Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy
Abstract
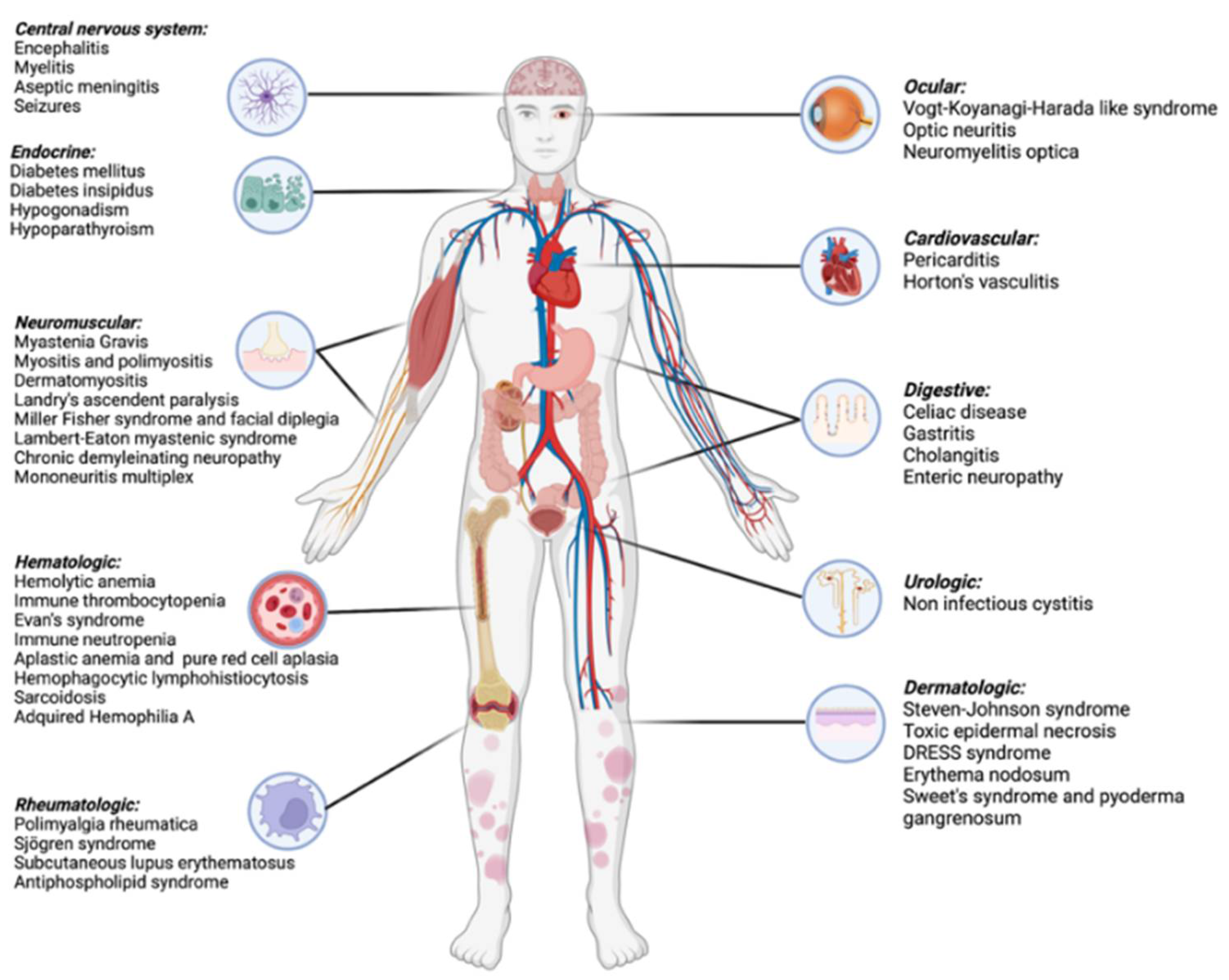
1. Introduction
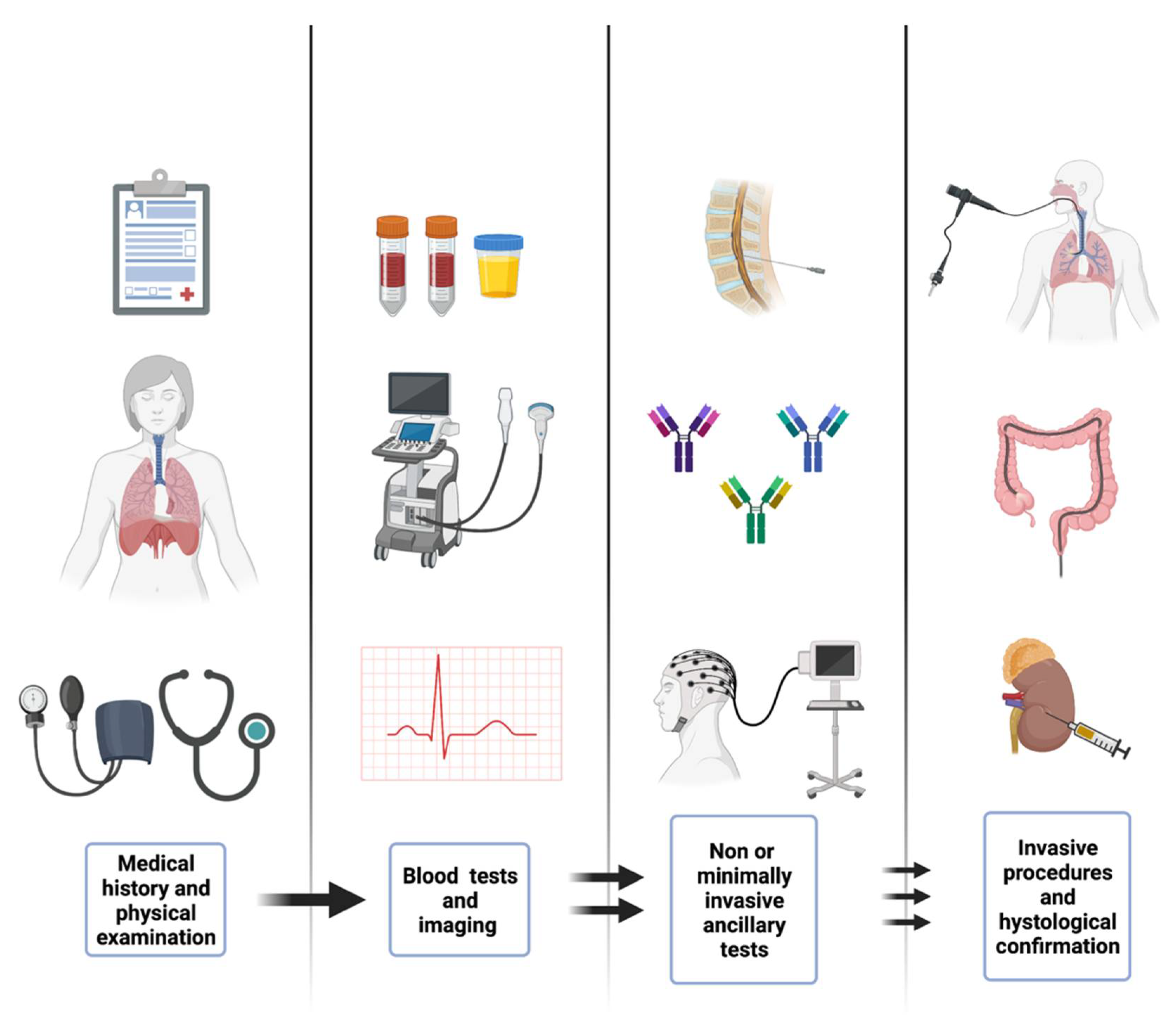
2. Materials and Methods
2.1. Neuromuscular Adverse Events
2.1.1. Myasthenia Gravis
2.1.2. Myopathies
2.1.3. Guillain–Barré Syndrome
2.2. Hematological Immune Related Adverse Events
2.2.1. Immune Cytopenias
2.2.2. Hemophagocytic Lymphohistiocytosis
2.2.3. Sarcoidosis-Like Reaction
2.2.4. Bleeding Disorders
2.3. Immune-Related Endocrinopathies
2.3.1. Type 1 Diabetes Mellitus
2.3.2. Hypoparathyroidism
2.3.3. Other Endocrinopathies
2.4. Dermatologic Immune-Related Adverse Events
2.4.1. Steven Johnson Syndrome and Toxic Epidermal Necrolysis
2.4.2. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS Syndrome)
2.4.3. Erythema Nodosum-Like Panniculitis
2.5. Digestive Immune-Related Adverse Events
2.5.1. Celiac Disease
2.5.2. Gastritis
2.5.3. Cholangitis
2.6. Cardiac Immune-Related Adverse Events
Pericardial Disease
2.7. Urologic Immune-Related Adverse Events
Non-Infectious Cystitis
2.8. Ocular Immune Related Adverse Events
Vogt–Koyanagi–Harada-Like Reaction
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C. Combination Strategies to Maximize the Benefits of Cancer Immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and Treating the Inflammatory Adverse Events of Cancer Immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Russell, J.; Lebbé, C.; Chmielowski, B.; Gambichler, T.; Grob, J.-J.; Kiecker, F.; Rabinowits, G.; Terheyden, P.; Zwiener, I.; et al. Efficacy and Safety of First-Line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018, 4, e180077. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Mazieres, J.; Rittmeyer, A.; Gadgeel, S.; Hida, T.; Gandara, D.R.; Cortinovis, D.L.; Barlesi, F.; Yu, W.; Matheny, C.; Ballinger, M.; et al. Atezolizumab Versus Docetaxel in Pretreated Patients With NSCLC: Final Results From the Randomized Phase 2 POPLAR and Phase 3 OAK Clinical Trials. J. Thorac. Oncol. 2021, 16, 140–150. [Google Scholar] [CrossRef]
- Tecentriq®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Tecentriq®; EMA Oficial: Amsterdam, The Netherlands, 2021.
- Bavencio®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Bavencio®; EMA Oficial: Amsterdam, The Netherlands, 2021.
- Opdivo®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Opdivo®; EMA Oficial: Amsterdam, The Netherlands, 2022.
- Imfinzi®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Imfinzi®; EMA Oficial: Amsterdam, The Netherlands, 2022.
- Keytruda®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Keytruda®; EMA Oficial: Amsterdam, The Netherlands, 2022.
- Yervoy®. In The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Propietary Medicinal Products (CPMP); European Public Assesment Report (EPAR): Yervoy®; EMA Oficial: Amsterdam, The Netherlands, 2022.
- Zimmer, L.; Goldinger, S.M.; Hofmann, L.; Loquai, C.; Ugurel, S.; Thomas, I.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Neurological, Respiratory, Musculoskeletal, Cardiac and Ocular Side-Effects of Anti-PD-1 Therapy. Eur. J. Cancer 2016, 60, 210–225. [Google Scholar] [CrossRef]
- Kao, J.C.; Brickshawana, A.; Liewluck, T. Neuromuscular Complications of Programmed Cell Death-1 (PD-1) Inhibitors. Curr. Neurol. Neurosci. Rep. 2018, 18, 63. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.-J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant Ipilimumab versus Placebo after Complete Resection of High-Risk Stage III Melanoma (EORTC 18071): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef]
- Kolb, N.A.; Trevino, C.R.; Waheed, W.; Sobhani, F.; Landry, K.K.; Thomas, A.A.; Hehir, M. Neuromuscular Complications of Immune Checkpoint Inhibitor Therapy: Neuromuscular Complications of Immune Checkpoint Inhibitor Therapy. Muscle Nerve 2018, 58, 10–22. [Google Scholar] [CrossRef]
- Makarious, D.; Horwood, K.; Coward, J.I.G. Myasthenia Gravis: An Emerging Toxicity of Immune Checkpoint Inhibitors. Eur. J. Cancer 2017, 82, 128–136. [Google Scholar] [CrossRef]
- March, K.L.; Samarin, M.J.; Sodhi, A.; Owens, R.E. Pembrolizumab-Induced Myasthenia Gravis: A Fatal Case Report. J. Oncol. Pharm. Pract. 2018, 24, 146–149. [Google Scholar] [CrossRef]
- Gonzalez, N.L.; Puwanant, A.; Lu, A.; Marks, S.M.; Živković, S.A. Myasthenia Triggered by Immune Checkpoint Inhibitors: New Case and Literature Review. Neuromuscul. Disord. 2017, 27, 266–268. [Google Scholar] [CrossRef]
- Nguyen, B.H.V.; Kuo, J.; Budiman, A.; Christie, H.; Ali, S. Two Cases of Clinical Myasthenia Gravis Associated with Pembrolizumab Use in Responding Melanoma Patients. Melanoma Res. 2017, 27, 152–154. [Google Scholar] [CrossRef]
- Shirai, T.; Sano, T.; Kamijo, F.; Saito, N.; Miyake, T.; Kodaira, M.; Katoh, N.; Nishie, K.; Okuyama, R.; Uhara, H. Acetylcholine Receptor Binding Antibody-Associated Myasthenia Gravis and Rhabdomyolysis Induced by Nivolumab in a Patient with Melanoma. Jpn. J. Clin. Oncol. 2016, 46, 86–88. [Google Scholar] [CrossRef]
- Kimura, T.; Fukushima, S.; Miyashita, A.; Aoi, J.; Jinnin, M.; Kosaka, T.; Ando, Y.; Matsukawa, M.; Inoue, H.; Kiyotani, K.; et al. Myasthenic Crisis and Polymyositis Induced by One Dose of Nivolumab. Cancer Sci. 2016, 107, 1055–1058. [Google Scholar] [CrossRef]
- Alnahhas, I.; Wong, J. A Case of New-onset Antibody-positive Myasthenia Gravis in a Patient Treated with Pembrolizumab for Melanoma. Muscle Nerve 2017, 55, E25–E26. [Google Scholar] [CrossRef]
- Polat, P.; Donofrio, P.D. Myasthenia Gravis Induced by Nivolumab Therapy in a Patient with Non-Small-Cell Lung Cancer: Noteworthy Cases. Muscle Nerve 2016, 54, 507. [Google Scholar] [CrossRef]
- Sciacca, G.; Nicoletti, A.; Rampello, L.; Noto, L.; Parra, H.J.S.; Zappia, M. Benign Form of Myasthenia Gravis after Nivolumab Treatment: Myasthenia after Nivolumab. Muscle Nerve 2016, 54, 507–509. [Google Scholar] [CrossRef]
- Chang, E.; Sabichi, A.L.; Sada, Y.H. Myasthenia Gravis After Nivolumab Therapy for Squamous Cell Carcinoma of the Bladder. J. Immunother. 2017, 40, 114–116. [Google Scholar] [CrossRef]
- Liao, B.; Shroff, S.; Kamiya-Matsuoka, C.; Tummala, S. Atypical Neurological Complications of Ipilimumab Therapy in Patients with Metastatic Melanoma. Neuro-Oncology 2014, 16, 589–593. [Google Scholar] [CrossRef]
- Johnson, D.B.; Saranga-Perry, V.; Lavin, P.J.M.; Burnette, W.B.; Clark, S.W.; Uskavitch, D.R.; Wallace, D.E.; Dickson, M.A.; Kudchadkar, R.R.; Sosman, J.A. Myasthenia Gravis Induced by Ipilimumab in Patients With Metastatic Melanoma. J. Clin. Oncol. 2015, 33, e122–e124. [Google Scholar] [CrossRef] [PubMed]
- Montes, V.; Sousa, S.; Pita, F.; Guerreiro, R.; Carmona, C. Myasthenia Gravis Induced by Ipilimumab in a Patient With Metastatic Melanoma. Front. Neurol. 2018, 9, 150. [Google Scholar] [CrossRef]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Otani, Y.; et al. Nivolumab-Related Myasthenia Gravis with Myositis and Myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar] [CrossRef]
- Liewluck, T.; Kao, J.C.; Mauermann, M.L. PD-1 Inhibitor-Associated Myopathies: Emerging Immune-Mediated Myopathies. J. Immunother. 2018, 41, 208–211. [Google Scholar] [CrossRef]
- Nakatani, Y.; Tanaka, N.; Enami, T.; Minami, S.; Okazaki, T.; Komuta, K. Lambert-Eaton Myasthenic Syndrome Caused by Nivolumab in a Patient with Squamous Cell Lung Cancer. Case Rep. Neurol. 2018, 10, 346–352. [Google Scholar] [CrossRef]
- Agrawal, K.; Agrawal, N. Lambert-Eaton Myasthenic Syndrome Secondary to Nivolumab and Ipilimumab in a Patient with Small-Cell Lung Cancer. Case Rep. Neurol. Med. 2019, 2019, 5353202. [Google Scholar] [CrossRef]
- Lee, J.H.; Baek, S.K.; Han, J.J.; Kim, H.J.; Lee, Y.-A.; Yoo, D.; Maeng, C.H. Lambert-Eaton Myasthenic Syndrome (LEMS) in a Patient with Lung Cancer under Treatment with Pembrolizumab: A Case Study. J. Chemother. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duplaine, A.; Prot, C.; Le-Masson, G.; Soulages, A.; Duval, F.; Dutriaux, C.; Prey, S. Myasthenia Gravis Lambert-Eaton Overlap Syndrome Induced by Nivolumab in a Metastatic Melanoma Patient. Neurol. Sci. 2021, 42, 5377–5378. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Gandhy, S.; Lancaster, E. Nivolumab-associated LAMBERT-EATON Myasthenic Syndrome and Cerebellar Dysfunction in a Patient with a Neuroendocrine Tumor. Muscle Nerve 2021, 63, E18–E21. [Google Scholar] [CrossRef] [PubMed]
- Kunii, E.; Owaki, S.; Yamada, K.; Yoshihara, M.; Yamaba, Y.; Takakuwa, O.; Toyoda, T.; Akita, K. Lambert-Eaton Myasthenic Syndrome Caused by Atezolizumab in a Patient with Small-Cell Lung Cancer. Intern. Med. 2022, 61, 1739–1742. [Google Scholar] [CrossRef]
- Sheik Ali, S.; Goddard, A.L.; Luke, J.J.; Donahue, H.; Todd, D.J.; Werchniak, A.; Vleugels, R.A. Drug-Associated Dermatomyositis Following Ipilimumab Therapy: A Novel Immune-Mediated Adverse Event Associated With Cytotoxic T-Lymphocyte Antigen 4 Blockade. JAMA Dermatol. 2015, 151, 195. [Google Scholar] [CrossRef]
- Bourgeois-Vionnet, J.; Joubert, B.; Bernard, E.; Sia, M.A.; Pante, V.; Fabien, N.; Honnorat, J.; Streichenberger, N. Nivolumab-Induced Myositis: A Case Report and a Literature Review. J. Neurol. Sci. 2018, 387, 51–53. [Google Scholar] [CrossRef]
- Kadota, H.; Gono, T.; Shirai, Y.; Okazaki, Y.; Takeno, M.; Kuwana, M. Immune Checkpoint Inhibitor-Induced Myositis: A Case Report and Literature Review. Curr. Rheumatol. Rep. 2019, 21, 10. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and Neuromuscular Side-Effects Induced by Immune Checkpoint Inhibitors. Eur. J. Cancer 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.-E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased Reporting of Fatal Immune Checkpoint Inhibitor-Associated Myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Huh, S.-Y.; Shin, S.-H.; Kim, M.K.; Lee, S.-Y.; Son, K.H.; Shin, H.Y. Emergence of Myasthenia Gravis with Myositis in a Patient Treated with Pembrolizumab for Thymic Cancer. J. Clin. Neurol. 2018, 14, 115. [Google Scholar] [CrossRef]
- Vallet, H.; Gaillet, A.; Weiss, N.; Vanhaecke, C.; Saheb, S.; Touitou, V.; Franck, N.; Kramkimel, N.; Borden, A.; Touat, M.; et al. Pembrolizumab-Induced Necrotic Myositis in a Patient with Metastatic Melanoma. Ann. Oncol. 2016, 27, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Crudele, L.; Leone, P.; Argentiero, A.; Guarascio, M.; Silvestris, N.; Vacca, A.; Racanelli, V. Immune Checkpoint Inhibitor-Related Myositis: From Biology to Bedside. Int. J. Mol. Sci. 2020, 21, 3054. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Lee, K.-Y.; Hu, C.-J.; Chung, C.-C. Coexisting Myasthenia Gravis, Myositis, and Polyneuropathy Induced by Ipilimumab and Nivolumab in a Patient with Non-Small-Cell Lung Cancer: A Case Report and Literature Review. Medicine 2017, 96, e9262. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Subudhi, S.K.; Gao, J.; Tannir, N.M.; Tu, S.-M.; Sharma, P. Acute Rhabdomyolysis with Severe Polymyositis Following Ipilimumab-Nivolumab Treatment in a Cancer Patient with Elevated Anti-Striated Muscle Antibody. J. Immunother. Cancer 2016, 4, 36. [Google Scholar] [CrossRef]
- Wilgenhof, S.; Neyns, B. Anti-CTLA-4 Antibody-Induced Guillain–Barré Syndrome in a Melanoma Patient. Ann. Oncol. 2011, 22, 991–993. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, Y.; Wang, X.; Zhao, B. Guillain–Barré Syndrome in Patients Treated with Immune Checkpoint Inhibitors. J. Neurol. 2021, 268, 2169–2174. [Google Scholar] [CrossRef]
- Yost, M.D.; Chou, C.Z.; Botha, H.; Block, M.S.; Liewluck, T. Facial Diplegia after Pembrolizumab Treatment: Facial Diplegia after Pembrolizumab Treatment. Muscle Nerve 2017, 56, E20–E21. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Kuwahara, M.; Kawai, S.; Nakahama, K.; Kusunoki, S. Acute Demyelinating Polyneuropathy Induced by Nivolumab. J. Neurol. Neurosurg. Psychiatry 2018, 89, 435–437. [Google Scholar] [CrossRef]
- Graus, F. Recommended Diagnostic Criteria for Paraneoplastic Neurological Syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef]
- De Maleissye, M.-F.; Nicolas, G.; Saiag, P. Pembrolizumab-Induced Demyelinating Polyradiculoneuropathy. N. Engl. J. Med. 2016, 375, 296–297. [Google Scholar] [CrossRef]
- Schneiderbauer, R.; Schneiderbauer, M.; Wick, W.; Enk, A.; Haenssle, H.; Hassel, J. PD-1 Antibody-Induced Guillain-Barré Syndrome in a Patient with Metastatic Melanoma. Acta Derm. Venereol. 2017, 97, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Dimachkie, M.M.; Saperstein, D.S. Acquired Immune Demyelinating Neuropathies. Contin. Lifelong Learn. Neurol. 2014, 20, 1241–1260. [Google Scholar] [CrossRef] [PubMed]
- Delanoy, N.; Michot, J.-M.; Comont, T.; Kramkimel, N.; Lazarovici, J.; Dupont, R.; Champiat, S.; Chahine, C.; Robert, C.; Herbaux, C.; et al. Haematological Immune-Related Adverse Events Induced by Anti-PD-1 or Anti-PD-L1 Immunotherapy: A Descriptive Observational Study. Lancet Haematol. 2019, 6, e48–e57. [Google Scholar] [CrossRef]
- Shiuan, E.; Beckermann, K.E.; Ozgun, A.; Kelly, C.; McKean, M.; McQuade, J.; Thompson, M.A.; Puzanov, I.; Greer, J.P.; Rapisuwon, S.; et al. Thrombocytopenia in Patients with Melanoma Receiving Immune Checkpoint Inhibitor Therapy. J. Immunother. Cancer 2017, 5, 8. [Google Scholar] [CrossRef]
- Omar, N.E.; El-Fass, K.A.; Abushouk, A.I.; Elbaghdady, N.; Barakat, A.E.M.; Noreldin, A.E.; Johar, D.; Yassin, M.; Hamad, A.; Elazzazy, S.; et al. Diagnosis and Management of Hematological Adverse Events Induced by Immune Checkpoint Inhibitors: A Systematic Review. Front. Immunol. 2020, 11, 1354. [Google Scholar] [CrossRef]
- Davis, E.J.; Salem, J.-E.; Young, A.; Green, J.R.; Ferrell, P.B.; Ancell, K.K.; Lebrun-Vignes, B.; Moslehi, J.J.; Johnson, D.B. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 2019, 24, 584–588. [Google Scholar] [CrossRef]
- Khellaf, M.; Michel, M.; Schaeffer, A.; Bierling, P.; Godeau, B. Assessment of a Therapeutic Strategy for Adults with Severe Autoimmune Thrombocytopenic Purpura Based on a Bleeding Score Rather than Platelet Count. Haematologica 2005, 90, 829–832. [Google Scholar]
- Algaze, S.D.; Park, W.; Harrington, T.J.; Mudad, R. Autoimmune Haemolytic Anaemia in a Patient with Advanced Lung Adenocarcinoma and Chronic Lymphocytic Leukaemia Receiving Nivolumab and Intravenous Immunoglobulin. BMJ Case Rep. 2018, 2018, bcr-2017-221801. [Google Scholar] [CrossRef]
- Go, R.S.; Winters, J.L.; Kay, N.E. How I Treat Autoimmune Hemolytic Anemia. Blood 2017, 129, 2971–2979. [Google Scholar] [CrossRef]
- Michot, J.M.; Lazarovici, J.; Tieu, A.; Champiat, S.; Voisin, A.L.; Ebbo, M.; Godeau, B.; Michel, M.; Ribrag, V.; Lambotte, O. Haematological Immune-Related Adverse Events with Immune Checkpoint Inhibitors, How to Manage? Eur. J. Cancer 2019, 122, 72–90. [Google Scholar] [CrossRef]
- Ramahi, A.; Chan, K.H.; Lim, S.L.; Shaaban, H.S. Cryoglobulinemia Unmasked by Nivolumab in a Patient with Hepatitis C-Induced Hepatocellular Carcinoma: A Case Report and Literature Review. Int. J. Crit. Illn. Inj. Sci. 2021, 11, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, B.; Musolino, A.; Tiseo, M. Anti-PD-1-Related Cryoglobulinemia during Treatment with Nivolumab in NSCLC Patient. Ann. Oncol. 2017, 28, 1405–1406. [Google Scholar] [CrossRef]
- Le Burel, S.; Champiat, S.; Routier, E.; Aspeslagh, S.; Albiges, L.; Szwebel, T.-A.; Michot, J.-M.; Chretien, P.; Mariette, X.; Voisin, A.-L.; et al. Onset of Connective Tissue Disease Following Anti-PD1/PD-L1 Cancer Immunotherapy. Ann. Rheum. Dis. 2018, 77, 468–470. [Google Scholar] [CrossRef]
- Grayson, P.C.; Sloan, J.M.; Niles, J.L.; Monach, P.A.; Merkel, P.A. Antineutrophil Cytoplasmic Antibodies, Autoimmune Neutropenia, and Vasculitis. Semin. Arthritis Rheum. 2011, 41, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wright, Z.; Brown, A. High-Grade Neutropenia in a Patient Successfully Treated with Nivolumab for Refractory Primary Mediastinal B-Cell Lymphoma. Blood Adv. 2017, 1, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, M.; Waller, E.K.; Jaye, D.L.; Lawson, D.H.; Ibrahim, R.; Papadopoulos, N.E.; Arellano, M.L. Neutropenia in a Patient Treated With Ipilimumab (Anti–CTLA-4 Antibody). J. Immunother. 2009, 32, 322–324. [Google Scholar] [CrossRef]
- Turgeman, I.; Wollner, M.; Hassoun, G.; Bonstein, L.; Bar-Sela, G. Severe Complicated Neutropenia in Two Patients with Metastatic Non-Small-Cell Lung Cancer Treated with Nivolumab. Anticancer Drugs 2017, 28, 811–814. [Google Scholar] [CrossRef]
- Killick, S.B.; Bown, N.; Cavenagh, J.; Dokal, I.; Foukaneli, T.; Hill, A.; Hillmen, P.; Ireland, R.; Kulasekararaj, A.; Mufti, G.; et al. Guidelines for the Diagnosis and Management of Adult Aplastic Anaemia. Br. J. Haematol. 2016, 172, 187–207. [Google Scholar] [CrossRef]
- Gordon, I.O.; Wade, T.; Chin, K.; Dickstein, J.; Gajewski, T.F. Immune-Mediated Red Cell Aplasia after Anti-CTLA-4 Immunotherapy for Metastatic Melanoma. Cancer Immunol. Immunother. 2009, 58, 1351–1353. [Google Scholar] [CrossRef]
- Nair, R.; Gheith, S.; Nair, S.G. Immunotherapy-Associated Hemolytic Anemia with Pure Red-Cell Aplasia. N. Engl. J. Med. 2016, 374, 1096–1097. [Google Scholar] [CrossRef]
- Yuki, A.; Takenouchi, T.; Takatsuka, S.; Ishiguro, T. A Case of Pure Red Cell Aplasia during Nivolumab Therapy for Cardiac Metastatic Melanoma. Melanoma Res. 2017, 27, 635–637. [Google Scholar] [CrossRef]
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.M.; Routier, E.; Robert, C. Experience in Daily Practice with Ipilimumab for the Treatment of Patients with Metastatic Melanoma: An Early Increase in Lymphocyte and Eosinophil Counts Is Associated with Improved Survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef]
- Gaba, L.; Victoria, I.; Pineda, E.; Fernandez, A.; Aya, F.; Prat, A.; Arance, A.M. Changes in Blood Eosinophilia during Anti-PD1 Therapy as a Predictor of Long Term Disease Control in Metastatic Melanoma. J. Clin. Oncol. 2015, 33 (Suppl. S15), 9069. [Google Scholar] [CrossRef]
- Lou, Y.; Marin-Acevedo, J.A.; Vishnu, P.; Manochakian, R.; Dholaria, B.; Soyano, A.; Luo, Y.; Zhang, Y.; Knutson, K.L. Hypereosinophilia in a Patient with Metastatic Non-Small-Cell Lung Cancer Treated with Antiprogrammed Cell Death 1 (Anti-PD-1) Therapy. Immunotherapy 2019, 11, 577–584. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-Related Adverse Events with Immune Checkpoint Blockade: A Comprehensive Review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Bernard-Tessier, A.; Jeanville, P.; Champiat, S.; Lazarovici, J.; Voisin, A.-L.; Mateus, C.; Lambotte, O.; Annereau, M.; Michot, J.-M. Immune-Related Eosinophilia Induced by Anti-Programmed Death 1 or Death-Ligand 1 Antibodies. Eur. J. Cancer 2017, 81, 135–137. [Google Scholar] [CrossRef]
- Helgadottir, H.; Kis, L.; Ljungman, P.; Larkin, J.; Kefford, R.; Ascierto, P.A.; Hansson, J.; Masucci, G. Lethal Aplastic Anemia Caused by Dual Immune Checkpoint Blockade in Metastatic Melanoma. Ann. Oncol. 2017, 28, 1672–1673. [Google Scholar] [CrossRef]
- Townsley, D.M.; Scheinberg, P.; Winkler, T.; Desmond, R.; Dumitriu, B.; Rios, O.; Weinstein, B.; Valdez, J.; Lotter, J.; Feng, X.; et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N. Engl. J. Med. 2017, 376, 1540–1550. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult Haemophagocytic Syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Shah, D.; Shrestha, R.; Ramlal, R.; Hatton, J.; Saeed, H. Pembrolizumab Associated Hemophagocytic Lymphohistiocytosis. Ann. Oncol. 2017, 28, 1403. [Google Scholar] [CrossRef]
- Kurozumi, A.; Takahashi, H.; Watanabe, T.; Iwasaki, Y. Two Cases of Lung Cancer with Hemophagocytic Lymphohistiocytosis Caused by Immune Checkpoint Inhibitors. Thorac. Cancer 2021, 12, 1625–1628. [Google Scholar] [CrossRef]
- Takahashi, H.; Koiwa, T.; Fujita, A.; Suzuki, T.; Tagashira, A.; Iwasaki, Y. A Case of Pembrolizumab-Induced Hemophagocytic Lymphohistiocytosis Successfully Treated with Pulse Glucocorticoid Therapy. Respir. Med. Case Rep. 2020, 30, 101097. [Google Scholar] [CrossRef]
- Noseda, R.; Bertoli, R.; Müller, L.; Ceschi, A. Haemophagocytic Lymphohistiocytosis in Patients Treated with Immune Checkpoint Inhibitors: Analysis of WHO Global Database of Individual Case Safety Reports. J. Immunother. Cancer 2019, 7, 117. [Google Scholar] [CrossRef]
- Mizuta, H.; Nakano, E.; Takahashi, A.; Koyama, T.; Namikawa, K.; Yamazaki, N. Hemophagocytic Lymphohistiocytosis with Advanced Malignant Melanoma Accompanied by Ipilimumab and Nivolumab: A Case Report and Literature Review. Dermatol. Ther. 2020, 33, e13321. [Google Scholar] [CrossRef]
- Lorenz, G.; Schul, L.; Bachmann, Q.; Angermann, S.; Slotta-Huspenina, J.; Heemann, U.; Küchle, C.; Schmaderer, C.; Jäger, M.; Tauber, R.; et al. Hemophagocytic Lymphohistiocytosis Secondary to Pembrolizumab Treatment with Insufficient Response to High-Dose Steroids. Rheumatology 2019, 58, 1106–1109. [Google Scholar] [CrossRef]
- Olivares-Hernández, A.; Figuero-Pérez, L.; Amores Martín, M.A.; Bellido Hernández, L.; Mezquita, L.; Vidal Tocino, M.D.R.; López Cadenas, F.; Gómez-Caminero López, F.; Escala-Cornejo, R.A.; Cruz Hernández, J.J. Response to Treatment with an Anti-Interleukin-6 Receptor Antibody (Tocilizumab) in a Patient with Hemophagocytic Syndrome Secondary to Immune Checkpoint Inhibitors. Case Rep. Oncol. Med. 2021, 2021, 6631859. [Google Scholar] [CrossRef]
- Torres-Jiménez, J.; Esteban-Villarrubia, J.; García-Abellás, P.; Cortés-Salgado, A.; Soria-Rivas, A.; Gajate-Borau, P.; Olmedo-García, M.E.; Corral-de la Fuente, E.; Lage-Alfranca, Y.; Gómez-Rueda, A.; et al. Sarcoidosis-like Reactions in Cancer Patients Treated with Immune Checkpoint Inhibitors: Experience in a Spanish Hospital. Clin. Transl. Oncol. 2021, 23, 1474–1480. [Google Scholar] [CrossRef]
- Le Burel, S.; Champiat, S.; Mateus, C.; Marabelle, A.; Michot, J.-M.; Robert, C.; Belkhir, R.; Soria, J.-C.; Laghouati, S.; Voisin, A.-L.; et al. Prevalence of Immune-Related Systemic Adverse Events in Patients Treated with Anti-Programmed Cell Death 1/Anti-Programmed Cell Death-Ligand 1 Agents: A Single-Centre Pharmacovigilance Database Analysis. Eur. J. Cancer 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Chopra, A.; Nautiyal, A.; Kalkanis, A.; Judson, M.A. Drug-Induced Sarcoidosis-Like Reactions. Chest 2018, 154, 664–677. [Google Scholar] [CrossRef]
- Thajudeen, B.; Madhrira, M.; Bracamonte, E.; Cranmer, L.D. Ipilimumab Granulomatous Interstitial Nephritis. Am. J. Ther. 2015, 22, e84–e87. [Google Scholar] [CrossRef]
- Reuss, J.E.; Kunk, P.R.; Stowman, A.M.; Gru, A.A.; Slingluff, C.L.; Gaughan, E.M. Sarcoidosis in the Setting of Combination Ipilimumab and Nivolumab Immunotherapy: A Case Report & Review of the Literature. J. Immunother. Cancer 2016, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Danlos, F.-X.; Pagès, C.; Baroudjian, B.; Vercellino, L.; Battistella, M.; Mimoun, M.; Jebali, M.; Bagot, M.; Tazi, A.; Lebbé, C. Nivolumab-Induced Sarcoid-Like Granulomatous Reaction in a Patient With Advanced Melanoma. Chest 2016, 149, e133–e136. [Google Scholar] [CrossRef] [PubMed]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- on behalf of the Society for Immunotherapy of Cancer Toxicity Management Working Group; Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Mateus, C.; Lambert, T. Hemophilia A Induced by Ipilimumab. N. Engl. J. Med. 2011, 365, 1747–1748. [Google Scholar] [CrossRef]
- Kato, R.; Hayashi, H.; Sano, K.; Handa, K.; Kumode, T.; Ueda, H.; Okuno, T.; Kawakami, H.; Matsumura, I.; Kudo, M.; et al. Nivolumab-Induced Hemophilia A Presenting as Gastric Ulcer Bleeding in a Patient With NSCLC. J. Thorac. Oncol. 2018, 13, e239–e241. [Google Scholar] [CrossRef]
- Gokozan, H.N.; Friedman, J.D.; Schmaier, A.H.; Downes, K.A.; Farah, L.A.; Reeves, H.M. Acquired Hemophilia A After Nivolumab Therapy in a Patient With Metastatic Squamous Cell Carcinoma of the Lung Successfully Managed With Rituximab. Clin. Lung Cancer 2019, 20, e560–e563. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Elia, G.; Ragusa, F.; Ruffilli, I.; Patrizio, A.; Galdiero, M.R.; Baldini, E.; Ulisse, S.; Marone, G.; et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int. J. Mol. Sci. 2019, 20, 2560. [Google Scholar] [CrossRef]
- Chang, L.-S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef]
- Deligiorgi, M.V.; Sagredou, S.; Vakkas, L.; Trafalis, D.T. The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries. Cancers 2021, 13, 5277. [Google Scholar] [CrossRef] [PubMed]
- Coniac, S.; Stoian, M. Updates In Endocrine Immune-Related Adverse Events in Oncology Immunotherapy. Acta Endocrinol. Buchar. 2021, 17, 286–289. [Google Scholar] [CrossRef]
- Quandt, Z.; Young, A.; Anderson, M. Immune Checkpoint Inhibitor Diabetes Mellitus: A Novel Form of Autoimmune Diabetes. Clin. Exp. Immunol. 2020, 200, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Imagawa, A.; Hosokawa, Y.; Baden, M.Y.; Kimura, T.; Uno, S.; Fukui, K.; Goto, K.; Uemura, M.; Eguchi, H.; et al. T-Lymphocyte Infiltration to Islets in the Pancreas of a Patient Who Developed Type 1 Diabetes After Administration of Immune Checkpoint Inhibitors. Diabetes Care 2019, 42, e116–e118. [Google Scholar] [CrossRef]
- Mellati, M.; Eaton, K.D.; Brooks-Worrell, B.M.; Hagopian, W.A.; Martins, R.; Palmer, J.P.; Hirsch, I.B. Anti–PD-1 and Anti–PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care 2015, 38, e137–e138. [Google Scholar] [CrossRef]
- For the consultation of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research; Baden, M.Y.; Imagawa, A.; Abiru, N.; Awata, T.; Ikegami, H.; Uchigata, Y.; Oikawa, Y.; Osawa, H.; Kajio, H.; et al. Characteristics and Clinical Course of Type 1 Diabetes Mellitus Related to Anti-Programmed Cell Death-1 Therapy. Diabetol. Int. 2019, 10, 58–66. [Google Scholar] [CrossRef]
- De Filette, J.M.K.; Pen, J.J.; Decoster, L.; Vissers, T.; Bravenboer, B.; Van der Auwera, B.J.; Gorus, F.K.; Roep, B.O.; Aspeslagh, S.; Neyns, B.; et al. Immune Checkpoint Inhibitors and Type 1 Diabetes Mellitus: A Case Report and Systematic Review. Eur. J. Endocrinol. 2019, 181, 363–374. [Google Scholar] [CrossRef]
- Imagawa, A.; Hanafusa, T.; Awata, T.; Ikegami, H.; Uchigata, Y.; Osawa, H.; Kawasaki, E.; Kawabata, Y.; Kobayashi, T.; Shimada, A.; et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-Onset Type 1 Diabetes Mellitus: New Diagnostic Criteria of Fulminant Type 1 Diabetes Mellitus (2012). J. Diabetes Investig. 2012, 3, 536–539. [Google Scholar] [CrossRef]
- George, A.S.; Fernandez, C.J.; Eapen, D.; Pappachan, J.M. Organ-Specific Adverse Events of Immune Checkpoint Inhibitor Therapy, with Special Reference to Endocrinopathies. TouchREVIEWS Endocrinol. 2021, 17, 21–32. [Google Scholar] [CrossRef]
- Stelmachowska-Banaś, M.; Czajka-Oraniec, I. Management of Endocrine Immune-Related Adverse Events of Immune Checkpoint Inhibitors: An Updated Review. Endocr. Connect. 2020, 9, R207–R228. [Google Scholar] [CrossRef]
- Nogueira, E.; Newsom-Davis, T.; Morganstein, D.L. Immunotherapy-Induced Endocrinopathies: Assessment, Management and Monitoring. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819896182. [Google Scholar] [CrossRef] [PubMed]
- Umeguchi, H.; Takenoshita, H.; Inoue, H.; Kurihara, Y.; Sakaguchi, C.; Yano, S.; Hasuzawa, N.; Sakamoto, S.; Sakamoto, R.; Ashida, K. Autoimmune-Related Primary Hypoparathyroidism Possibly Induced by the Administration of Pembrolizumab: A Case Report. J. Oncol. Pract. 2018, 14, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Win, M.A.; Thein, K.Z.; Qdaisat, A.; Yeung, S.-C.J. Acute Symptomatic Hypocalcemia from Immune Checkpoint Therapy-Induced Hypoparathyroidism. Am. J. Emerg. Med. 2017, 35, 1039.e5–1039.e79. [Google Scholar] [CrossRef]
- Mahmood, I.; Kuhadiya, N.D.; Gonzalaes, M. Pembrolizumab-Associated Hypoparathyroidism: A Single Case Report. AACE Clin. Case Rep. 2020, 7, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Shoback, D.M.; Bilezikian, J.P.; Costa, A.G.; Dempster, D.; Dralle, H.; Khan, A.A.; Peacock, M.; Raffaelli, M.; Silva, B.C.; Thakker, R.V.; et al. Presentation of Hypoparathyroidism: Etiologies and Clinical Features. J. Clin. Endocrinol. Metab. 2016, 101, 2300–2312. [Google Scholar] [CrossRef]
- Trinh, B.; Sanchez, G.O.; Herzig, P.; Läubli, H. Inflammation-Induced Hypoparathyroidism Triggered by Combination Immune Checkpoint Blockade for Melanoma. J. Immunother. Cancer 2019, 7, 52. [Google Scholar] [CrossRef]
- Piranavan, P.; Li, Y.; Brown, E.; Kemp, E.H.; Trivedi, N. Immune Checkpoint Inhibitor-Induced Hypoparathyroidism Associated With Calcium-Sensing Receptor-Activating Autoantibodies. J. Clin. Endocrinol. Metab. 2019, 104, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Lupi, I.; Brancatella, A.; Cetani, F.; Latrofa, F.; Kemp, E.H.; Marcocci, C. Activating Antibodies to The Calcium-Sensing Receptor in Immunotherapy-Induced Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1581–1588. [Google Scholar] [CrossRef]
- El Kawkgi, O.M.; Li, D.; Kotwal, A.; Wermers, R.A. Hypoparathyroidism: An Uncommon Complication Associated With Immune Checkpoint Inhibitor Therapy. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 821–825. [Google Scholar] [CrossRef]
- Gafni, R.I.; Collins, M.T. Hypoparathyroidism. N. Engl. J. Med. 2019, 380, 1738–1747. [Google Scholar] [CrossRef]
- Nalluru, S.S.; Piranavan, P.; Ning, Y.; Ackula, H.; Siddiqui, A.D.; Trivedi, N. Hypocalcemia with Immune Checkpoint Inhibitors: The Disparity among Various Reports. Int. J. Endocrinol. 2020, 2020, 7459268. [Google Scholar] [CrossRef] [PubMed]
- Dadu, R.; Rodgers, T.E.; Trinh, V.A.; Kemp, E.H.; Cubb, T.D.; Patel, S.; Simon, J.M.; Burton, E.M.; Tawbi, H. Calcium-Sensing Receptor Autoantibody-Mediated Hypoparathyroidism Associated with Immune Checkpoint Inhibitor Therapy: Diagnosis and Long-Term Follow-Up. J. Immunother. Cancer 2020, 8, e000687. [Google Scholar] [CrossRef] [PubMed]
- Dillard, T.; Yedinak, C.G.; Alumkal, J.; Fleseriu, M. Anti-CTLA-4 Antibody Therapy Associated Autoimmune Hypophysitis: Serious Immune Related Adverse Events across a Spectrum of Cancer Subtypes. Pituitary 2010, 13, 29–38. [Google Scholar] [CrossRef]
- Lythgoe, H.; Baildam, E.; Beresford, M.W.; Cleary, G.; McCann, L.J.; Pain, C.E. Tocilizumab as a Potential Therapeutic Option for Children with Severe, Refractory Juvenile Localized Scleroderma. Rheumatology 2018, 57, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, A.; Strigari, L.; Corsello, A.; Paragliola, R.M.; Iannantuono, G.M.; Salvatori, R.; Corsello, S.M.; Torino, F. Grading Central Diabetes Insipidus Induced by Immune Checkpoint Inhibitors: A Challenging Task. Front. Endocrinol. 2022, 13, 840971. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, A.; Strigari, L.; Corsello, A.; Paragliola, R.M.; Falzone, L.; Salvatori, R.; Corsello, S.M.; Torino, F. Immune Checkpoint Inhibitor-Induced Central Diabetes Insipidus: Looking for the Needle in the Haystack or a Very Rare Side-Effect to Promptly Diagnose? Front. Oncol. 2022, 12, 798517. [Google Scholar] [CrossRef]
- Nallapaneni, N.N.; Mourya, R.; Bhatt, V.R.; Malhotra, S.; Ganti, A.K.; Tendulkar, K.K. Ipilimumab-Induced Hypophysitis and Uveitis in a Patient With Metastatic Melanoma and a History of Ipilimumab-Induced Skin Rash. J. Natl. Compr. Canc. Netw. 2014, 12, 1077–1081. [Google Scholar] [CrossRef]
- Tan, M.H.; Iyengar, R.; Mizokami-Stout, K.; Yentz, S.; MacEachern, M.P.; Shen, L.Y.; Redman, B.; Gianchandani, R. Spectrum of Immune Checkpoint Inhibitors-Induced Endocrinopathies in Cancer Patients: A Scoping Review of Case Reports. Clin. Diabetes Endocrinol. 2019, 5, 1. [Google Scholar] [CrossRef]
- Zhao, C.; Tella, S.H.; Del Rivero, J.; Kommalapati, A.; Ebenuwa, I.; Gulley, J.; Strauss, J.; Brownell, I. Anti–PD-L1 Treatment Induced Central Diabetes Insipidus. J. Clin. Endocrinol. Metab. 2017, 103, 365–369. [Google Scholar] [CrossRef]
- Tshuma, N.; Glynn, N.; Evanson, J.; Powles, T.; Drake, W.M. Hypothalamitis and Severe Hypothalamic Dysfunction Associated with Anti–Programmed Cell Death Ligand 1 Antibody Treatment. Eur. J. Cancer 2018, 104, 247–249. [Google Scholar] [CrossRef]
- Lupu, J.; Pages, C.; Laly, P.; Delyon, J.; Laloi, M.; Petit, A.; Basset-Seguin, N.; Oueslati, I.; Zagdanski, A.-M.; Young, J.; et al. Transient Pituitary ACTH-Dependent Cushing Syndrome Caused by an Immune Checkpoint Inhibitor Combination. Melanoma Res. 2017, 27, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C. Immune Checkpoint Inhibitor-Related Hypogonadism and Infertility: A Neglected Issue in Immuno-Oncology. J. Immunother. Cancer 2021, 9, e002220. [Google Scholar] [CrossRef] [PubMed]
- Garon-Czmil, J.; Petitpain, N.; Rouby, F.; Sassier, M.; Babai, S.; Yéléhé-Okouma, M.; Weryha, G.; Klein, M.; Gillet, P. Immune Check Point Inhibitors-Induced Hypophysitis: A Retrospective Analysis of the French Pharmacovigilance Database. Sci. Rep. 2019, 9, 19419. [Google Scholar] [CrossRef] [PubMed]
- Brunet-Possenti, F.; Opsomer, M.A.; Gomez, L.; Ouzaid, I.; Descamps, V. Immune Checkpoint Inhibitors-Related Orchitis. Ann. Oncol. 2017, 28, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Quach, H.T.; Robbins, C.J.; Balko, J.M.; Chiu, C.Y.; Miller, S.; Wilson, M.R.; Nelson, G.E.; Johnson, D.B. Severe Epididymo-Orchitis and Encephalitis Complicating Anti-PD-1 Therapy. Oncologist 2019, 24, 872–876. [Google Scholar] [CrossRef]
- Scovell, J.M.; Benz, K.; Samarska, I.; Kohn, T.P.; Hooper, J.E.; Matoso, A.; Herati, A.S. Association of Impaired Spermatogenesis With the Use of Immune Checkpoint Inhibitors in Patients With Metastatic Melanoma. JAMA Oncol. 2020, 6, 1297. [Google Scholar] [CrossRef]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous Adverse Effects of the Immune Checkpoint Inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Saw, S.; Lee, H.Y.; Ng, Q.S. Pembrolizumab-Induced Stevens–Johnson Syndrome in Non-Melanoma Patients. Eur. J. Cancer 2017, 81, 237–239. [Google Scholar] [CrossRef]
- Cao, J.; Li, Q.; Zhi, X.; Yang, F.; Zhu, W.; Zhou, T.; Hou, X.; Chen, D. Pembrolizumab-Induced Autoimmune Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis with Myositis and Myocarditis in a Patient with Esophagogastric Junction Carcinoma: A Case Report. Transl. Cancer Res. 2021, 10, 3870–3876. [Google Scholar] [CrossRef]
- Choudhary, S.; McLeod, M.; Torchia, D.; Romanelli, P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J. Clin. Aesthetic Dermatol. 2013, 6, 31–37. [Google Scholar]
- Mirza, S.; Hill, E.; Ludlow, S.P.; Nanjappa, S. Checkpoint Inhibitor-Associated Drug Reaction with Eosinophilia and Systemic Symptom Syndrome. Melanoma Res. 2017, 27, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Berking, C.; Bergmann, T.; Bockmeyer, C.L.; Eigentler, T.; Fluck, M.; et al. The Price of Tumor Control: An Analysis of Rare Side Effects of Anti-CTLA-4 Therapy in Metastatic Melanoma from the Ipilimumab Network. PLoS ONE 2013, 8, e53745. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Thuraisingam, T.; Chergui, M.; Nguyen, K. Nivolumab-Associated DRESS Syndrome: A Case Report. JAAD Case Rep. 2019, 5, 216–218. [Google Scholar] [CrossRef][Green Version]
- Tetzlaff, M.T.; Jazaeri, A.A.; Torres-Cabala, C.A.; Korivi, B.R.; Landon, G.A.; Nagarajan, P.; Choksi, A.; Chen, L.; Uemura, M.; Aung, P.P.; et al. Erythema Nodosum-like Panniculitis Mimicking Disease Recurrence: A Novel Toxicity from Immune Checkpoint Blockade Therapy-Report of 2 Patients. J. Cutan. Pathol. 2017, 44, 1080–1086. [Google Scholar] [CrossRef]
- Pach, J.; Moody, K.; Ring, N.; Panse, G.; Zhang, M.; Deverapalli, S.; Leventhal, J. Erythema Nodosum-like Panniculitis Associated with Immune Checkpoint Inhibitor Therapy: Two Cases Reporting a Rare Cutaneous Adverse Event. JAAD Case Rep. 2021, 13, 118–120. [Google Scholar] [CrossRef]
- Munoz, J.; Guillot, B.; Girard, C.; Dereure, O.; Du-Thanh, A. First Report of Ipilimumab-induced G Rover Disease. Br. J. Dermatol. 2014, 171, 1236–1237. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Buser, T.; Willi, N.; Rothschild, S.I.; Wicki, A.; Schiller, P.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Grover’s-like Drug Eruption in a Patient with Metastatic Melanoma under Ipilimumab Therapy. J. Immunother. Cancer 2016, 4, 47. [Google Scholar] [CrossRef][Green Version]
- Uemura, M.; Faˈak, F.; Haymaker, C.; McQuail, N.; Sirmans, E.; Hudgens, C.W.; Barbara, L.; Bernatchez, C.; Curry, J.L.; Hwu, P.; et al. A Case Report of Grover’s Disease from Immunotherapy-a Skin Toxicity Induced by Inhibition of CTLA-4 but Not PD-1. J. Immunother. Cancer 2016, 4, 55. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Gutierrez, A.K.; Baer, A.N.; Albayda, J.; Manno, R.L.; Haque, U.; Lipson, E.J.; Bleich, K.B.; Shah, A.A.; Naidoo, J.; et al. Inflammatory Arthritis and Sicca Syndrome Induced by Nivolumab and Ipilimumab. Ann. Rheum. Dis. 2017, 76, 43–50. [Google Scholar] [CrossRef]
- Shenoy, N.; Esplin, B.; Barbosa, N.; Wieland, C.; Thanarajasingam, U.; Markovic, S. Pembrolizumab Induced Severe Sclerodermoid Reaction. Ann. Oncol. 2017, 28, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.L.; Tetzlaff, M.T.; Nagarajan, P.; Drucker, C.; Diab, A.; Hymes, S.R.; Duvic, M.; Hwu, W.-J.; Wargo, J.A.; Torres-Cabala, C.A.; et al. Diverse Types of Dermatologic Toxicities from Immune Checkpoint Blockade Therapy: Dermatologic Toxicities from Immune Checkpoint Blockade. J. Cutan. Pathol. 2017, 44, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Freites-Martinez, A.; Kwong, B.Y.; Rieger, K.E.; Coit, D.G.; Colevas, A.D.; Lacouture, M.E. Eruptive Keratoacanthomas Associated With Pembrolizumab Therapy. JAMA Dermatol. 2017, 153, 694. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Sharma, R.; Fernandez-Penas, P. Ipilimumab-Induced Acute Generalized Exanthematous Pustulosis in a Patient with Metastatic Melanoma. Melanoma Res. 2016, 26, 417–420. [Google Scholar] [CrossRef]
- Gormley, R.; Wanat, K.; Elenitsas, R.; Giles, J.; McGettigan, S.; Schuchter, L.; Takeshita, J. Ipilimumab-Associated Sweet Syndrome in a Melanoma Patient. J. Am. Acad. Dermatol. 2014, 71, e211–e213. [Google Scholar] [CrossRef]
- Kyllo, R.L.; Parker, M.K.; Rosman, I.; Musiek, A.C. Ipilimumab-Associated Sweet Syndrome in a Patient with High-Risk Melanoma. J. Am. Acad. Dermatol. 2014, 70, e85–e86. [Google Scholar] [CrossRef]
- Pintova, S.; Sidhu, H.; Friedlander, P.A.; Holcombe, R.F. Sweet’s Syndrome in a Patient with Metastatic Melanoma after Ipilimumab Therapy. Melanoma Res. 2013, 23, 498–501. [Google Scholar] [CrossRef]
- Gambichler, T.; Strutzmann, S.; Tannapfel, A.; Susok, L. Paraneoplastic Acral Vascular Syndrome in a Patient with Metastatic Melanoma under Immune Checkpoint Blockade. BMC Cancer 2017, 17, 327. [Google Scholar] [CrossRef]
- Bastuji-Garin, S.; Rzany, B.; Stern, R.S.; Shear, N.H.; Naldi, L.; Roujeau, J.C. Clinical Classification of Cases of Toxic Epidermal Necrolysis, Stevens-Johnson Syndrome, and Erythema Multiforme. Arch. Dermatol. 1993, 129, 92–96. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, G.; He, Z.; Zheng, Y.; Gao, S.; Li, J.; Ling, Y.; Yu, X.; Qiu, K.; Wu, J. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis in Patients Treated with Immune Checkpoint Inhibitors: A Safety Analysis of Clinical Trials and FDA Pharmacovigilance Database. eClinicalMedicine 2021, 37, 100951. [Google Scholar] [CrossRef]
- Vivar, K.L.; Deschaine, M.; Messina, J.; Divine, J.M.; Rabionet, A.; Patel, N.; Harrington, M.A.; Seminario-Vidal, L. Epidermal Programmed Cell Death-ligand 1 Expression in TEN Associated with Nivolumab Therapy. J. Cutan. Pathol. 2017, 44, 381–384. [Google Scholar] [CrossRef]
- Hsu, D.Y.; Brieva, J.; Silverberg, N.B.; Silverberg, J.I. Morbidity and Mortality of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J. Investig. Dermatol. 2016, 136, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Chodosh, J.; Fathy, C.; Goverman, J.; Mitchell, C.; Saeed, H.N. Multidisciplinary Care in Stevens-Johnson Syndrome. Ther. Adv. Chronic Dis. 2020, 11, 204062231989446. [Google Scholar] [CrossRef]
- Maity, S.; Banerjee, I.; Sinha, R.; Jha, H.; Ghosh, P.; Mustafi, S. Nikolsky’s Sign: A Pathognomic Boon. J. Fam. Med. Prim. Care 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A.; McDonough, P.H.; Lee, B.W. Toxic Epidermal Necrolysis. Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J. Am. Acad. Dermatol. 2013, 69, 173.e1–173.e13. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A.; McDonough, P.H.; Lee, B.W. Toxic Epidermal Necrolysis. Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J. Am. Acad. Dermatol. 2013, 69, 187.e1–187.e16. [Google Scholar] [CrossRef]
- Goldinger, S.M.; Stieger, P.; Meier, B.; Micaletto, S.; Contassot, E.; French, L.E.; Dummer, R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin. Cancer Res. 2016, 22, 4023–4029. [Google Scholar] [CrossRef]
- Molina, G.E.; Yu, Z.; Foreman, R.K.; Reynolds, K.L.; Chen, S.T. Generalized Bullous Mucocutaneous Eruption Mimicking Stevens-Johnson Syndrome in the Setting of Immune Checkpoint Inhibition: A Multicenter Case Series. J. Am. Acad. Dermatol. 2020, 83, 1475–1477. [Google Scholar] [CrossRef]
- Kardaun, S.H.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.B.; Mockenhaupt, M.; Roujeau, J.C. Variability in the Clinical Pattern of Cutaneous Side-Effects of Drugs with Systemic Symptoms: Does a DRESS Syndrome Really Exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Original Multisystem Adverse Drug Reaction. Results from the Prospective RegiSCAR Study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef]
- Pérez-Garza, D.M.; Chavez-Alvarez, S.; Ocampo-Candiani, J.; Gomez-Flores, M. Erythema Nodosum: A Practical Approach and Diagnostic Algorithm. Am. J. Clin. Dermatol. 2021, 22, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Leong, K.F.; Lam, J.M. Erythema Nodosum. World J. Pediatr. 2018, 14, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Shiomi, H.; Fujiwara, A.; Yoshihara, K.; Yoshioka, R.; Kawata, S.; Ota, S.; Yuri, Y.; Takashima, T.; Aizawa, N.; et al. Clinical Characteristics of ICI-Related Pancreatitis and Cholangitis Including Radiographic and Endoscopic Findings. Healthcare 2022, 10, 763. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Gentile, N.M.; D’Souza, A.; Fujii, L.L.; Wu, T.-T.; Murray, J.A. Association between Ipilimumab and Celiac Disease. Mayo Clin. Proc. 2013, 88, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.; Hoibian, S.; Boucraut, A.; Ratone, J.-P.; Stoffaes, L.; Dano, D.; Louvel-Perrot, D.; Chanez, B.; Chretien, A.-S.; Madroszyk, A.; et al. Celiac Disease After Administration of Immune Checkpoint Inhibitors: A Case Report. Front. Immunol. 2021, 12, 799666. [Google Scholar] [CrossRef] [PubMed]
- Badran, Y.R.; Shih, A.; Leet, D.; Mooradian, M.J.; Coromilas, A.; Chen, J.; Kem, M.; Zheng, H.; Borowsky, J.; Misdraji, J.; et al. Immune Checkpoint Inhibitor-Associated Celiac Disease. J. Immunother. Cancer 2020, 8, e000958. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Benzvi, C. Checkpoint Inhibitors and Induction of Celiac Disease-like Condition. Biomedicines 2022, 10, 609. [Google Scholar] [CrossRef]
- Woodford, R.; Briscoe, K.; Tustin, R.; Jain, A. Immunotherapy-Related Gastritis: Two Case Reports and Literature Review. Clin. Med. Insights Oncol. 2021, 15, 11795549211028570. [Google Scholar] [CrossRef]
- Collins, M.; Michot, J.M.; Danlos, F.X.; Mussini, C.; Soularue, E.; Mateus, C.; Loirat, D.; Buisson, A.; Rosa, I.; Lambotte, O.; et al. Inflammatory Gastrointestinal Diseases Associated with PD-1 Blockade Antibodies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2860–2865. [Google Scholar] [CrossRef]
- Johncilla, M.; Grover, S.; Zhang, X.; Jain, D.; Srivastava, A. Morphological Spectrum of Immune Check-Point Inhibitor Therapy-Associated Gastritis. Histopathology 2020, 76, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Placke, J.-M.; Rawitzer, J.; Reis, H.; Rashidi-Alavijeh, J.; Livingstone, E.; Ugurel, S.; Hadaschik, E.; Griewank, K.; Schmid, K.W.; Schadendorf, D.; et al. Apoptotic Gastritis in Melanoma Patients Treated With PD-1-Based Immune Checkpoint Inhibition-Clinical and Histopathological Findings Including the Diagnostic Value of Anti-Caspase-3 Immunohistochemistry. Front. Oncol. 2021, 11, 725549. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, M.; Romero, F.A.; Argüello, E.; Kyi, C.; Postow, M.A.; Redelman-Sidi, G. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Sagvand, B.T.; Hwang, D.G.; Legesse, T.; Cross, R.K. Isolated Gastritis Secondary to Immune Checkpoint Inhibitors Complicated by Superimposed Cytomegalovirus Infection. ACG Case Rep. J. 2022, 9, e00747. [Google Scholar] [CrossRef]
- Aqel, N.M.; Tanner, P.; Drury, A.; Francis, N.D.; Henry, K. Cytomegalovirus Gastritis with Perforation and Gastrocolic Fistula Formation. Histopathology 1991, 18, 165–168. [Google Scholar] [CrossRef]
- Kawakami, H.; Tanizaki, J.; Tanaka, K.; Haratani, K.; Hayashi, H.; Takeda, M.; Kamata, K.; Takenaka, M.; Kimura, M.; Chikugo, T.; et al. Imaging and Clinicopathological Features of Nivolumab-Related Cholangitis in Patients with Non-Small Cell Lung Cancer. Investig. New Drugs 2017, 35, 529–536. [Google Scholar] [CrossRef]
- Kashima, J.; Okuma, Y.; Shimizuguchi, R.; Chiba, K. Bile Duct Obstruction in a Patient Treated with Nivolumab as Second-Line Chemotherapy for Advanced Non-Small-Cell Lung Cancer: A Case Report. Cancer Immunol. Immunother. CII 2018, 67, 61–65. [Google Scholar] [CrossRef]
- Koya, Y.; Shibata, M.; Shinohara, N.; Nebuya, S.; Oe, S.; Honma, Y.; Senju, M.; Sato, N.; Harada, M. Secondary Sclerosing Cholangitis with Hemobilia Induced by Pembrolizumab: Case Report and Review of Published Work. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019, 49, 950–956. [Google Scholar] [CrossRef]
- McClure, T.; Cui, W.; Asadi, K.; John, T.; Testro, A. Case of Nivolumab-Induced Sclerosing Cholangitis: Lessons from Long-Term Follow-Up. BMJ Open Gastroenterol. 2020, 7, e000487. [Google Scholar] [CrossRef]
- Sato, K.; Hayashi, M.; Abe, K.; Fujita, M.; Takahashi, A.; Ohira, H. Pembrolizumab-Induced Sclerosing Cholangitis in a Lung Adenocarcinoma Patient with a Remarkable Response to Chemotherapy: A Case Report. Clin. J. Gastroenterol. 2020, 13, 1310–1314. [Google Scholar] [CrossRef]
- Sawada, K.; Shonaka, T.; Nishikawa, Y.; Hasegawa, K.; Hayashi, H.; Hasebe, T.; Nakajima, S.; Ikuta, K.; Fujiya, M.; Furukawa, H.; et al. Successful Treatment of Nivolumab-Related Cholangitis with Prednisolone: A Case Report and Review of the Literature. Intern. Med. Tokyo Jpn. 2019, 58, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Onofrio, F.Q.; Hirschfield, G.M.; Gulamhusein, A.F. A Practical Review of Primary Biliary Cholangitis for the Gastroenterologist. Gastroenterol. Hepatol. 2019, 15, 145–154. [Google Scholar]
- Botros, M.; Sikaris, K.A. The de Ritis Ratio: The Test of Time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Wah-Suarez, M.I.; Danford, C.J.; Patwardhan, V.R.; Jiang, Z.G.; Bonder, A. Hyperlipidaemia in Primary Biliary Cholangitis: Treatment, Safety and Efficacy. Frontline Gastroenterol. 2019, 10, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Dawe, D.E. Nivolumab-Induced Secondary Sclerosing Cholangitis with Deterioration Despite Immunosuppression. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, e205–e206. [Google Scholar] [CrossRef] [PubMed]
- Ederhy, S.; Dolladille, C.; Thuny, F.; Alexandre, J.; Cohen, A. Takotsubo Syndrome in Patients with Cancer Treated with Immune Checkpoint Inhibitors: A New Adverse Cardiac Complication. Eur. J. Heart Fail. 2019, 21, 945–947. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef]
- De Almeida, D.V.P.; Gomes, J.R.; Haddad, F.J.; Buzaid, A.C. Immune-Mediated Pericarditis With Pericardial Tamponade During Nivolumab Therapy. J. Immunother. 2018, 41, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Nesfeder, J.; Elsensohn, A.N.; Thind, M.; Lennon, J.; Domsky, S. Pericardial Effusion with Tamponade Physiology Induced by Nivolumab. Int. J. Cardiol. 2016, 222, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Mirshahidi, H.; Nagaraj, G.; Hsueh, C.-T. Conservative Management of Nivolumab-Induced Pericardial Effusion: A Case Report and Review of Literature. Exp. Hematol. Oncol. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, I.; Wolf, I. Nivolumab-Induced Pericardial Tamponade: A Case Report and Discussion. Cardiology 2017, 136, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Drobni, Z.D.; Zafar, A.; Quinaglia, T.; Hartmann, S.; Gilman, H.K.; Raghu, V.K.; Gongora, C.; Sise, M.E.; Alvi, R.M.; et al. Pericardial Disease in Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002771. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Corrections to “Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv264–iv266. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Draibe, J.B.; García-Carro, C.; Martinez-Valenzuela, L.; Agraz, I.; Fulladosa, X.; Bolufer, M.; Tango, A.; Torras, J.; Soler, M.J. Acute Tubulointerstitial Nephritis Induced by Checkpoint Inhibitors versus Classical Acute Tubulointerstitial Nephritis: Are They the Same Disease? Clin. Kidney J. 2021, 14, 884–890. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological Features of Acute Kidney Injury Associated with Immune Checkpoint Inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-Programmed-Death-Receptor-1 Treatment with Pembrolizumab in Ipilimumab-Refractory Advanced Melanoma: A Randomised Dose-Comparison Cohort of a Phase 1 Trial. Lancet Lond. Engl. 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- He, X.; Tu, R.; Zeng, S.; He, Z.; Liu, S.; Fang, Y. Non-Bacterial Cystitis Secondary to Pembrolizumab: A Case Report and Review of the Literature. Curr. Probl. Cancer 2022, 46, 100863. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, B.; Geng, Z.; Geng, Q. Toripalimab: The First Domestic Anti-Tumor PD-1 Antibody in China. Front. Immunol. 2021, 12, 730666. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Stebbing, J.; Wang, Z.; Peng, L. Immunotherapy-Related Cystitis: Case Report and Review of the Literature. OncoTargets Ther. 2021, 14, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, W.; Yang, Y.; Xiao, Y.; Cui, Y.; Duan, J.; He, Q.; Jin, J.; Wu, S. Expression of Programmed Death Ligand-1 on Bladder Tissues Is Detected in a Clinically and Histologically Well-Defined Interstitial Cystitis Cohort. Neurourol. Urodyn. 2018, 37, 1396–1404. [Google Scholar] [CrossRef]
- Ueki, Y.; Matsuki, M.; Kubo, T.; Morita, R.; Hirohashi, Y.; Sato, S.; Horibe, R.; Matsuo, K.; Tsukahara, T.; Kanaseki, T.; et al. Non-Bacterial Cystitis with Increased Expression of Programmed Death-Ligand 1 in the Urothelium: An Unusual Immune-Related Adverse Event during Treatment with Pembrolizumab for Lung Adenocarcinoma. IJU Case Rep. 2020, 3, 266–269. [Google Scholar] [CrossRef]
- Bersanelli, M.; Santoni, M.; Ticinesi, A.; Buti, S. The Urinary Microbiome and Anticancer Immunotherapy: The Potentially Hidden Role of Unculturable Microbes. Target. Oncol. 2019, 14, 247–252. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A. Immune-Related Ocular Toxicities in Solid Tumor Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review. Expert Rev. Anticancer Ther. 2017, 17, 387–394. [Google Scholar] [CrossRef]
- Mori, S.; Kurimoto, T.; Ueda, K.; Enomoto, H.; Sakamoto, M.; Keshi, Y.; Yamada, Y.; Nakamura, M. Optic Neuritis Possibly Induced by Anti-PD-L1 Antibody Treatment in a Patient with Non-Small Cell Lung Carcinoma. Case Rep. Ophthalmol. 2018, 9, 348–356. [Google Scholar] [CrossRef]
- Weiss, D.; Cantré, D.; Zettl, U.K.; Storch, A.; Prudlo, J. Lethal Form of a Late-Onset Aquaporin-4 Antibody-Positive NMOSD Related to the Immune Checkpoint Inhibitor Nivolumab. J. Neurol. 2022, 269, 2778–2780. [Google Scholar] [CrossRef] [PubMed]
- Narumi, Y.; Yoshida, R.; Minami, Y.; Yamamoto, Y.; Takeguchi, S.; Kano, K.; Takahashi, K.; Saito, T.; Sawada, J.; Terui, H.; et al. Neuromyelitis Optica Spectrum Disorder Secondary to Treatment with Anti-PD-1 Antibody Nivolumab: The First Report. BMC Cancer 2018, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Khimani, K.; Patel, S.P.; Whyte, A.; Al-Zubidi, N. Case Report: Neuromyelitis Optica After Treatment of Uveal Melanoma With Nivolumab and Ipilimumab. Front. Oncol. 2022, 12, 806501. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Ueno, T.; Suzuki, C.; Nunomura, J.; Igarashi, S.; Sato, T.; Tomiyama, M. Aquaporin-4 Antibody Positive Neuromyelitis Optica Spectrum Disorder Associated with Esophageal Cancer. J. Neuroimmunol. 2017, 309, 38–40. [Google Scholar] [CrossRef]
- Nowosielski, M.; Di Pauli, F.; Iglseder, S.; Wagner, M.; Hoellweger, N.; Nguyen, V.A.; Gruber, J.; Stockhammer, G. Encephalomyeloneuritis and Arthritis after Treatment with Immune Checkpoint Inhibitors. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e773. [Google Scholar] [CrossRef]
- Noble, C.W.; Gangaputra, S.S.; Thompson, I.A.; Yuan, A.; Apolo, A.B.; Lee, J.-M.; Papaliodis, G.N.; Kodati, S.; Bishop, R.; Magone, M.T.; et al. Ocular Adverse Events Following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul. Immunol. Inflamm. 2020, 28, 854–859. [Google Scholar] [CrossRef]
- O’Keefe, G.A.D.; Rao, N.A. Vogt-Koyanagi-Harada Disease. Surv. Ophthalmol. 2017, 62, 1–25. [Google Scholar] [CrossRef]
- Bricout, M.; Petre, A.; Amini-Adle, M.; Bezza, W.; Seve, P.; Kodjikian, L.; Dalle, S.; Thomas, L. Vogt-Koyanagi-Harada–like Syndrome Complicating Pembrolizumab Treatment for Metastatic Melanoma. J. Immunother. 2017, 40, 77–82. [Google Scholar] [CrossRef]
- Read, R.W.; Holland, G.N.; Rao, N.A.; Tabbara, K.F.; Ohno, S.; Arellanes-Garcia, L.; Pivetti-Pezzi, P.; Tessler, H.H.; Usui, M. Revised Diagnostic Criteria for Vogt-Koyanagi-Harada Disease: Report of an International Committee on Nomenclature. Am. J. Ophthalmol. 2001, 131, 6. [Google Scholar]
- Shinoda, K.; Imamura, Y.; Matsumoto, C.S.; Mizota, A.; Ando, Y. Wavy and Elevated Retinal Pigment Epithelial Line in Optical Coherence Tomographic Images of Eyes with Atypical Vogt–Koyanagi–Harada Disease. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1399–1402. [Google Scholar] [CrossRef]
- Arai, T.; Harada, K.; Usui, Y.; Irisawa, R.; Tsuboi, R. Case of Acute Anterior Uveitis and Vogt-Koyanagi-Harada Syndrome-like Eruptions Induced by Nivolumab in a Melanoma Patient. J. Dermatol. 2017, 44, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Theillac, C.; Straub, M.; Breton, A.-L.; Thomas, L.; Dalle, S. Bilateral Uveitis and Macular Edema Induced by Nivolumab: A Case Report. BMC Ophthalmol. 2017, 17, 227. [Google Scholar] [CrossRef] [PubMed]
- Ushio, R.; Yamamoto, M.; Miyasaka, A.; Muraoka, T.; Kanaoka, H.; Tamura, H.; Kaneko, A.; Izawa, A.; Hirama, N.; Teranishi, S.; et al. Nivolumab-Induced Vogt-Koyanagi-Harada-like Syndrome and Adrenocortical Insufficiency with Long-Term Survival in a Patient with Non-Small-Cell Lung Cancer. Intern. Med. 2021, 60, 3593–3598. [Google Scholar] [CrossRef] [PubMed]
| References | Age (y), Sex | Cancer Histology and Stage | ICI Drugs | Time to Onset | Findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Nakatani et al. [35] | 73; female | Stage IV non-small cell lung cancer | Nivolumab | 20 weeks | Bilateral ptosis, limb weakness, photophobia, hyporeflexia, autonomic dysfunction. Positive anti-P/Q-type voltage-gated calcium channel (VGCC) antibodies (-ab) | Pyridostigmine; 3,4-diaminopyridine, low-dose corticosteroids. Initially restarted nivolumab, then definite discontinuation. | Progressive disease |
| Agrawal and Agrawal [36] | 59; male | Stage IV non-small cell lung cancer | Nivolumab/ Ipilimumab | 16 weeks | Gait disturbance, limb weakness, hyporeflexia No information regarding Abs status | Pyridostigmine; corticosteroids. ICI discontinuation | Progressive disease |
| Lee et al. [37] | 80; male | Squamous and large cell neuroendocrine lung carcinoma | Pembrolizumab | 10 months | Upper and lower limb weakness VGCC-ab not available | ICI discontinuation, corticosteroids, azathioprine | Maintained response for 12 months after ICI discontinuation |
| Duplaine et al. [38] | 58; female | Extensive stage small cell lung carcinoma | Nivolumab | 8 months | Ptosis, dysphagia. Proximal muscle weakness, hyporeflexia, autonomic dysfunction. Concomitant MG and LEMS with positive AChR-ab and anti-P/N-type VGCC-ab | Corticosteroids, anticholinesterase drugs, amifampridine, plasmapheresis, IVIG ICI discontinuation | Oligoprogressive brain disease treated with stereotactic surgery. Complete response up to 27 months after ICI discontinuation |
| Gill et al. [39] | 58; female | Stage IV melanoma | Nivolumab | 3 weeks | Diplopia, gait disturbance. Positive anti-P/Q-type VGCC-ab | 3,4-diaminopyridine, prednisone, IVIG, rituximab | No disease recurrence after 24 months after ICI withdrawal |
| Kunii et al. [40] | 74; male | Extensive stage small cell lung cancer | Atezolizumab | 12 months | Upper and lower limbs weakness, fatigue. Slight gait disturbance and absent patellar reflexes. Positive anti-P/Q-type VGCC-ab | High-dose corticosteroids, IVIG, ICI discontinuation | Progressive disease |
| Electromyographic and Other Findings | Autoantibodies | Differential Diagnosis and Workup | Treatment | |
|---|---|---|---|---|
| Myasthenia gravis | Muscle action potential decrement at baseline on low-rate repetitive stimulation [31] | AChR-Ab positive in up to 2/3 of patients. Anti-MuSK-Ab almost always negative [17] | Lambert–Eaton myasthenic syndrome, check for concurrent myopathies or myocarditis [31] | Pyridostigmine, high-dose IV corticosteroids, intravenous immunoglobulins (IVIG), and plasmapheresis [19,27,31] |
| Myopathies | Myopathic pattern with fibrillation and myopathic recruitment [42,43] | Anti-Ro/SSA, anti-DNAPK, anti-PM-Scl, anti-Scl70, anti-Jo-1, anti-MDA5, anti-TIF-1, anti-Mi-2, anti-NXP2 [47,48] | Check for concurrent myasthenia gravis or myocarditis [31] | High-dose IV corticosteroids, IVIG, plasmapheresis, infliximab [17,25,34,42,46,47,49,50] |
| Guillain–Barré Syndrome | EMG: Acute demyelinating polyradiculoneuropathy, prolonged distal latencies, low conduction velocities. CSF: mild pleocytosis with lymphocytic predominance (50%), hyperproteinorrhaquia, albumincytologic dissociation (44%) [54] | Anti-gangliosides antibodies occasionally positive [54] | Botulism, tick paralysis, intermediate syndrome in organophosphate poisoning, meningeal carcinomatosis, Lyme disease, West Nile virus flaccid paralysis. Stroke, brainstem metastases, and multiple sclerosis to be considered if total ophthalmoplegia is present [51,53,54,55] | IVIG and plasmapheresis. Some reports of response to high-dose corticosteroids [17,57,58] |
| Immune-Related Cytopenia | Bone Marrow Findings |
|---|---|
| Immune thrombocytopenia | Moderate hypercellularity and increased megakaryocytes |
| Hemolytic anemia (AIHA) | Not mandatory for diagnosis. Consider if an underlying cause is deemed possible. Erythroid hyperplasia may be found |
| Pure red cell aplasia | Erythroid hypoplasia accompanied by a granulocytic hyperplasia and adequate numbers of mature-appearing megakaryocytes in an otherwise normocellular bone marrow |
| Immune neutropenia | Blockage in granulocyte maturation (44%), granulocytic lineage hypoplasia (22%), or a near normal granulocytic lineage and bone marrow smear in up to 33% |
| Aplastic anemia | Marked hypocellularity (less than 20%) without blasts, and no reticulin fibrosis |
| Steven Johnson syndrome [145,146] |
| Toxic epidermal necrolysis [146,147] |
| Drug reaction with eosinophilia and systemic symptoms [148,149,150] |
| Erythema nodosum-like panniculitis [151,152] |
| Grover’s disease [153,154,155] |
| Sjögren’s syndrome [156] |
| Scleroderma reaction [157] |
| Urticaria [158] |
| Eruptive keratoacanthomas [159] |
| Neutrophilic dermatoses (Sweet’s syndrome, pyoderma gangrenosum) [160,161,162,163] |
| Necrotizing vasculitis [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarrán-Artahona, V.; Laguna, J.-C.; Gorría, T.; Torres-Jiménez, J.; Pascal, M.; Mezquita, L. Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy. Diagnostics 2022, 12, 2091. https://doi.org/10.3390/diagnostics12092091
Albarrán-Artahona V, Laguna J-C, Gorría T, Torres-Jiménez J, Pascal M, Mezquita L. Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy. Diagnostics. 2022; 12(9):2091. https://doi.org/10.3390/diagnostics12092091
Chicago/Turabian StyleAlbarrán-Artahona, Víctor, Juan-Carlos Laguna, Teresa Gorría, Javier Torres-Jiménez, Mariona Pascal, and Laura Mezquita. 2022. "Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy" Diagnostics 12, no. 9: 2091. https://doi.org/10.3390/diagnostics12092091
APA StyleAlbarrán-Artahona, V., Laguna, J.-C., Gorría, T., Torres-Jiménez, J., Pascal, M., & Mezquita, L. (2022). Immune-Related Uncommon Adverse Events in Patients with Cancer Treated with Immunotherapy. Diagnostics, 12(9), 2091. https://doi.org/10.3390/diagnostics12092091







