New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden
Abstract
1. Introduction
2. Methods
2.1. AD and Mucosal Pigmentations
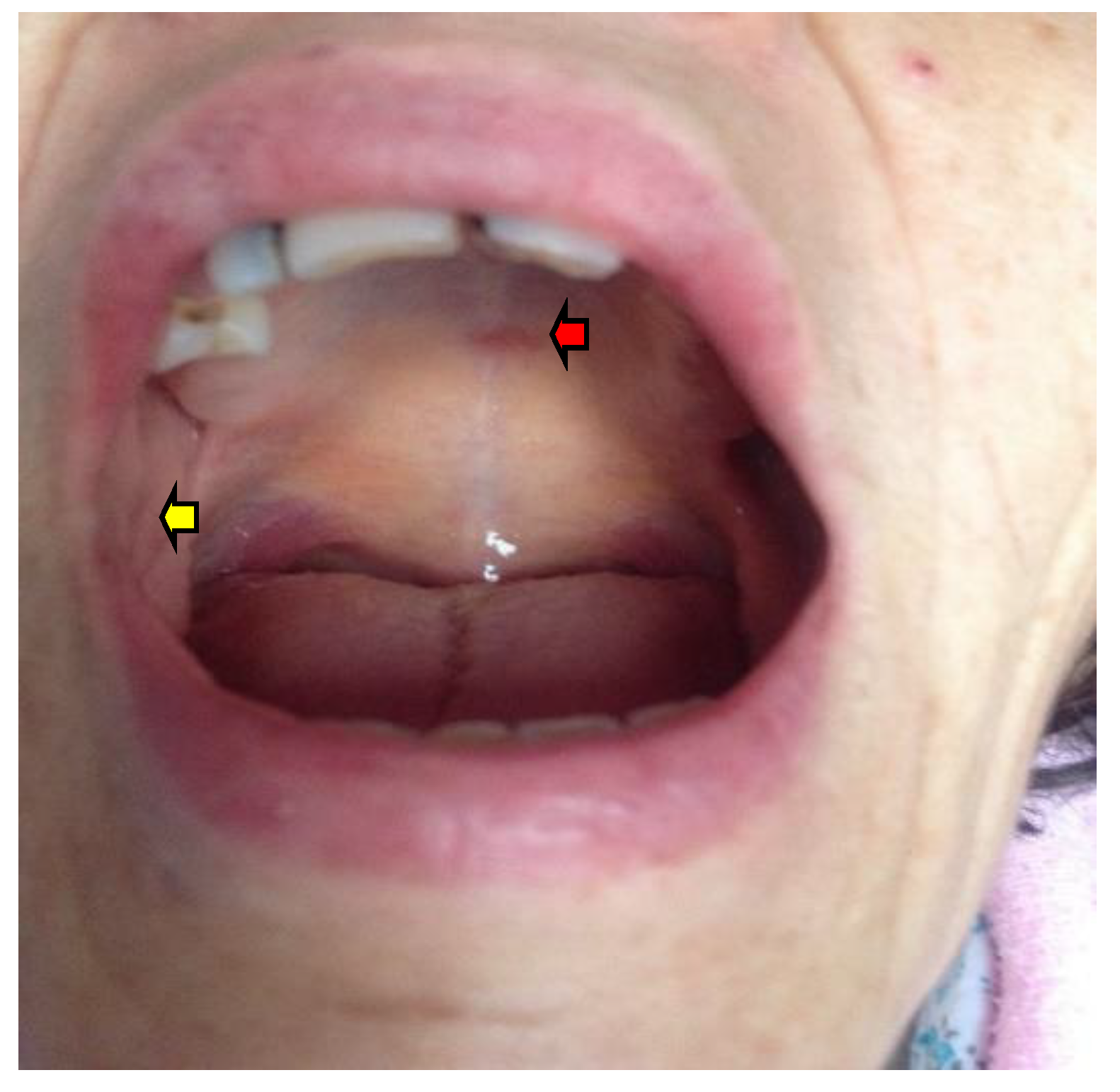
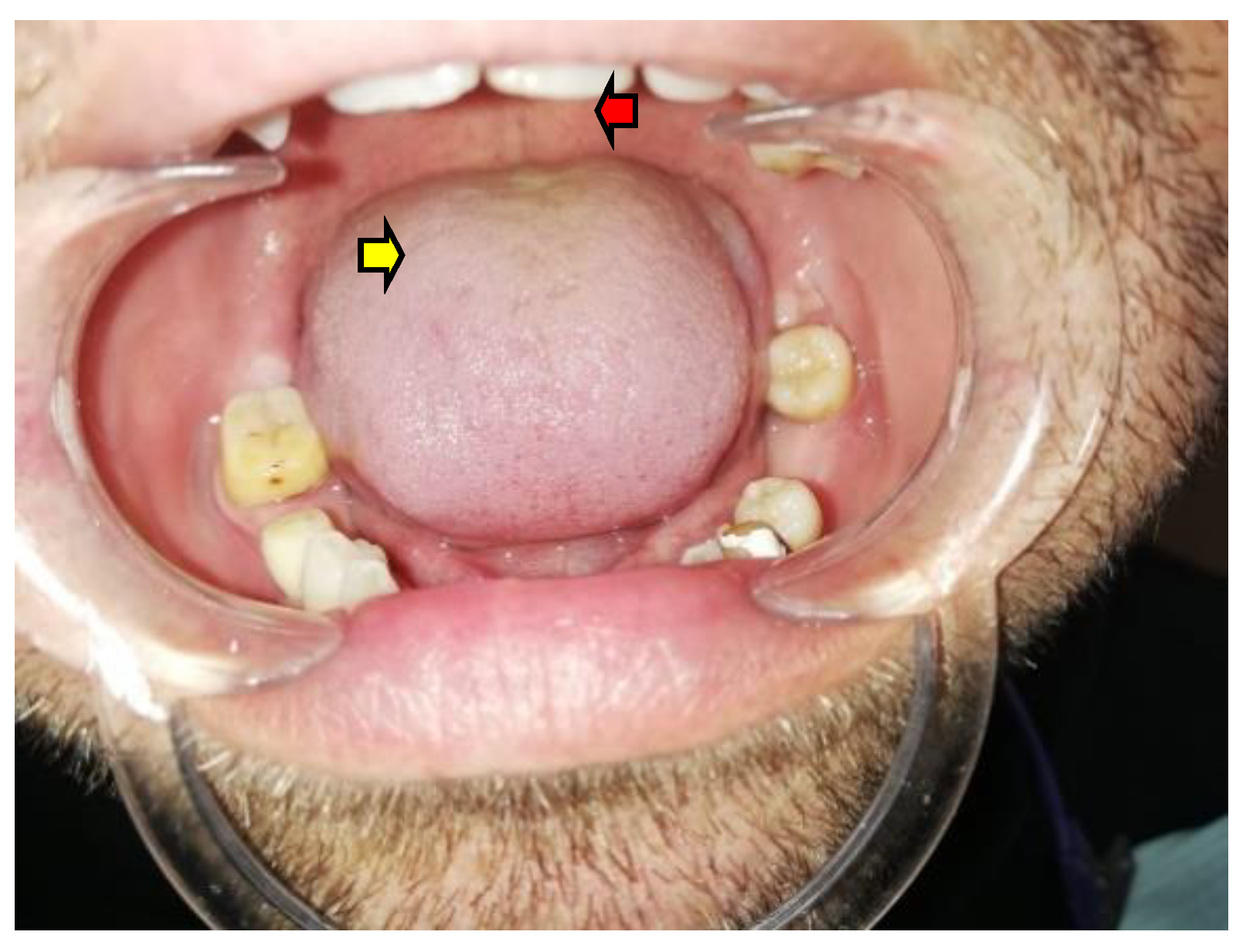
2.1.1. Oral Mucosa Pigmentation in AD
2.1.2. AD-Related Tongue Anomalies of Color
2.2. Spectrum of AD-Related Comorbidities: Oral Health Perspective
2.2.1. Oral Candidiasis in Patients with APS1
2.2.2. Early-Onset APS-Related Hypoparathyroidism and Dental Anomalies
2.2.3. From AD-Related type 1 DM to Periodontal Disease
2.2.4. Anemia in AD Individuals
2.2.5. Oral Features of Other Non-DM, Autoimmune Comorbidities in AD Patients
2.2.6. Exceptional Entities of AD with Potential Oral Manifestations
2.3. Oral Surgery Procedures in Patients with AD
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| AGEs | advanced glycation end-products |
| AD | Addison disease |
| APS | autoimmune polyglandular syndrome |
| APECED | autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy |
| CD | celiac disease |
| DM | diabetes mellitus |
| DMFT index | decayed, missing, and filled teeth index |
| HLA | human leukocyte antigen |
| 25OHD | 25-hydroxyvitamin D |
| IL | interleukin |
| MMP | metalloproteinase |
| MSH | melanocyte stimulating hormone |
| MCR | melanocortin receptor |
| PD | periodontal disease |
| POMC | proopiomelanocortin |
| PMN | polymorphonuclear leukocyte |
| PTH | parathormone |
| TNF | tumor necrosis factor |
| QL | quality of life |
| VDD | vitamin D deficiency |
References
- Nowotny, H.; Ahmed, S.F.; Bensing, S.; Beun, J.G.; Brösamle, M.; Chifu, I.; Claahsen van der Grinten, H.; Clemente, M.; Falhammar, H.; Hahner, S.; et al. Therapy options for adrenal insufficiency and recommendations for the management of adrenal crisis. Endocrine 2021, 71, 586–594. [Google Scholar] [CrossRef]
- Husebye, E.S.; Pearce, S.H.; Krone, N.P.; Kämpe, O. Adrenal insufficiency. Lancet 2021, 397, 613–629. [Google Scholar] [CrossRef]
- Rehman, H.U.; Lohrenz, S. Hyperpigmentation of the Tongue and Buccal Mucosa. Am. Fam. Physician 2020, 102, 181–182. [Google Scholar] [PubMed]
- Sarkar, S.B.; Sarkar, S.; Ghosh, S.; Bandyopadhyay, S. Addison’s disease. Contemp. Clin. Dent. 2012, 3, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Napier, C.; Pearce, S.H. Current and emerging therapies for Addison’s disease. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 147–153. [Google Scholar] [CrossRef]
- Michelet, R.; Melin, J.; Parra-Guillen, Z.P.; Neumann, U.; Whitaker, J.M.; Stachanow, V.; Huisinga, W.; Porter, J.; Blankenstein, O.; Ross, R.J.; et al. Paediatric population pharmacokinetic modelling to assess hydrocortisone replacement dosing regimens in young children. Eur. J. Endocrinol. 2020, 183, 357–368. [Google Scholar] [CrossRef]
- Oprea, A.; Bonnet, N.C.G.; Pollé, O.; Lysy, P.A. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018818821294. [Google Scholar] [CrossRef]
- Giordano, R.; Guaraldi, F.; Berardelli, R.; Karamouzis, I.; D’Angelo, V.; Zichi, C.; Grottoli, S.; Ghigo, E.; Arvat, E. Dual-release Hydrocortisone in Addison’s Disease—A Review of the Literature. Eur. Endocrinol. 2014, 10, 75–78. [Google Scholar] [CrossRef][Green Version]
- Mundell, L.; Lindemann, R.; Douglas, J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017, 6, e000209. [Google Scholar] [CrossRef]
- Arshad, M.F.; Debono, M. Current and future treatment options for adrenal insufficiency. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 303–311. [Google Scholar] [CrossRef]
- Sandru, F.; Petrova, E.; Petrov, B.; Draghici, A.; Ghemigian, A.; Carsote, M.; Dumitrascu, A.; Mehedintu, C.; Dumitrascu, M.C. Not just weight loss. Ro J. Med. Pract. 2021, 16, 529–532. [Google Scholar] [CrossRef]
- Hasenmajer, V.; Bonaventura, I.; Minnetti, M.; Sada, V.; Sbardella, E.; Isidori, A.M. Non-Canonical Effects of ACTH: Insights Into Adrenal Insufficiency. Front. Endocrinol. 2021, 12, 701263. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L. Stress-induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, T.V.; Chan, L.F.; Clark, A.J.L. Pathophysiology of melanocortin receptors and their accessory proteins. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, O.; Liapakis, G.; Venihaki, M. Role of Central and Peripheral CRH in Skin. Curr. Mol. Pharmacol. 2018, 11, 72–80. [Google Scholar] [CrossRef]
- Tutal, E.; Yılmazer, D.; Demirci, T.; Cakır, E.; Gültekin, S.S.; Celep, B.; Topaloğlu, O.; Çakal, E. A rare case of ectopic ACTH syndrome originating from malignant renal paraganglioma. Arch. Endocrinol. Metab. 2017, 61, 291–295. [Google Scholar] [CrossRef][Green Version]
- Mehta, R.; Lam-Chung, C.E.; Hinojosa-Amaya, J.M.; Roldán-Sarmiento, P.; Guillen-Placencia, M.F.; Villanueva-Rodriguez, G.; Juarez-Leon, O.A.; Leon-Domínguez, J.; Grajales-Gómez, M.; Ventura-Gallegos, J.L.; et al. High Molecular Weight ACTH-Precursor Presence in a Metastatic Pancreatic Neuroendocrine Tumor Causing Severe Ectopic Cushing’s Syndrome: A Case Report. Front. Endocrinol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Tan, H.; Chen, D.; Yu, Y.; Yu, K.; He, W.; Cai, B.; Jiang, S.; Tang, Y.; Tong, N.; An, Z. Unusual ectopic ACTH syndrome in a patient with orbital neuroendocrine tumor, resulted false-positive outcome of BIPSS:a case report. BMC Endocr. Disord. 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Sandru, F.; Dumitrascu, M.C.; Albu, S.E.; Carsote, M.; Valea, A. Hyperpigmentation and ACTH—An overview of literature. Rom. Med. J. 2019, 66, 309–312. [Google Scholar] [CrossRef]
- Morar, A.; Ghemigian, A.; Tupea, C.; Petrova, E.; Popescu, M.; Carsote, M.; Dumitru, N.; Valea, A. Twist of endocrine scenario: Approach of ectopic Cushing syndrome (review). Rom. J. Med. Pract. 2020, 15, 405–409. [Google Scholar] [CrossRef]
- Ranawaka, N.; Welikumbura, N.H. Addison’s disease as a primary manifestation of extrapulmonary tuberculosis; A case report. Indian J. Tuberc. 2021, 68, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Soedarso, M.A.; Nugroho, K.H.; Meira Dewi, K.A. A case report: Addison disease caused by adrenal tuberculosis. Urol Case Rep. 2018, 20, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.F.S.; Oliveira, J.M.; Kater, C.E. Crisis? What crisis? Abdominal pain and darkening skin in Addison’s disease. Lancet 2020, 396, 498. [Google Scholar] [CrossRef]
- Fredette, M.E.; Topor, L.S. Case 3: Emesis and Oral Hyperpigmentation in a 17-year-old Girl. Pediatr. Rev. 2018, 39, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Flores, A.; Cassarino, D.S. Histopathologic Findings of Cutaneous Hyperpigmentation in Addison Disease and Immunostain of the Melanocytic Population. Am. J. Dermatopathol. 2017, 39, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Gondak, R.O.; da Silva-Jorge, R.; Jorge, J.; Lopes, M.A.; Vargas, P.A. Oral pigmented lesions: Clinicopathologic features and review of the literature. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e919–e924. [Google Scholar] [CrossRef] [PubMed]
- das Chagas e Silva de Carvalho, L.F.; Farina, V.H.; Cabral, L.A.G.; Brandão, A.A.H.; Coletta RDella Almeida, J.D. Immunohistochemi cal features of multifocal melanoacanthoma in the hard palate: A case report. BMC Res. Notes 2013, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, K.; Ramesh, V.; Balamurali, P.D.; Singh, N. Oral melanoacan thoma of a rare intraoral site: Case report and review of literature. Int. J. Clin. Pediatr. Dent. 2013, 6, 40–43. [Google Scholar] [CrossRef]
- Maymone, M.B.C.; Greer, R.O.; Burdine, L.K.; Dao-Cheng, A.; Venkatesh, S.; Sahitya, P.C.; Maymone, A.C.; Kesecker, J.; Vashi, N.A. Benign oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 43–56. [Google Scholar] [CrossRef]
- Maymone, M.B.C.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.D.; Maymone, A.C.; Vashi, N.A. Premalignant and malignant oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 59–71. [Google Scholar] [CrossRef]
- Dantas, T.S.; Nascimento, I.V.D.; Verde, M.E.Q.L.; Alves, A.P.N.N.; Sousa, F.B.; Mota, M.R.L. Multifocal oral melanoacanthoma associated with Addison’s disease and hyperthyroidism: A case report. Arch. Endocrinol. Metab. 2017, 61, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Cantudo-Sanagustín, E.; Gutiérrez-Corrales, A.; Vigo-Martínez, M.; Serrera-Figallo, M.Á.; Torres-Lagares, D.; Gutiérrez-Pérez, J.L. Pathogenesis and clinicohistopathological caractheristics of melanoacanthoma: A systematic review. J. Clin. Exp. Dent. 2016, 8, e327–e336. [Google Scholar] [CrossRef]
- Lee, K.; Lian, C.; Vaidya, A.; Tsibris, H.C. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020, 6, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Ben-Ari, J.; Nemet, D.; Eliakim, A. The interpretation of color—An endocrine cause of skin discoloration mimicking cyanosis. J. Pediatr. Endocrinol. Metab. 2013, 26, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Manna, R.; Bianchi, A.; Gerardino, L.; Cipolla, C.; Rigante, D.; Landolfi, R. Neuropsychiatric symptoms, oral pigmentation and fever as revealing hints of autoimmune Addison’s disease. Minerva Endocrinol. 2021. [Google Scholar] [CrossRef]
- Alawi, F. Pigmented lesions of the oral cavity: An update. Dent. Clin. N. Am. 2013, 57, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Rosebush, M.S.; Briody, A.N.; Cordell, K.G. Black and Brown: Non-neoplastic Pigmentation of the Oral Mucosa. Head Neck Pathol. 2019, 13, 47–55. [Google Scholar] [CrossRef]
- Sputa-Grzegrzolka, P.; Wozniak, Z.; Akutko, K.; Pytrus, T.; Baran, W.; Calik, J.; Glatzel-Plucinska, N.; Domagala, Z.; Podhorska-Okolow, M.; Stawarski, A.; et al. Laugier-Hunziker syndrome: A case report of the pediatric patient and review of the literature. Int. J. Dermatol. 2020, 59, 1513–1519. [Google Scholar] [CrossRef]
- Miličević, T.; Žaja, I.; Tešanović, D.; Radman, M. Laugier-Hunziker syndrome in endocrine clinical practice. Endocrinol. Diabetes Metab Case Rep. 2018, 2018, 18-0025. [Google Scholar] [CrossRef] [PubMed]
- Lalosevic, J.; Zivanovic, D.; Skiljevic, D.; Medenica, L. Laugier-Hunziker syndrome—Case report. Bras Dermatol. 2015, 90 (Suppl. S1), 223–225. [Google Scholar] [CrossRef]
- Dika, E.; Starace, M.; Lambertini, M.; Patrizi, A.; Veronesi, G.; Alessandrini, A.; Piraccini, B.M. Oral and nail pigmentations: A useful parallelism for the clinician. J. Dtsch. Dermatol. Ges. 2020, 18, 7–14. [Google Scholar] [CrossRef]
- Malpartida-Carrillo, V.; Tinedo-Lopez, P.L.; Guerrero, M.E.; Amaya-Pajares, S.P.; Özcan, M.; Rösing, C.K. Periodontal phenotype: A review of historical and current classifications evaluating different methods and characteristics. J. Esthet. Restor. Dent. 2021, 33, 432–445. [Google Scholar] [CrossRef]
- Wémeau, J.L.; Proust-Lemoine, E.; Ryndak, A.; Vanhove, L. Thyroid autoimmunity and polyglandular endocrine syndromes. Hormones 2013, 12, 39–45. [Google Scholar] [CrossRef]
- Al-Zahrani, M.S.; Alhassani, A.A.; Zawawi, K.H. Clinical manifestations of gastrointestinal diseases in the oral cavity. Saudi Dent. J. 2021, 33, 835–841. [Google Scholar] [CrossRef]
- Gameiro, R.S.; Reis, A.I.A.; Grilo, A.C.; Noronha, C. Following leads: Connecting dysphagia to mixed connective tissue disease. BMJ Case Rep. 2018, 2018, bcr2017223699. [Google Scholar] [CrossRef]
- Goto, A.; Kokabu, S.; Dusadeemeelap, C.; Kawaue, H.; Matsubara, T.; Tominaga, K.; Addison, W.N. Tongue Muscle for the Analysis of Head Muscle Regeneration Dynamics. J. Dent. Res. 2022, 22, 220345221075966. [Google Scholar] [CrossRef]
- Seeker, P.; Osswald, S. Tongue Discoloration. N. Engl. J. Med. 2021, 384, e102. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, P.; Muñoz-Aceituno, E.; Vargas, E. Hyperpigmentation of the tongue. Cutis 2020, 106, E21–E22. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Ring, C.; Yang, S. Pigmented Fungiform Papillae of the Tongue. JAMA Dermatol. 2020, 156, 1249. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Santosh, A.B.R.; Hariyani, N.; Ernawati, D.S.; Cecilia, P.H. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: A systematic review. J. Oral Biol. Craniofac. Res. 2021, 11, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Panchal, N. Pigmented Lesions. Dermatol. Clin. 2020, 38, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Acquired Asymptomatic Blue Tongue: A Report of Exogenous Agent-associated Tongue Dyschromia and Review of Blue Tongue Etiologies. Cureus 2019, 11, e6243. [Google Scholar] [CrossRef] [PubMed]
- Tenório, J.R.; Tuma, M.M.; Andrade, N.S.; Santana, T.; Gallottini, M. Oral manifestations of autoimmune polyglandular syndrome type 1. Spec. Care Dentist. 2022. [Google Scholar] [CrossRef]
- Nisticò, D.; Bossini, B.; Benvenuto, S.; Pellegrin, M.C.; Tornese, G. Pediatric Adrenal Insufficiency: Challenges and Solutions. Ther. Clin. Risk Manag. 2022, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Halabi, I.; Barohom, M.N.; Peleg, S.; Trougouboff, P.; Elias-Assad, G.; Agbaria, R.; Tenenbaum-Rakover, Y. Case Report: Severe Hypocalcemic Episodes due to Autoimmune Enteropathy. Front. Endocrinol. 2021, 12, 645279. [Google Scholar] [CrossRef]
- Cranston, T.; Boon, H.; Olesen, M.K.; Ryan, F.J.; Shears, D.; London, R.; Rostom, H.; Elajnaf, T.; Thakker, R.V.; Hannan, F.M. Spectrum of germline AIRE mutations causing APS-1 and familial hypoparathyroidism. Eur. J. Endocrinol. 2022, 187, 111–122. [Google Scholar] [CrossRef]
- Peterson, P.; Kisand, K.; Kluger, N.; Ranki, A. Loss of AIRE-Mediated Immune Tolerance and the Skin. J. Investig. Dermatol. 2022, 142 Pt B, 760–767. [Google Scholar] [CrossRef]
- Philippot, Q.; Casanova, J.L.; Puel, A. Candidiasis in patients with APS-1: Low IL-17, high IFN-γ, or both? Curr. Opin. Immunol. 2021, 72, 318–323. [Google Scholar] [CrossRef]
- Oikonomou, V.; Break, T.J.; Gaffen, S.L.; Moutsopoulos, N.M.; Lionakis, M.S. Infections in the monogenic autoimmune syndrome APECED. Curr. Opin. Immunol. 2021, 72, 286–297. [Google Scholar] [CrossRef]
- Puel, A.; Bastard, P.; Bustamante, J.; Casanova, J.L. Human autoantibodies underlying infectious diseases. J. Exp. Med. 2022, 219, e20211387. [Google Scholar] [CrossRef]
- Besnard, M.; Padonou, F.; Provin, N.; Giraud, M.; Guillonneau, C. AIRE deficiency, from preclinical models to human APECED disease. Dis. Model Mech. 2021, 14, dmm046359. [Google Scholar] [CrossRef] [PubMed]
- Sivabalan, S.; Mahadevan, S.; Srinath, M.V. Recurrent oral thrush. Indian J. Pediatr. 2014, 81, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Ponranjini, V.C.; Jayachandran, S.; Kayal, L.; Bakyalakshmi, K. Autoimmune polyglandular syndrome type 1. J. Clin. Imaging Sci. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Sanjeevi, A.; Asirvatham, A.R.; Balachandran, K.; Mahadevan, S. Atypical presentation of autoimmune polyglandular syndrome type 1 in the fifth decade. BMJ Case Rep. 2021, 14, e241680. [Google Scholar] [CrossRef]
- Ferré, E.M.N.; Schmitt, M.M.; Lionakis, M.S. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Front Pediatr. 2021, 9, 723532. [Google Scholar] [CrossRef]
- Sharifinejad, N.; Zaki-Dizaji, M.; Tebyanian, S.; Zainaldain, H.; Jamee, M.; Rizvi, F.S.; Hosseinzadeh, S.; Fayyaz, F.; Hamedifar, H.; Sabzevari, A.; et al. Clinical, immunological, and genetic features in 938 patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED): A systematic review. Expert Rev. Clin. Immunol. 2021, 17, 807–817. [Google Scholar] [CrossRef]
- Garelli, S.; Dalla Costa, M.; Sabbadin, C.; Barollo, S.; Rubin, B.; Scarpa, R.; Masiero, S.; Fierabracci, A.; Bizzarri, C.; Crinò, A.; et al. Autoimmune polyendocrine syndrome type 1: An Italian survey on 158 patients. J. Endocrinol. Investig. 2021, 44, 2493–2510. [Google Scholar] [CrossRef]
- Humbert, L.; Cornu, M.; Proust-Lemoine, E.; Bayry, J.; Wemeau, J.L.; Vantyghem, M.C.; Sendid, B. Chronic Mucocutaneous Candidiasis in Autoimmune Polyendocrine Syndrome Type 1. Front. Immunol. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Shephard, M.K.; Schifter, M.; Palme, C.E. Multiple oral squamous cell carcinomas associated with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e36–e42. [Google Scholar] [CrossRef]
- Borchers, J.; Mäkitie, O.; Laakso, S. Infections and demanding endocrine care contribute to increased mortality in patients with APECED. Eur. J. Endocrinol. 2021, 185, K13–K17. [Google Scholar] [CrossRef]
- Borchers, J.; Pukkala, E.; Mäkitie, O.; Laakso, S. Patients With APECED Have Increased Early Mortality Due to Endocrine Causes, Malignancies and infections. J. Clin. Endocrinol. Metab. 2020, 105, e2207-13. [Google Scholar] [CrossRef] [PubMed]
- Nouraei, H.; Jahromi, M.G.; Jahromi, L.R.; Zomorodian, K.; Pakshir, K. Potential Pathogenicity of Candida Species Isolated from Oral Cavity of Patients with Diabetes Mellitus. Biomed Res. Int. 2021, 2021, 9982744. [Google Scholar] [CrossRef]
- Pallavan, B.; Ramesh, V.; Dhanasekaran, B.P.; Oza, N.; Indu, S.; Govindarajan, V. Comparison and correlation of candidal colonization in diabetic patients and normal individuals. J. Diabetes Metab. Disord. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Perniola, R.; Fierabracci, A.; Falorni, A. Autoimmune Addison’s Disease as Part of the Autoimmune Polyglandular Syndrome Type 1: Historical Overview and Current Evidence. Front. Immunol. 2021, 12, 606860. [Google Scholar] [CrossRef]
- Fierabracci, A.; Lanzillotta, M.; Vorgučin, I.; Palma, A.; Katanić, D.; Betterle, C. Report of two siblings with APECED in Serbia: Is there a founder effect of c.769C>T AIRE genotype? Ital. J. Pediatr. 2021, 47, 126. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, A.A.; Pemberton, M.N.; Waring, D.T. The dental manifestations and orthodontic implications of hypoparathyroidism in childhood. J. Orthod. 2018, 45, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kamarthi, N.; Venkatraman, S.; Patil, P.B. Dental findings in the diagnosis of idiopathic hypoparathyroidism. Ann. Saudi Med. 2013, 33, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Hejlesen, J.; Underbjerg, L.; Gjørup, H.; Bloch-Zupan, A.; Sikjaer, T.; Rejnmark, L.; Haubek, D. Dental Findings in Patients With Non-surgical Hypoparathyroidism and Pseudohypoparathyroidism: A Systematic Review. Front. Physiol. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Gupta, D.; Sekhn, S.G. Oral manifestations of parathyroid disorders and its dental management. J. Dent. Allied Sci. 2014, 3, 34–38. [Google Scholar] [CrossRef]
- Giusti, F.; Brandi, M.L. Clinical Presentation of Hypoparathyroidism. Front. Horm. Res. 2019, 51, 139–146. [Google Scholar] [CrossRef]
- Al-Azem, H.; Khan, A.A. Hypoparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Bouça, B.; Nogueira, A.; Caetano, J.; Cardoso, R.; Dinis, I.; Mirante, A. Clinical characteristics of polyglandular autoimmune syndromes in pediatric age: An observational study. J. Pediatr. Endocrinol. Metab. 2022, 35, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.A.; Joshi, H.R.; Straseski, J.A. Mistaken Identity: The Role of Autoantibodies in Endocrine Disease. J. Appl. Lab. Med. 2022, 7, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Røyrvik, E.C.; Husebye, E.S. The genetics of autoimmune Addison disease: Past, present and future. Nat. Rev. Endocrinol. 2022, 18, 399–412. [Google Scholar] [CrossRef]
- Prinz, N.; Tittel, S.R.; Bachran, R.; Birnbacher, R.; Brückel, J.; Dunstheimer, D.; Haberland, H.; Hess, M.; Karges, W.; Oeverink, R.; et al. Characteristics of Patients with Type 1 Diabetes and Additional Autoimmune Disease in the DPV Registry. J. Clin. Endocrinol. Metab. 2021, 106, e3381–e3389. [Google Scholar] [CrossRef]
- Silva, N.; Abusleme, L.; Bravo, D.; Dutzan, N.; Garcia-Sesnich, J.; Vernal, R.; Hernández, M.; Gamonal, J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015, 23, 329–355. [Google Scholar] [CrossRef] [PubMed]
- John, V.; Alqallaf, H.; De Bedout, T. Periodontal Disease and Systemic Diseases: An Update for the Clinician. J. Indiana Dent. Assoc. 2016, 95, 16–23. [Google Scholar] [PubMed]
- Sun, S.; Mao, Z.; Wang, H. Relationship between periodontitis and diabetes: A bibliometrics analysis. Ann. Transl. Med. 2022, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Mn, P.; Menon, P.K.; Radeideh, A.; Varma, S.; Thomas, S.; Varughese, N.; Hamed, G.M. A Clinical Study on the Circadian Rhythm of Salivary Cortisol on Aggressive Periodontitis and Its Correlation with Clinical Parameters using Electrochemiluminescence Immunoassay Method. J. Contemp. Dent. Pract. 2019, 20, 482–488. [Google Scholar]
- Natto, Z.S.; Hameedaldain, A. Methodological Quality Assessment of Meta-analyses and Systematic Reviews of the Relationship Between Periodontal and Systemic Diseases. J. Evid. Based Dent. Pract. 2019, 19, 131–139. [Google Scholar] [CrossRef]
- Marchio, V.; Derchi, G.; Cinquini, C.; Miceli, M.; Gabriele, M.; Alfonsi, F.; Barone, A. Tissue level implants in healthy versus medically compromised patients: A cohort comparative study. Minerva Stomatol. 2020, 69, 295–301. [Google Scholar] [CrossRef]
- Elebrashy, I.; Yousief, E.; Saif, A. Primary anti-phospholipid antibody syndrome causing recurrent venous thrombosis and thrombocytopenia in a patient with Addison’s disease. JRSM Open 2014, 5, 2054270414562985. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.A.; Casarin, M.; Monajemzadeh, S.; Bezerra, B.B.; Lux, R.; Pirih, F.Q. The Microbiome in Periodontitis and Diabetes. Front. Oral Health 2022, 3, 859209. [Google Scholar] [CrossRef]
- Wilson, M.L. Prediabetes: Beyond the Borderline. Nurs. Clin. N. Am. 2017, 52, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Kudiyirickal, M.G.; Pappachan, J.M. Diabetes mellitus and oral health. Endocrine 2015, 49, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Barlattani, A., Jr.; Perrone, M.A.; Basili, M.; Miranda, M.; Costacurta, M.; Gualtieri, P.; Pujia, A.; Merra, G.; Bollero, P. Obesity, bariatric surgery and periodontal disease: A literature update. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, Y.; Keceli, H.G.; Helvaci, N.; Erbas, T.; Nohutcu, R.M. The tendency of reduced periodontal destruction in acromegalic patients showing similar inflammatory status with periodontitis patients. Endocrine 2019, 66, 622–633. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, S.; Bhansali, A.; Gupta, M.; Jain, A.; Bhaskar, N.; Kaur, R.K. Impact of concurrent diabetes on periodontal health in patients with acromegaly. Sci. Rep. 2020, 10, 19170. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, M.; Xia, L.; Fang, Z.; Yu, S.; Gao, J.; Feng, Q.; Yang, P. Alteration of salivary microbiome in periodontitis with or without type-2 diabetes mellitus and metformin treatment. Sci. Rep. 2020, 10, 15363. [Google Scholar] [CrossRef] [PubMed]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Wang, Y.; Wang, Z.; Ding, W.; Sun, X.; He, K.; Feng, Q.; Zhang, X. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging 2020, 12, 13090–13114. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xiao, Z.; Wen, C.; Ge, C.; Liu, L.; Xu, K.; Cao, S. Correlation between salivary developmental endothelial locus-1, interleukin 17 expression level and severity of periodontal disease in patients with type 2 diabetes mellitus. Am. J. Transl. Res. 2021, 13, 11704–11710. [Google Scholar] [PubMed]
- Holmstrup, P.; Plemons, J.; Meyle, J. Non-plaque-induced gingival diseases. J. Periodontol. 2018, 89 (Suppl. S1), S28–S45. [Google Scholar] [CrossRef] [PubMed]
- Özçaka, Ö.; Ceyhan-Öztürk, B.; Gümüş, P.; Akcalı, A.; Nalbantsoy, A.; Buduneli, N. Clinical periodontal status and inflammatory cytokines in gestational diabetes mellitus. Arch. Oral. Biol. 2016, 72, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.K.; Seo, M.; Lee, Y.S.; Moon, S.S. Association of periodontitis with insulin resistance, β-cell function, and impaired fasting glucose before onset of diabetes. Endocr. J. 2015, 62, 981–989. [Google Scholar] [CrossRef]
- Buysschaert, M.; Medina, J.L.; Bergman, M.; Shah, A.; Lonier, J. Prediabetes and associated disorders. Endocrine 2015, 48, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Akcalı, A.; Bostanci, N.; Özçaka, Ö.; Öztürk-Ceyhan, B.; Gümüş, P.; Buduneli, N.; Belibasakis, G.N. Association between polycystic ovary syndrome, oral microbiota and systemic antibody responses. PLoS ONE 2014, 9, e108074. [Google Scholar] [CrossRef]
- Khocht, A.; Albandar, J.M. Aggressive forms of periodontitis secondary to systemic disorders. Periodontol 2000 2014, 65, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Timonen, P.; Saxlin, T.; Knuuttila, M.; Suominen, A.L.; Jula, A.; Tervonen, T.; Ylöstalo, P. Role of insulin sensitivity and beta cell function in the development of periodontal disease in adults without diabetes. J. Clin. Periodontol. 2013, 40, 1079–1086. [Google Scholar] [CrossRef]
- Gul, S.S.; Zardawi, F.M.; Abdulkareem, A.A.; Shaikh, M.S.; Al-Rawi, N.H.; Zafar, M.S. Efficacy of MMP-8 Level in Gingival Crevicular Fluid to Predict the Outcome of Nonsurgical Periodontal Treatment: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 3131. [Google Scholar] [CrossRef]
- Umeizudike, K.; Räisänen, I.; Gupta, S.; Nwhator, S.; Grigoriadis, A.; Sakellari, D.; Sorsa, T. Active matrix metalloproteinase-8: A potential biomarker of oral systemic link. Clin. Exp. Dent. Res. 2022, 8, 359–365. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, E.; Radaic, A.; Yu, X.; Yang, S.; Yu, C.; Xiao, S.; Ye, C. Diagnostic potential and future directions of matrix metalloproteinases as biomarkers in gingival crevicular fluid of oral and systemic diseases. Int. J. Biol. Macromol. 2021, 188, 180–196. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Ghassib, I.; Chen, Z.; Zhu, J.; Wang, H.L. Use of IL-1 β, IL-6, TNF-α, and MMP-8 biomarkers to distinguish peri-implant diseases: A systematic review and meta-analysis. Clin Implant Dent. Relat. Res. 2019, 21, 190–207. [Google Scholar] [CrossRef]
- Al-Majid, A.; Alassiri, S.; Rathnayake, N.; Tervahartiala, T.; Gieselmann, D.R.; Sorsa, T. Matrix Metalloproteinase-8 as an Inflammatory and Prevention Biomarker in Periodontal and Peri-Implant Diseases. Int. J. Dent. 2018, 2018, 7891323. [Google Scholar] [CrossRef]
- de Morais, E.F.; Dantas, A.N.; PinheiRom, J.C.; Leite, R.B.; Galvao Barboza, C.A.; de Vasconcelos Gurgel, B.C.; de Almeida Freitas, R. Matrix metalloproteinase-8 analysis in patients with periodontal disease with prediabetes or type 2 diabetes mellitus: A systematic review. Arch. Oral Biol. 2018, 87, 43–51. [Google Scholar] [CrossRef]
- Chopra, A.; Jayasinghe, T.N.; Eberhard, J. Are Inflamed Periodontal Tissues Endogenous Source of Advanced Glycation End-Products (AGEs) in Individuals with and without Diabetes Mellitus? A Systematic Review. Biomolecules 2022, 12, 642. [Google Scholar] [CrossRef]
- Goubar, T.; Torpy, D.J.; McGrath, S.; Rushworth, R.L. Prehospital Management of Acute Addison Disease: Audit of Patients Attending a Referral Hospital in a Regional Area. J. Endocr. Soc. 2019, 3, 2194–2203. [Google Scholar] [CrossRef]
- Angelousi, A.; Larger, E. Anaemia, a common but often unrecognized risk in diabetic patients: A review. Diabetes Metab. 2015, 41, 18–27. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M. Diagnosis of Addison’s disease in a patient with newly diagnosed renal insufficiency having a context of autoimmune poly-endocrine syndrome. Acta Med. Transilv. 2016, 21, 45–47. [Google Scholar]
- Hussain, S.B.; Leira, Y.; Zehra, S.A.; Botelho, J.; Machado, V.; Ciurtin, C.; D’Aiuto, F.; Orlandi, M. Periodontitis and Systemic Lupus Erythematosus: A systematic review and meta-analysis. J. Periodontal. Res. 2022, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, M.; Pedroni, G.; Bigi, L.; Apponi, R.; Murri Dello Diago, A.; Dattola, A.; Farnetani, F.; Pellacani, G. Acquired White Oral Lesions with Specific Patterns: Oral Lichen Planus and Lupus Erythematosus. Dermatol. Pract. Concept 2021, 11, e2021074. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Patschan, S.; Patschan, D.; Ziebolz, D. Oral-Health-Related Quality of Life in Adult Patients with Rheumatic Diseases—A Systematic Review. J. Clin. Med. 2020, 9, 1172. [Google Scholar] [CrossRef]
- Baumrin, E.; Webster, G.; Werth, V.P. Addison Disease and Discoid Lupus Erythematosus: A Rare Association of Polyglandular Autoimmune Syndrome Type II. J. Clin. Rheumatol. 2016, 22, 382–383. [Google Scholar] [CrossRef]
- Borysewicz-Sańczyk, H.; Sawicka, B.; Michalak, J.; Wójtowicz, J.; Dobreńko, E.; Konstantynowicz, J.; Kemp, E.H.; Thakker, R.V.; Allgrove, J.; Hannan, F.M.; et al. Case report: A 10-year-old girl with primary hypoparathyroidism and systemic lupus erythematosus. J. Pediatr. Endocrinol. Metab. 2020, 33, 1231–1235. [Google Scholar] [CrossRef]
- Ozmeric, N.; Bissada, N.; da Silva, A.P.B. The Association between Inflammatory Bowel Disease and Periodontal Conditions: Is There a Common Bacterial Etiology? J. Int. Acad. Periodontol. 2018, 20, 40–51. [Google Scholar]
- Nijakowski, K.; Gruszczyński, D.; Surdacka, A. Oral Health Status in Patients with Inflammatory Bowel Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11521. [Google Scholar] [CrossRef]
- Marruganti, C.; Discepoli, N.; Gaeta, C.; Franciosi, G.; Ferrari, M.; Grandini, S. Dental Caries Occurrence in Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Caries Res. 2021, 55, 485–495. [Google Scholar] [CrossRef]
- Qiu, Y.; Mao, R.; Chen, M.H. A De Novo Arisen Case of Primary Adrenal Insufficiency in an Adolescent Patient With Crohn Disease: A Case report. Medicine 2015, 94, e818. [Google Scholar] [CrossRef]
- Mitsinikos, T.; Shillingford, N.; Cynamon, H.; Bhardwaj, V. Autoimmune Gastritis in Pediatrics: A Review of 3 Cases. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 252–257. [Google Scholar] [CrossRef]
- Rodriguez-Castro, K.I.; Franceschi, M.; Miraglia, C.; Russo, M.; Nouvenne, A.; Leandro, G.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F. Autoimmune diseases in autoimmune atrophic gastritis. Acta Biomed. 2018, 89, 100–103. [Google Scholar] [CrossRef]
- Agrawala, R.K.; Sahoo, S.K.; Choudhury, A.K.; Mohanty, B.K.; Baliarsinha, A.K. Pigmentation in vitamin B12 deficiency masquerading Addison’s pigmentation: A rare presentation. Indian J. Endocrinol. Metab. 2013, 17 (Suppl. S1), S254–S256. [Google Scholar] [CrossRef]
- Gaba, N.; Gaba, S.; Singla, M.; Gupta, M. Type 2 Autoimmune Polyglandular Syndrome Presenting with Hyperpigmentation and Amenorrhea. Cureus 2020, 12, e7772. [Google Scholar] [CrossRef]
- Fernández Miró, M.; Colom Comí, C.; Godoy Lorenzo, R. Autoinmune polyendocrinopathy. Med. Clin. 2021, 157, 241–246. [Google Scholar] [CrossRef]
- Mikosch, P.; Aistleitner, A.; Oehrlein, M.; Trifina-Mikosch, E. Hashimoto’s thyroiditis and coexisting disorders in correlation with HLA status-an overview. Wien. Med. Wochenschr. 2021. [Google Scholar] [CrossRef]
- Meling Stokland, A.E.; Ueland, G.; Lima, K.; Grønning, K.; Finnes, T.E.; Svendsen, M.; Ewa Tomkowicz, A.; Emblem Holte, S.; Therese Sollid, S.; Debowska, A.; et al. Autoimmune Thyroid Disorders in Autoimmune Addison Disease. J. Clin. Endocrinol. Metab. 2022, 107, e2331–e2338. [Google Scholar] [CrossRef]
- Wémeau, J.L.; Do Cao, C.; Ladsous, M. Oral and dental expression of thyroid diseases. Presse Med. 2017, 46, 864–868. [Google Scholar] [CrossRef]
- Ursomanno, B.L.; Cohen, R.E.; Levine, M.J.; Yerke, L.M. The Effect of Hypothyroidism on Bone Loss at Dental Implants. J. Oral Implantol. 2021, 47, 131–134. [Google Scholar] [CrossRef]
- Penna-Martinez, M.; Meyer, G.; Wolff, A.B.; Skinningsrud, B.; Betterle, C.; Falorni, A.; Ollier, W.; Undlien, D.; Husebye, E.; Pearce, S.; et al. Vitamin D status and pathway genes in five European autoimmune Addison’s disease cohorts. Eur. J. Endocrinol. 2021, 184, 373–381. [Google Scholar] [CrossRef]
- Beckett, D.M.; Broadbent, J.M.; Loch, C.; Mahoney, E.K.; Drummond, B.K.; Wheeler, B.J. Dental Consequences of Vitamin D Deficiency during Pregnancy and Early Infancy-An Observational Study. Int. J. Environ. Res. Public Health 2022, 19, 1932. [Google Scholar] [CrossRef] [PubMed]
- Alsulaimani, L.; Alqarni, A.; Almarghlani, A.; Hassoubah, M. The Relationship Between Low Serum Vitamin D Level and Early Dental Implant Failure: A Systematic Review. Cureus 2022, 14, e21264. [Google Scholar] [CrossRef] [PubMed]
- Piperea-Sianu, D.; Ceau, A.; Carsote, M.; Croitoru, A.G.; Cristea, S. Association between peridontal disease and osteoporosis. Rom. J. Rheumatol. 2017, XXVI, 107–114. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int. J. Implant Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Tabrizi, R.; Mohajerani, H.; Jafari, S.; Tümer, M.K. Does the serum level of vitamin D affect marginal bone loss around dental implants? Int. J. Oral Maxillofac. Surg. 2022, 51, 832–836. [Google Scholar] [CrossRef]
- Asamoah, E. Levothyroxine sodium oral solution to control thyroid function in a patient with hypothyroidism and celiac disease. Clin. Case Rep. 2021, 9, e04170. [Google Scholar] [CrossRef]
- Tarar, Z.I.; Zafar, M.U.; Farooq, U.; Basar, O.; Tahan, V.; Daglilar, E. The Progression of Celiac Disease, Diagnostic Modalities, and Treatment Options. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211053702. [Google Scholar] [CrossRef]
- Miconi, F.; Savarese, E.; Miconi, G.; Cabiati, G.; Rapaccini, V.; Principi, N.; Esposito, S. Unusual Onset of Celiac Disease and Addison’s Disease in a 12-Year-Old Boy. Int. J. Environ. Res. Public Health 2017, 14, 855. [Google Scholar] [CrossRef]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef]
- Liu, J.; Lundemann, A.J.; Reibel, J.; Pedersen, A.M.L. Salivary gland involvement and oral health in patients with coeliac disease. Eur. J. Oral Sci. 2022, 130, e12861. [Google Scholar] [CrossRef]
- Alsadat, F.A.; Alamoudi, N.M.; El-Housseiny, A.A.; Felemban, O.M.; Dardeer, F.M.; Saadah, O.I. Oral and dental manifestations of celiac disease in children: A case-control study. BMC Oral Health 2021, 21, 669. [Google Scholar] [CrossRef] [PubMed]
- Nota, A.; Abati, S.; Bosco, F.; Rota, I.; Polizzi, E.; Tecco, S. General Health, Systemic Diseases and Oral Status in Adult Patients with Coeliac Disease. Nutrients 2020, 12, 3836. [Google Scholar] [CrossRef] [PubMed]
- Arya PV, A.; Kumar, J.; Unnikrishnan, D.; Raj, R. Case of autoimmune polyglandular syndrome type 2: How we uncovered the diagnosis. BMJ Case Rep. 2019, 12, e227187. [Google Scholar] [CrossRef] [PubMed]
- Dahir, A.M.; Thomsen, S.F. Comorbidities in vitiligo: Comprehensive review. Int. J. Dermatol. 2018, 57, 1157–1164. [Google Scholar] [CrossRef]
- Lause, M.; Kamboj, A.; Fernandez Faith, E. Dermatologic manifestations of endocrine disorders. Transl. Pediatr. 2017, 6, 300–312. [Google Scholar] [CrossRef]
- Nagarajan, A.; Masthan, M.K.; Sankar, L.S.; Narayanasamy, A.B.; Elumalai, R. Oral manifestations of vitiligo. Indian J. Dermatol. 2015, 60, 103. [Google Scholar] [CrossRef]
- Gaiani, F.; Gismondi, P.; Minelli, R.; Casadio, G.; de’Angelis, N.; Fornaroli, F.; de’Angelis, G.L.; Manfredi, M. Case report of a familial triple: A syndrome and review of the literature. Medicine 2020, 99, e20474. [Google Scholar] [CrossRef]
- Nakamura, J.; Hikichi, T.; Inoue, H.; Watanabe, K.; Kikuchi, H.; Takagi, T.; Suzuki, R.; Sugimoto, M.; Konno, N.; Waragai, Y.; et al. Per-oral endoscopic myotomy for esophageal achalasia in a case of Allgrove syndrome. Clin. J. Gastroenterol. 2018, 11, 273–277. [Google Scholar] [CrossRef]
- Dhar, M.; Verma, N.; Singh, R.B.; Pai, V.K. Triple A to triple S: From diagnosis, to anesthetic management of Allgrove syndrome. J. Clin. Anesth. 2016, 33, 141–143. [Google Scholar] [CrossRef]
- Pogliaghi, G.; Cangiano, B.; Duminuco, P.; Vezzoli, V.; Bonomi, M. Triple-A Syndrome (TAS): An In-Depth Overview on Genetic and Phenotype Heterogeneity. Protein Pept. Lett. 2020, 27, 1192–1203. [Google Scholar] [CrossRef]
- Tadini, G.; Besagni, F.; Callea, M.; Brena, M.; Rossi, L.C.; Angiero, F.; Crippa, R. Allgrove syndrome: A report of a unique case characterised by peculiar dental findings resembling those of ectodermal dysplasia. Eur. J. Paediatr. Dent. 2015, 16, 324–326. [Google Scholar] [PubMed]
- Folk, G.A.; Nelson, B.L. Oral Histoplasmosis. Head Neck Pathol. 2017, 11, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chandy, D.D.; Bharani, T.; Marak, R.S.K.; Yadav, S.; Dabadghao, P.; Gupta, S.; Sahoo, S.K.; Pandey, R.; Bhatia, E. Clinical outcomes and cortical reserve in adrenal histoplasmosis-A retrospective follow-up study of 40 patients. Clin. Endocrinol. 2019, 90, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Aggarwal, I.; Dutta, D.; Ghosh, A.G.; Chatterjee, G.; Chowdhury, S. Acquired perforating dermatosis and Addison’s disease due to disseminated histoplasmosis: Presentation and clinical outcomes. Dermato-endocrinology 2013, 5, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Facco, E.; Bacci, C.; Zanette, G. Hypnosis as sole anesthesia for oral surgery: The egg of Columbus. J. Am. Dent. Assoc. 2021, 152, 756–762. [Google Scholar] [CrossRef]
- Woodcock, T.; Barker, P.; Daniel, S.; Fletcher, S.; Wass, J.A.H.; Tomlinson, J.W.; Misra, U.; Dattani, M.; Arlt, W.; Vercueil, A. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: Guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia 2020, 75, 654–663. [Google Scholar] [CrossRef]
- Isidori, A.M.; Arnaldi, G.; Boscaro, M.; Falorni, A.; Giordano, C.; Giordano, R.; Pivonello, R.; Pozza, C.; Sbardella, E.; Simeoli, C.; et al. Italian Society of Endocrinology.Towards the tailoring of glucocorticoid replacement in adrenal insufficiency: The Italian Society of Endocrinology Expert Opinion. J. Endocrinol. Investig. 2020, 43, 683–696. [Google Scholar] [CrossRef]
- Strietzel, F.P.; Schmidt-Westhausen, A.M.; Neumann, K.; Reichart, P.A.; Jackowski, J. Implants in patients with oral manifestations of autoimmune or muco-cutaneous diseases–A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e217–e230. [Google Scholar] [CrossRef]
- Vissink, A.; Spijkervet, F.; Raghoebar, G.M. The medically compromised patient: Are dental implants a feasible option? Oral Dis. 2018, 24, 253–260. [Google Scholar] [CrossRef]
- Reichart, P.A.; Schmidt-Westhausen, A.M.; Khongkhunthian, P.; Strietzel, F.P. Dental implants in patients with oral mucosal diseases—A systematic review. J. Oral Rehabil. 2016, 43, 388–399. [Google Scholar] [CrossRef]
- Esimekara, J.O.; Perez, A.; Courvoisier, D.S.; Scolozzi, P. Dental implants in patients suffering from autoimmune diseases: A systematic critical review. J. Stomatol. Oral Maxillofac. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.W.; Khader, R.; Cobetto, G.; Yepes, J.F.; Karounos, D.G.; Miller, C.S. Risk of adrenal crisis in dental patients: Results of a systematic search of the literature. J. Am. Dent. Assoc. 2013, 144, 152–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carmona, D.; Ouanounou, A. Adrenal Insufficiency and Its Implications on Dental Treatment. Compend. Contin. Educ. Dent. 2021, 42, 422–428. [Google Scholar] [PubMed]
- Henderson, S. What steroid supplementation is required for a patient with primary adrenal insufficiency undergoing a dental procedure? Dent. Update 2014, 41, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Guignat, L. Therapeutic patient education in adrenal insufficiency. Ann. Endocrinol. 2018, 79, 167–173. [Google Scholar] [CrossRef]
- Guignat, L.; Proust-Lemoine, E.; Reznik, Y.; Zenaty, D. Group 6. Modalities and frequency of monitoring of patients with adrenal insufficiency. Patient education. Ann. Endocrinol. 2017, 78, 544–558. [Google Scholar] [CrossRef]
- Falorni, A.; Minarelli, V.; Morelli, S. Therapy of adrenal insufficiency: An update. Endocrine 2013, 43, 514–528. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugălă, N.M.; Carsote, M.; Stoica, L.E.; Albulescu, D.M.; Ţuculină, M.J.; Preda, S.A.; Boicea, A.-R.; Alexandru, D.O. New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden. Diagnostics 2022, 12, 2080. https://doi.org/10.3390/diagnostics12092080
Bugălă NM, Carsote M, Stoica LE, Albulescu DM, Ţuculină MJ, Preda SA, Boicea A-R, Alexandru DO. New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden. Diagnostics. 2022; 12(9):2080. https://doi.org/10.3390/diagnostics12092080
Chicago/Turabian StyleBugălă, Narcis Mihăiţă, Mara Carsote, Loredana Elena Stoica, Dana Maria Albulescu, Mihaela Jana Ţuculină, Smaranda Adelina Preda, Ancuta-Ramona Boicea, and Dragoș Ovidiu Alexandru. 2022. "New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden" Diagnostics 12, no. 9: 2080. https://doi.org/10.3390/diagnostics12092080
APA StyleBugălă, N. M., Carsote, M., Stoica, L. E., Albulescu, D. M., Ţuculină, M. J., Preda, S. A., Boicea, A.-R., & Alexandru, D. O. (2022). New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden. Diagnostics, 12(9), 2080. https://doi.org/10.3390/diagnostics12092080





