Salisbury, D.F. Reduced auditory segmentation potentials in first-episode schizophrenia. Schizophr. Res. 2018, 195, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Bruder, G.E.; Friedman, D.; Tenke, C.E.; Amador, X.F.; Clark, S.C.; Malaspina, D.; Gorman, J.M. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. Int. J. Psychophysiol. 1999, 34, 249–265. [Google Scholar] [CrossRef]
- Klein, S.D.; Shekels, L.L.; McGuire, K.A.; Sponheim, S.R. Neural anomalies during vigilance in schizophrenia: Diagnostic specificity and genetic associations. NeuroImage: Clin. 2020, 28, 102414. [Google Scholar] [CrossRef]
- Sklar, A.L.; Coffman, B.A.; Haas, G.; Ghuman, A.; Cho, R.; Salisbury, D.F. Inefficient visual search strategies in the first-episode schizophrenia spectrum. Schizophr. Res. 2020, 224, 126–132. [Google Scholar] [CrossRef]
- Clementz, B.A.; Wang, J.; Keil, A. Normal Electrocortical Facilitation but Abnormal Target Identification during Visual Sustained Attention in Schizophrenia. J. Neurosci. 2008, 28, 13411–13418. [Google Scholar] [CrossRef]
- Dichter, G.S.; van der Stelt, O.; Boch, J.L.; Belger, A. Relations Among Intelligence, Executive Function, and P300 Event Related Potentials in Schizophrenia. J. Nerv. Ment. Dis. 2006, 194, 179–187. [Google Scholar] [CrossRef]
- Francisco, A.A.; Horsthuis, D.J.; Popiel, M.; Foxe, J.J.; Molholm, S. Atypical response inhibition and error processing in 22q11.2 Deletion Syndrome and schizophrenia: Towards neuromarkers of disease progression and risk. NeuroImage: Clin. 2020, 27, 102351. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Wydell, T.N.; Liao, J.; Wang, F.; Quan, W.; Tian, J.; Wang, P.; Liu, J.; Dong, W. Electrophysiological basis of reading related phonological impairment in Chinese speakers with schizophrenia: An ERP study. Psychiatry Res. Neuroimaging 2017, 261, 65–71. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, D.; Tan, S.; Song, C.; Cui, J.; Fan, F.; Zhu, X.; Zou, Y.; Luo, Y. Neural correlates of the abolished self-referential memory effect in schizophrenia. Psychol. Med. 2014, 44, 477–487. [Google Scholar] [CrossRef]
- Andersen, E.H.; Campbell, A.M.; Schipul, S.E.; Bellion, C.M.; Donkers, F.C.; Evans, A.M.; Belger, A. Electrophysiological Correlates of Aberrant Motivated Attention and Salience Processing in Unaffected Relatives of Schizophrenia Patients. Clin. EEG Neurosci. 2016, 47, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Sumiyoshi, T.; Kawasaki, Y.; Matsui, M.; Arai, H.; Kurachi, M. Electrophysiological basis for the ability of olanzapine to improve verbal memory and functional outcome in patients with schizophrenia: A LORETA analysis of P300. Schizophr. Res. 2008, 101, 320–330. [Google Scholar] [CrossRef]
- Johnston, P.J.; Stojanov, W.; Devir, H.; Schall, U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur. J. Neurosci. 2005, 22, 1221–1232. [Google Scholar] [CrossRef]
- Kruiper, C.; Fagerlund, B.; Nielsen, M.; Düring, S.; Jensen, M.H.; Ebdrup, B.H.; Glenthøj, B.Y.; Oranje, B. Associations between P3a and P3b amplitudes and cognition in antipsychotic-naïve first-episode schizophrenia patients. Psychol. Med. 2019, 49, 868–875. [Google Scholar] [CrossRef]
- Chang, W.-H.; Chen, K.-C.; Yang, Y.-K.; Chen, P.-S.; Lu, R.-B.; Yeh, T.-L.; Wang, C.S.-M.; Lee, I.-H. Association between auditory P300, psychopathology, and memory function in drug-naïve schizophrenia. Kaohsiung J. Med. Sci. 2014, 30, 133–138. [Google Scholar] [CrossRef]
- Ertekin, E.; Üçok, A.; Keskin-Ergen, Y.; Devrim-Üçok, M. Deficits in Go and NoGo P3 potentials in patients with schizophrenia. Psychiatry Res. 2017, 254, 126–132. [Google Scholar] [CrossRef]
- Galletly, C.A.; MacFarlane, A.C.; Clark, C.R. Impaired updating of working memory in schizophrenia. Int. J. Psychophysiol. 2007, 63, 265–274. [Google Scholar] [CrossRef]
- Heidrich, A.; Strik, W.K. Auditory P300 topography and neuropsychological test performance: Evidence for left hemispheric dysfunction in schizophrenia. Biol. Psychiatry 1997, 41, 327–335. [Google Scholar] [CrossRef]
- Higuchi, Y.; Sumiyoshi, T.; Tateno, T.; Nakajima, S.; Sasabayashi, D.; Nishiyama, S.; Mizukami, Y.; Takahashi, T.; Suzuki, M. Prolonged P300 Latency in Antipsychotic-Free Subjects with At-Risk Mental States Who Later Developed Schizophrenia. J. Pers. Med. 2021, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, T.H.; Kim, J.-H.; Hong, H.; Lee, T.Y.; Lee, Y.; Salisbury, D.F.; Kwon, J.S. Decomposing P300 into correlates of genetic risk and current symptoms in schizophrenia: An inter-trial variability analysis. Schizophr. Res. 2018, 192, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.H.; Koelman, J.H.T.M.; Linszen, D.H.; Bour, L.J.; Dingemans, P.M.; Ongerboer de Visser, B.W. Clinical and neuropsychological correlates of the P300 in schizophrenia. Schizophr. Res. 2002, 55, 105–113. [Google Scholar] [CrossRef]
- Schreiber, H.; Stolz-Born, G.; Kornhuber, H.H.; Born, J. Investigation of electrophysiological correlates of attention and information processing as vulnerability indicators for schizophrenia. J. Psychophysiol. 1998, 12, 286–300. [Google Scholar]
- Shajahan, P.M.; O’Carroll, R.E.; Glabus, M.F.; Ebmeier, K.P.; Blackwood, D.H.R. Correlation of auditory ‘oddball’ P300 with verbal memory deficits in schizophrenia. Psychol. Med. 1997, 27, 579–586. [Google Scholar] [CrossRef]
- Schall, U.; Catts, S.V.; Chaturvedi, S.; Liebert, B.; Redenbach, J.; Karayanidis, F.; Ward, P.B. The effect of clozapine therapy on frontal lobe dysfunction in schizophrenia: Neuropsychology and event-related potential measures. Int. J. Neuropsychopharmacol. 1998, 1, 19–29. [Google Scholar] [CrossRef]
- Boudewyn, M.A.; Carter, C.S.; Long, D.L.; Traxler, M.J.; Lesh, T.A.; Mangun, G.R.; Swaab, T.Y. Language context processing deficits in schizophrenia: The role of attentional engagement. Neuropsychologia 2017, 96, 262–273. [Google Scholar] [CrossRef]
- Jackson, F.; Foti, D.; Kotov, R.; Perlman, G.; Mathalon, D.H.; Proudfit, G.H. An incongruent reality: The N400 in relation to psychosis and recovery. Schizophr. Res. 2014, 160, 208–215. [Google Scholar] [CrossRef]
- Lepock, J.R.; Ahmed, S.; Mizrahi, R.; Gerritsen, C.J.; Maheandiran, M.; Bagby, R.M.; Korostil, M.; Kiang, M. N400 event-related brain potential as an index of real-world and neurocognitive function in patients at clinical high risk for schizophrenia. Early Interv. Psychiatry 2021, 15, 68–75. [Google Scholar] [CrossRef]
- Alain, C.; McNeely, H.E.; He, Y.; Christensen, B.K.; West, R. Neurophysiological Evidence of Error-monitoring Deficits in Patients with Schizophrenia. Cereb. Cortex 2002, 12, 840–846. [Google Scholar] [CrossRef]
- Chan, C.C.; Trachik, B.J.; Bedwell, J.S. An event-related potential investigation of error monitoring in adults with a history of psychosis. Clin. Neurophysiol. 2015, 126, 1717–1726. [Google Scholar] [CrossRef][Green Version]
- Foti, D.; Perlman, G.; Bromet, E.J.; Harvey, P.D.; Hajcak, G.; Mathalon, D.H.; Kotov, R. Pathways from performance monitoring to negative symptoms and functional outcomes in psychotic disorders. Psychol. Med. 2020, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Chou, T.-L.; Lai, W.-S.; Hsieh, M.H.; Liu, C.-C.; Liu, C.-M.; Hwu, H.-G. P50, N100, and P200 Auditory Sensory Gating Deficits in Schizophrenia Patients. Front. Psychiatry 2020, 11, 868. [Google Scholar] [CrossRef]
- Foxe, J.J.; Yeap, S.; Snyder, A.C.; Kelly, S.P.; Thakore, J.H.; Molholm, S. The N1 auditory evoked potential component as an endophenotype for schizophrenia: High-density electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Heinze, H.J.; Mangun, G.R. Electrophysiological signs of sustained and transient attention to spatial locations. Neuropsychologia 1995, 33, 889–908. [Google Scholar] [CrossRef]
- Coull, J.T. Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog. Neurobiol. 1998, 55, 343–361. [Google Scholar] [CrossRef]
- Onitsuka, T.; Oribe, N.; Nakamura, I.; Kanba, S. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 461–470. [Google Scholar] [CrossRef]
- Earls, H.A.; Curran, T.; Mittal, V. Deficits in Early Stages of Face Processing in Schizophrenia: A Systematic Review of the P100 Component. Schizophr. Bull. 2016, 42, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Tada, M.; Kirihara, K.; Yahata, N.; Hashimoto, R.; Araki, T.; Kasai, K. Auditory mismatch negativity and P3a in response to duration and frequency changes in the early stages of psychosis. Schizophr. Res. 2013, 150, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef]
- Fitzgerald, K.; Todd, J. Making Sense of Mismatch Negativity. Front. Psychiatry 2020, 11, 468. [Google Scholar] [CrossRef]
- Näätänen, R.; Escera, C. Mismatch Negativity: Clinical and Other Applications. Audiol. Neurotol. 2000, 5, 105–110. [Google Scholar] [CrossRef]
- Avissar, M.; Xie, S.; Vail, B.; Lopez-Calderon, J.; Wang, Y.; Javitt, D.C. Meta-analysis of mismatch negativity to simple versus complex deviants in schizophrenia. Schizophr. Res. 2018, 191, 25–34. [Google Scholar] [CrossRef]
- Erickson, M.A.; Ruffle, A.; Gold, J.M. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biol. Psychiatry 2016, 79, 980–987. [Google Scholar] [CrossRef]
- Crowley, K.E.; Colrain, I.M. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clin. Neurophysiol. 2004, 115, 732–744. [Google Scholar] [CrossRef]
- Ferreira-Santos, F.; Silveira, C.; Almeida, P.R.; Palha, A.; Barbosa, F.; Marques-Teixeira, J. The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clin. Neurophysiol. 2012, 123, 1300–1308. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected Studies of the Event-Related Potential. Int. J. Med Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knight, R.T. Cognitive neurophysiology: Event-related potentials. Handb. Clin. Neurol. 2019, 160, 543–558. [Google Scholar] [CrossRef]
- Raghavan, D.V.; Bharathi, P.; Shanmugiah, A.; Jeyaprakash, R. P300 and neuropsychological measurements in patients with schizophrenia and their healthy biological siblings. Indian J. Psychiatry 2016, 58, 454–458. [Google Scholar] [CrossRef]
- Hamilton, H.K.; Roach, B.J.; Mathalon, D.H. Forecasting Remission from the Psychosis Risk Syndrome with Mismatch Negativity and P300: Potentials and Pitfalls. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2021, 6, 178–187. [Google Scholar] [CrossRef]
- Bramon, E.; Rabe-Hesketh, S.; Sham, P.; Murray, R.M.; Frangou, S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004, 70, 315–329. [Google Scholar] [CrossRef]
- Bramon, E.; McDonald, C.; Croft, R.J.; Landau, S.; Filbey, F.; Gruzelier, J.H.; Sham, P.C.; Frangou, S.; Murray, R.M. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. NeuroImage 2005, 27, 960–968. [Google Scholar] [CrossRef]
- Strandburg, R.J.; Marsh, J.T.; Brown, W.S.; Asarnow, R.F.; Guthrie, D.; Higa, J.; Yee-Bradburay, C.M.; Nuechterlein, K.H. Reduced attention-related negative potentials in schizophrenic adults. Psychophysiology 1994, 31, 272–281. [Google Scholar] [CrossRef]
- Volpe, U.; Mucci, A.; Bucci, P.; Merlotti, E.; Galderisi, S.; Maj, M. The cortical generators of P3a and P3b: A LORETA study. Brain Res. Bull. 2007, 73, 220–230. [Google Scholar] [CrossRef]
- Kutas, M.; Federmeier, K.D. Thirty Years and Counting: Finding Meaning in the N400 Component of the Event-Related Brain Potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Kiang, M.; Gerritsen, C.J. The N400 event-related brain potential response: A window on deficits in predicting meaning in schizophrenia. Int. J. Psychophysiol. 2019, 145, 65–69. [Google Scholar] [CrossRef]
- Yeung, N.; Summerfield, C. Metacognition in human decision-making: Confidence and error monitoring. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1310–1321. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Christ, S.; Hohnsbein, J. ERP components on reaction errors and their functional significance: A tutorial. Biol. Psychol. 2000, 51, 87–107. [Google Scholar] [CrossRef]
- Orr, J.M.; Carrasco, M. The Role of the Error Positivity in the Conscious Perception of Errors. J. Neurosci. 2011, 31, 5891–5892. [Google Scholar] [CrossRef]
- Ferrarelli, F.; Tononi, G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr. Res. 2017, 180, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; Amzica, F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res. Online SRO 1998, 1, 1–10. [Google Scholar] [PubMed]
- Walker, M.P.; Stickgold, R. Sleep-Dependent Learning and Memory Consolidation. Neuron 2004, 44, 121–133. [Google Scholar] [CrossRef]
- Baandrup, L.; Christensen, J.A.E.; Fagerlund, B.; Jennum, P. Investigation of sleep spindle activity and morphology as predictors of neurocognitive functioning in medicated patients with schizophrenia. J. Sleep Res. 2019, 28, e12672. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, U.; Simpkin, A.J.; Demanuele, C.; Wamsley, E.; Marston, H.M.; Jones, M.W. Distributed slow-wave dynamics during sleep predict memory consolidation and its impairment in schizophrenia. NPJ Schizophr. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Forest, G.; Poulin, J.; Daoust, A.-M.; Lussier, I.; Stip, E.; Godbout, R. Attention and non-REM sleep in neuroleptic-naive persons with schizophrenia and control participants. Psychiatry Res. 2007, 149, 33–40. [Google Scholar] [CrossRef]
- Gerstenberg, M.; Furrer, M.; Tesler, N.; Franscini, M.; Walitza, S.; Huber, R. Reduced sleep spindle density in adolescent patients with early-onset schizophrenia compared to major depressive disorder and healthy controls. Schizophr. Res. 2020, 221, 20–28. [Google Scholar] [CrossRef]
- Göder, R.; Graf, A.; Ballhausen, F.; Weinhold, S.; Baier, P.C.; Junghanns, K.; Prehn-Kristensen, A. Impairment of sleep-related memory consolidation in schizophrenia: Relevance of sleep spindles? Sleep Med. 2015, 16, 564–569. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Cashmere, J.D.; Miewald, J.; Yeragani, V.K. Decreased nonlinear complexity and chaos during sleep in first episode schizophrenia: A preliminary report. Schizophr. Res. 2004, 71, 263–272. [Google Scholar] [CrossRef]
- Manoach, D.S.; Demanuele, C.; Wamsley, E.J.; Vangel, M.; Montrose, D.M.; Miewald, J.; Kupfer, D.; Buysse, D.; Stickgold, R.; Keshavan, M.S. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front. Hum. Neurosci. 2014, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Sartory, G.; van Beekum, A.; Lohrmann, T.; Pietrowsky, R. Sleep-related cognitive function and the K-complex in schizophrenia. Behav. Brain Res. 2012, 234, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, E.J.; Tucker, M.A.; Shinn, A.K.; Ono, K.E.; McKinley, S.K.; Ely, A.V.; Goff, D.C.; Stickgold, R.; Manoach, D.S. Reduced Sleep Spindles and Spindle Coherence in Schizophrenia: Mechanisms of Impaired Memory Consolidation? Biol. Psychiatry 2012, 71, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Sugita, Y.; Koga, E.; Shirakawa, S.; Inoue, K.; Uchida, S.; Kuwahara, H.; Kousaka, M.; Kobayashi, T.; Tsuji, Y.; et al. Proposed supplements and amendments to ‘A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin. Neurosci. 2001, 55, 305–310. [Google Scholar] [CrossRef]
- Schabus, M.; Hoedlmoser, K.; Pecherstorfer, T.; Anderer, P.; Gruber, G.; Parapatics, S.; Sauter, C.; Kloesch, G.; Klimesch, W.; Saletu, B.; et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008, 1191, 127–135. [Google Scholar] [CrossRef]
- Lee, K.-H.; Williams, L.M.; Breakspear, M.; Gordon, E. Synchronous Gamma activity: A review and contribution to an integrative neuroscience model of schizophrenia. Brain Res. Brain Res. Rev. 2003, 41, 57–78. [Google Scholar] [CrossRef]
- Choi, K.-M.; Kim, J.-Y.; Kim, Y.-W.; Han, J.-W.; Im, C.-H.; Lee, S.-H. Comparative analysis of default mode networks in major psychiatric disorders using resting-state EEG. Sci. Rep. 2021, 11, 22007. [Google Scholar] [CrossRef]
- Corcoran, A.W.; Alday, P.M.; Schlesewsky, M.; Bornkessel-Schlesewsky, I. Toward a reliable, automated method of individual alpha frequency (IAF) quantification. Psychophysiology 2018, 55, e13064. [Google Scholar] [CrossRef]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Schmiedek, F.; Lövdén, M.; Lindenberger, U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 2013, 50, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Yeum, T.-S.; Kang, U.G. Reduction in Alpha Peak Frequency and Coherence on Quantitative Electroencephalography in Patients with Schizophrenia. J. Korean Med. Sci. 2018, 33, e179. [Google Scholar] [CrossRef]
- Light, G.A.; Näätänen, R. Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proc. Natl. Acad. Sci. USA 2013, 110, 15175–15176. [Google Scholar] [CrossRef]
- Light, G.A.; Swerdlow, N.R.; Rissling, A.J.; Radant, A.; Sugar, C.A.; Sprock, J.; Pela, M.; Geyer, M.A.; Braff, D.L. Characterization of Neurophysiologic and Neurocognitive Biomarkers for Use in Genomic and Clinical Outcome Studies of Schizophrenia. PLoS ONE 2012, 7, e39434. [Google Scholar] [CrossRef]
- Paitel, E.R.; Samii, M.R.; Nielson, K.A. A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer’s disease. Behav. Brain Res. 2021, 396, 112904. [Google Scholar] [CrossRef]
- Tsolaki, A.; Kosmidou, V.; Hadjileontiadis, L.; Kompatsiaris, I.; Tsolaki, M. Brain source localization of MMN, P300 and N400: Aging and gender differences. Brain Res. 2015, 1603, 32–49. [Google Scholar] [CrossRef]
- Schiff, S.; Valenti, P.; Andrea, P.; Lot, M.; Bisiacchi, P.; Gatta, A.; Amodio, P. The effect of aging on auditory components of event-related brain potentials. Clin. Neurophysiol. 2008, 119, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Winkelman, J.W. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr. Res. 2006, 82, 251–260. [Google Scholar] [CrossRef]
- Fogel, S.M.; Smith, C.T. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011, 35, 1154–1165. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Short, M.A.; Gradisar, M. Sleep spindles and cognitive performance across adolescence: A meta-analytic review. J. Adolesc. 2018, 66, 55–70. [Google Scholar] [CrossRef]
- Barros, C.; Silva, C.A.; Pinheiro, A.P. Advanced EEG-based learning approaches to predict schizophrenia: Promises and pitfalls. Artif. Intell. Med. 2021, 114, 102039. [Google Scholar] [CrossRef]
- Thieme, A.; Belgrave, D.; Doherty, G. Machine Learning in Mental Health: A Systematic Review of the HCI Literature to Support the Development of Effective and Implementable ML Systems. ACM Trans. Comput. Hum. Interact. 2020, 27, 1–53. [Google Scholar] [CrossRef]
- Bracher-Smith, M.; Crawford, K.; Escott-Price, V. Machine learning for genetic prediction of psychiatric disorders: A systematic review. Mol. Psychiatry 2021, 26, 70–79. [Google Scholar] [CrossRef]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R.; et al. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef]
- Hasey, G.M.; Kiang, M. A Review of Recent Literature Employing Electroencephalographic Techniques to Study the Pathophysiology, Phenomenology, and Treatment Response of Schizophrenia. Curr. Psychiatry Rep. 2013, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, A.H.; Popescu, F.C.; Rentzsch, J.; Gallinat, J. Critical Evaluation of Auditory Event-Related Potential Deficits in Schizophrenia: Evidence from Large-Scale Single-Subject Pattern Classification. Schizophr. Bull. 2013, 40, 1062–1071. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.; Zhang, J.; Wang, C.; Zhao, X.; Chang, Q.; Wang, H.; Wang, C.-Y. Discriminating schizophrenia disease progression using a P50 sensory gating task with dense-array EEG, clinical assessments, and cognitive tests. Expert Rev. Neurother. 2019, 19, 459–470. [Google Scholar] [CrossRef]
- Chang, Q.; Liu, M.; Tian, Q.; Wang, H.; Luo, Y.; Zhang, J.; Wang, C.-Y. EEG-Based Brain Functional Connectivity in First-Episode Schizophrenia Patients, Ultra-High-Risk Individuals, and Healthy Controls During P50 Suppression. Front. Hum. Neurosci. 2019, 13, 379. [Google Scholar] [CrossRef]
- Koutsouleris, N.; Dwyer, D.B.; Degenhardt, F.; Maj, C.; Urquijo-Castro, M.F.; Sanfelici, R.; Popovic, D.; Oeztuerk, O.; Haas, S.S.; Weiske, J.; et al. Multimodal Machine Learning Workflows for Prediction of Psychosis in Patients with Clinical High-Risk Syndromes and Recent-Onset Depression. JAMA Psychiatry 2021, 78, 195–209. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).