Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review
Abstract
1. Introduction
2. Materials and Methods
3. Main Findings of the Literature (Review) Quest
3.1. Experimental Animal Models
3.2. Primary Intra-Abdominal Melanoma
3.2.1. Originating in the Gastrointestinal Tract
From the Esophagus
From the Stomach
From the Colon
From the Pancreas
From the Ovary
From the Anorectal Region
From the Gallbladder
From the Small Intestine
From the Adrenals
3.2.2. Association with Extracutaneous Blue Naevi
3.3. Secondary Intra-Abdominal Melanoma
3.3.1. Primary Intraocular Melanoma and Its Metastases
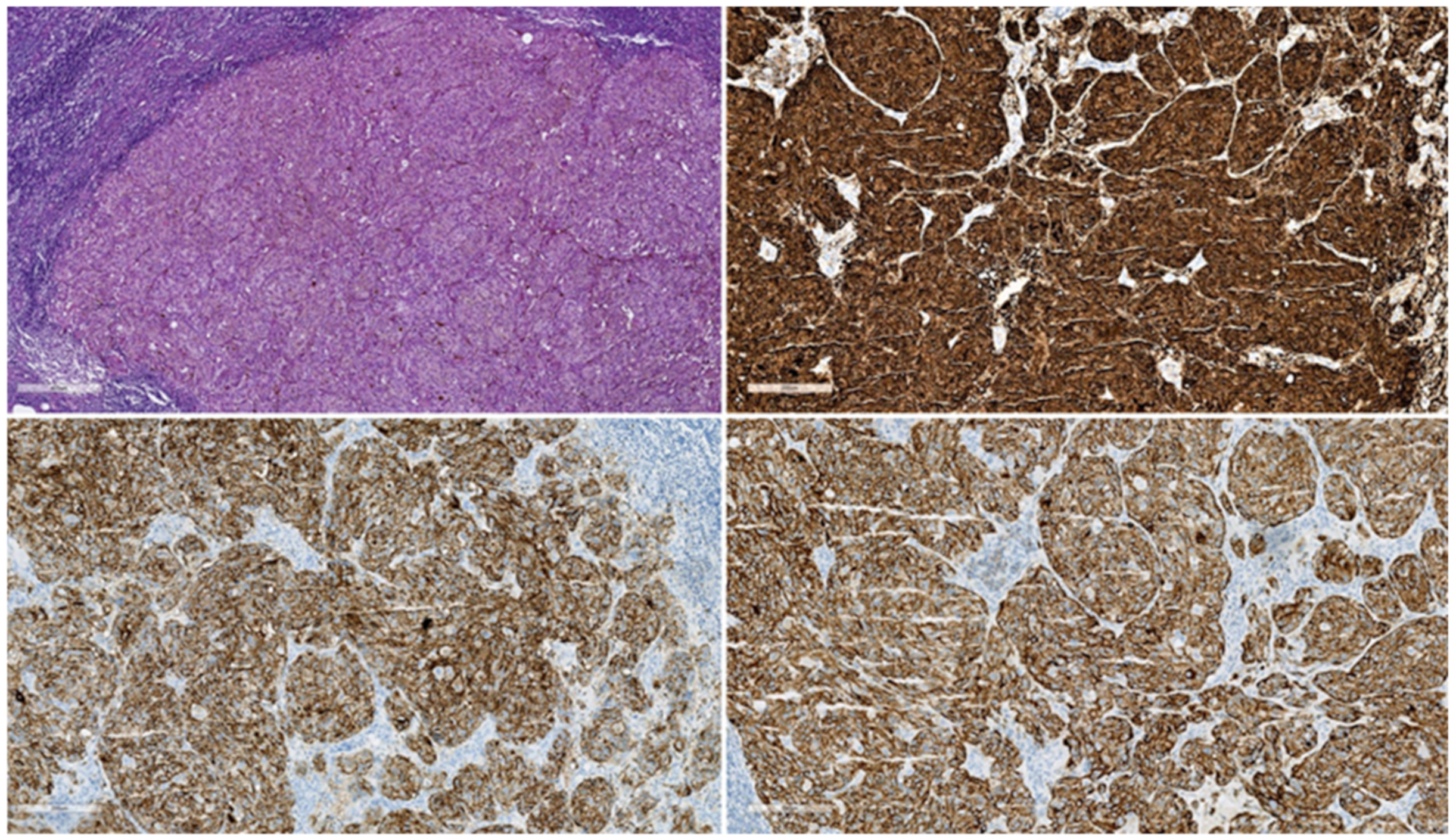
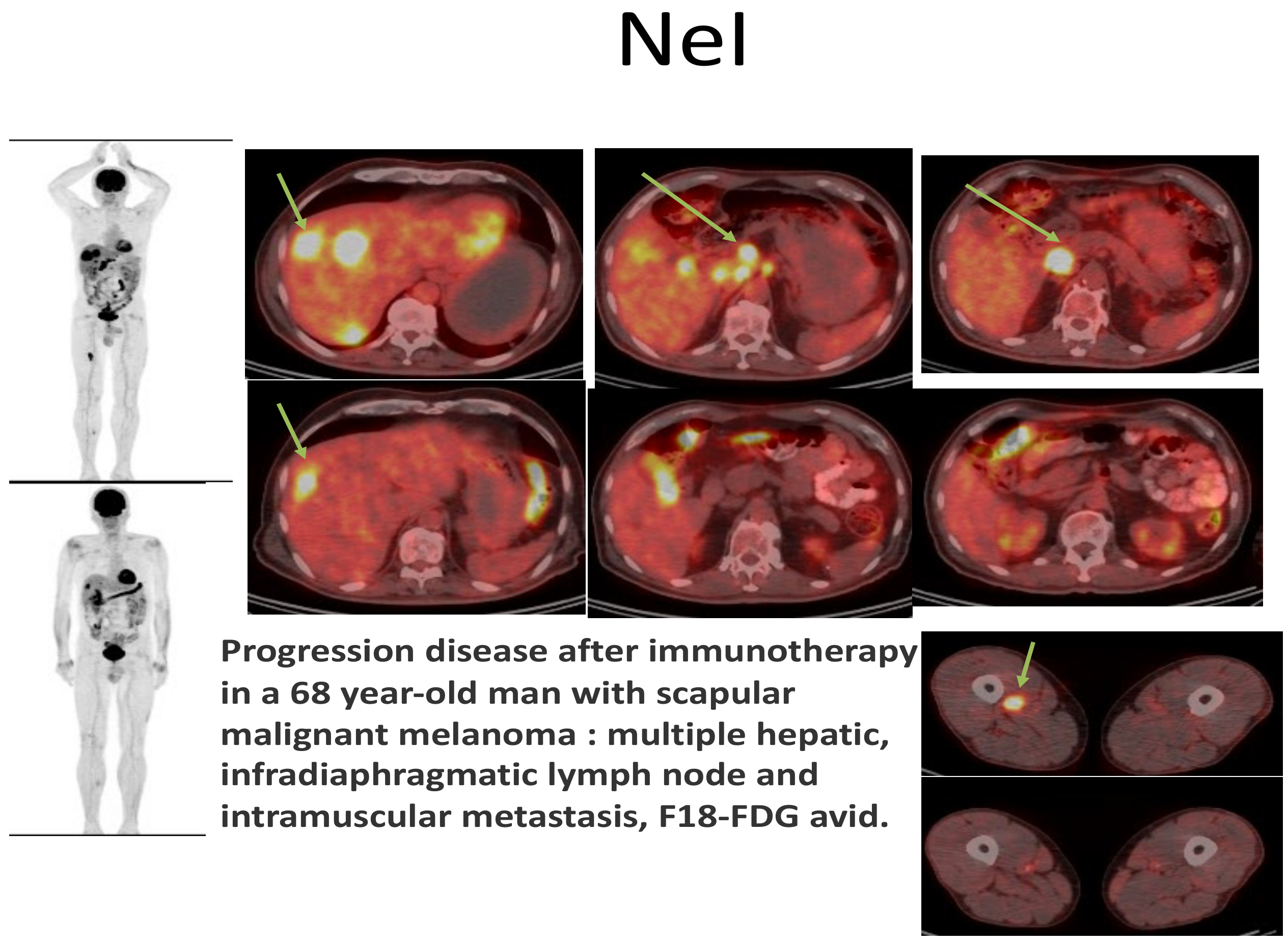
3.3.2. Cutaneous Malignant Melanoma and Its Metastases
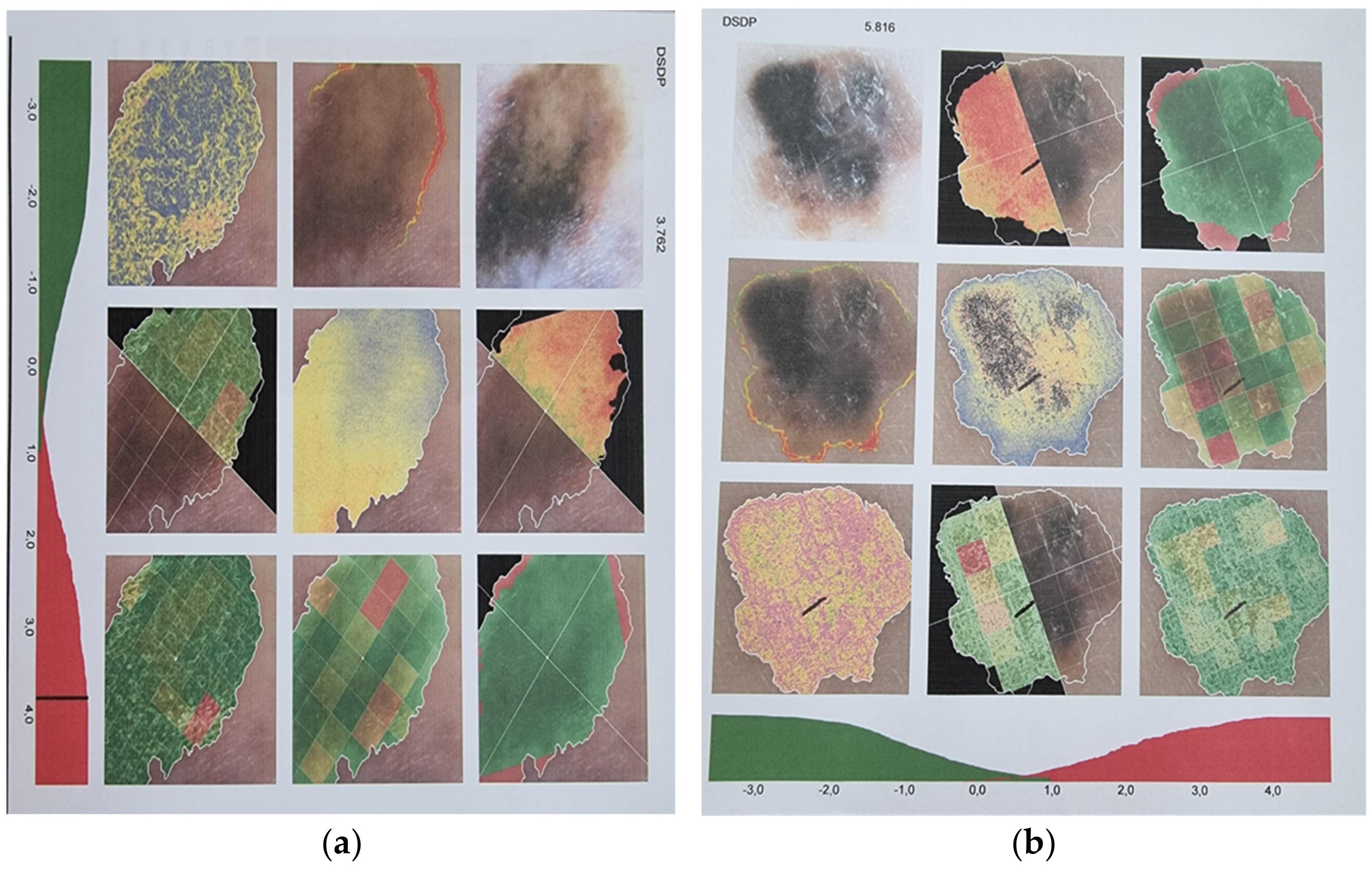
Clinical Aspects
Paraclinical Aspects
3.4. Special Discussions: Melanoma of Unknown Primary, Achromic/Amelanotic Melanoma, Melanoma of the Lower Genital Tract, and Melanoma of the Urinary Tract
3.4.1. Melanoma of Unknown Primary
3.4.2. Achromic/Amelanotic Melanoma
3.4.3. Melanoma of the Lower Genital Tract
3.4.4. Melanoma of the Urinary Tract/Apparatus
The Prostate Gland
The Urinary Bladder
The Urethra
The Ureter
3.5. Melanoma at the Level of the Umbilicus
3.6. Particularities of Surgical Techniques and Other Treatment Options
3.7. Presenting in an Emergency Setting
4. Prognosis
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.G.; Bagwan, I.; Board, R.E.; Capper, S.; Coupland, S.E.; Glen, J.; Lalondrelle, S.; Mayberry, A.; Muneer, A.; Nugent, K.; et al. Ano-uro-genital mucosal melanoma UK national guidelines. Eur. J. Cancer 2020, 135, 22–30. [Google Scholar] [CrossRef]
- Dueñas, C.S.; Esarte, S.G.; Fernández, J.F.J.; Diaz, M.M.; Picazo, R.I. Anorectal melanoma: An atypical cause of rectorrhagia. Gastroenterol. Y Hepatol. 2017, 40, 623–625. [Google Scholar] [CrossRef]
- Davies, E.J.; Terlizzo, M.; Hayes, A.J. Melanoma. Surgery 2021, 40, 46–52. [Google Scholar] [CrossRef]
- Faries, M.B.; Han, D.; Reintgen, M.; Kerivan, L.; Reintgen, D.; Caracò, C. Lymph node metastasis in melanoma: A debate on the significance of nodal metastases, conditional survival analysis and clinical trials. Clin. Exp. Metastasis 2018, 35, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Vuyyuru, R.; Kageyama, K.; Terai, M.; Ohara, M.; Cheng, H.; Manser, T.; Mastrangelo, M.J.; Aplin, A.E.; Sato, T. Establishment and Characterization of Orthotopic Mouse Models for Human Uveal Melanoma Hepatic Colonization. Am. J. Pathol. 2016, 186, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Sonoda, K.; Fujii, K.; Ishikawa, K.; Shiraishi, N.; Kitano, S. A convenient murine model for the study of intra-abdominal lymph node metastasis. Oncol. Rep. 2004, 12, 115–118. [Google Scholar] [CrossRef]
- Thaiss, W.M.; Gatidis, S.; Sartorius, T.; Machann, J.; Peter, A.; Eigentler, T.K.; Nikolaou, K.; Pichler, B.J.; Kneilling, M. Noninvasive, longitudinal imaging-based analysis of body adipose tissue and water composition in a melanoma mouse model and in immune checkpoint inhibitor-treated metastatic melanoma patients. Cancer Immunol. Immunother. 2021, 70, 1263–1275. [Google Scholar] [CrossRef]
- Nakayama, J.; Kokuba, H.; Kobayashi, J.; Yoshida, Y.; Hori, Y. Experimental approaches for the treatment of murine B16 melanomas of various sizes. I: Local injection of ethanol with a combination of interleukin-2 or microwaval hyperthermia for B16 melanomas with a size of less than 7 mm in diameter. J. Dermatol. Sci. 1997, 15, 75–81. [Google Scholar] [CrossRef]
- Park, H.-J. CARI III Inhibits Tumor Growth in a Melanoma-Bearing Mouse Model through Induction of G0/G1 Cell Cycle Arrest. Molecules 2014, 19, 14383–14395. [Google Scholar] [CrossRef]
- Ms, T.M.L.; Hoffman, R.M.; Bouvet, M. Advantages of patient-derived orthotopic mouse models and genetic reporters for developing fluorescence-guided surgery. J. Surg. Oncol. 2018, 118, 253–264. [Google Scholar] [CrossRef]
- Fjeld, J.G.; Vergote, I.; De Vos, L.; Nustad, K. Radio-immunotargeting in experimental animal models of intraperitoneal cancer. Acta Obstet. Gynecol. Scand. 1992, 71, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Mazurak, V.C. Evidence and Mechanisms of Fat Depletion in Cancer. Nutrients 2014, 6, 5280–5297. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2017, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Omata, D.; Munakata, L.; Maruyama, K.; Suzuki, R. Ultrasound and microbubble-mediated drug delivery and immunotherapy. J. Med Ultrason. 2022, 236–244. [Google Scholar] [CrossRef]
- Bisceglia, M.; Perri, F.; Tucci, A.; Tardio, M.; Panniello, G.; Vita, G.; Pasquinelli, G. Primary Malignant Melanoma of the Esophagus. Adv. Anat. Pathol. 2011, 18, 235–252. [Google Scholar] [CrossRef]
- Schizas, D.; Tomara, N.; Katsaros, I.; Sakellariou, S.; Machairas, N.; Paspala, A.; Tsilimigras, D.I.; Papanikolaou, I.S.; Mantas, D. Primary gastric melanoma in adult population: A systematic review of the literature. ANZ J. Surg. 2021, 91, 269–275. [Google Scholar] [CrossRef]
- Miliaras, S.; A Ziogas, I.; Mylonas, K.S.; Papadopoulos, V.N. Primary malignant melanoma of the ascending colon. BMJ Case Rep. 2018, 2018, bcr2017223282. [Google Scholar] [CrossRef]
- Jin, Y.; Ran, C.; Li, F.; Cheng, N. Melanoma of unknown primary in the pancreas: Should it be considered primary? BMC Surg. 2020, 20, 76. [Google Scholar] [CrossRef]
- Shroff, C.P.; Borges, A.M.; Deodhar, K. Primary Melanoma of the Ovary in a 25 Year Old Primigravida—A Case Report. Tumori J. 1989, 75, 72–75. [Google Scholar] [CrossRef]
- Carter, T.J.; George, C.; Harwood, C.; Nathan, P. Melanoma in pregnancy: Diagnosis and management in early-stage and advanced disease. Eur. J. Cancer 2022, 166, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Zikry, J.; Korta, D.Z.; Chapman, L.W.; Linden, K.G. Melanoma arising in an ovarian cystic teratoma: A systematic review of presentation, treatment, and outcomes. Arch. Gynecol. Obstet. 2017, 296, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, M.; Nowak, K.; Kordek, A.; Cymbaluk-Płoska, A. Therapeutic Management of Rare Primary Ovarian Neoplasms: Carcinosarcoma, Leiomyosarcoma, Melanoma and Carcinoid. Int. J. Environ. Res. Public Health 2021, 18, 7819. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Cheng, Z.; Song, S.; Kuang, D.; Zhu, X. Anal Malignant Melanoma Manifesting Hepatic Metastases Shown on FDG PET/CT. Clin. Nucl. Med. 2018, 43, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; Scorsi, A.; Amodio, P.; Goglia, A.; Palmieri, R.M. Primary malignant melanoma of the gallbladder: An outstandingly rare tumor. Clin. Exp. Med. 2015, 16, 479–480. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, C.W.; Kim, S.-W.; Lee, J.L.; Yoon, Y.S.; Park, I.J.; Lim, S.-B.; Yu, C.S.; Kim, J.C. Primary malignant melanoma of the small intestine: A report of 2 cases and a review of the literature. Ann. Surg. Treat. Res. 2018, 94, 274–278. [Google Scholar] [CrossRef]
- Sinagra, E. Ileal Melanoma, a Rare Cause of Small Bowel Obstruction: Report of a Case, and Short Literature Review. Curr. Radiopharm. 2019, 13, 56–62. [Google Scholar] [CrossRef]
- Xu, B.; Hong, Y.; Jin, M.; Li, M.; Wang, C.; Wang, X. Primary adrenal malignant melanoma. A case report and review of literature. Medicine 2017, 96, e8956. [Google Scholar] [CrossRef]
- Shah, K.; Folpe, A.L.; Miller, M.; Morgan, J.A.; Raut, C.P.; Doyle, L.A. Primary intra-abdominal melanoma arising in association with extracutaneous blue naevus: A report of two cases. Histopathology 2021, 78, 281–289. [Google Scholar] [CrossRef]
- Hawryluk, E.B.; Moustafa, D.; Bartenstein, D.; Brahmbhatt, M.; Cordoro, K.; Gardner, L.; Gauthier, A.; Grossman, D.; Gupta, D.; Hunt, R.D.; et al. A retrospective multicenter study of fatal pediatric melanoma. J. Am. Acad. Dermatol. 2020, 83, 1274–1281. [Google Scholar] [CrossRef]
- Jain, P.; Aronow, M.E.; Chawla, B. Advances in Clinical Management of Uveal Melanoma. Adv. Ophthalmol. Optom. 2020, 5, 103–118. [Google Scholar] [CrossRef]
- Kaštelan, S.; Gverović-Antunica, A.; Beketić-Orešković, L.; Kasun, B.; Hat, K. Uveal melanoma: An overview of management and prognosis. Libr. Oncol. Croat. J. Oncol. 2018, 46, 95–104. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.M.; Piperno-Neumann, S.; Grossniklaus, H.E.; Kivelä, T.T. Metastatic uveal melanoma: The final frontier. Prog. Retin. Eye Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kohoutova, D.; Worku, D.; Aziz, H.; Teare, J.; Weir, J.; Larkin, J. Malignant Melanoma of the Gastrointestinal Tract: Symptoms, Diagnosis, and Current Treatment Options. Cells 2021, 10, 327. [Google Scholar] [CrossRef]
- Kanno, Y.; Noda, Y.; Koshita, S.; Ogawa, T.; Masu, K.; Oikawa, M.; Okada, T.; Akazawa, N.; Sawai, T.; Ito, K. Surgically resected pancreatic metastasis from nasal malignant melanoma: Case report and literature review. Clin. J. Gastroenterol. 2019, 12, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Shamim, S.A.; Tripathy, S.; Rastogi, S.; Barwad, A.; Prakash, S. Isolated Pancreatic Metastasis from Choroidal Melanoma After 10 Years of Enucleation Mimicking Neuroendocine Tumor on 68Ga-DOTANOC PET/CT. Clin. Nucl. Med. 2021, 46, e21–e22. [Google Scholar] [CrossRef]
- Trna, J.; Novotný, I.; Tesaříková, P.; Múčková, K.; Poprach, A. Case report of melanoma metastasizing to the pancreas. Gastroenterol. Hepatol. 2018, 72, 212–216. [Google Scholar] [CrossRef]
- Paling, M.R.; Shawker, T.H.; Love, I.L. The sonographic appearance of metastatic malignant melanoma. J. Ultrasound Med. 1982, 1, 75–78. [Google Scholar] [CrossRef]
- Kuan, A.K.; I Jackson, F.; Hanson, J. Multimodality Detection of Metastatic Melanoma. J. R. Soc. Med. 1988, 81, 579–582. [Google Scholar] [CrossRef]
- Meli, R.J.; Ros, P.R. MR appearance of intra-abdominal metastatic melanoma. Magn. Reson. Imaging 1992, 10, 705–708. [Google Scholar] [CrossRef]
- Gritters, L.S.; Francis, I.R.; Zasadny, K.R.; Wahl, R.L. Initial assessment of positron emission tomography using 2-fluorine-18-fluoro-2-deoxy-D-glucose in the imaging of malignant melanoma. J. Nucl. Med. 1993, 34. Available online: https://pubmed.ncbi.nlm.nih.gov/8355058/ (accessed on 17 May 2022).
- Eigtved, A.; Andersson, A.P.; Dahlstrøm, K.; Rabøl, A.; Jensen, M.; Holm, S.; Sørensen, S.S.; Drzewiecki, K.T.; Højgaard, L.; Friberg, L. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur. J. Pediatr. 2000, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tatlidil, R.; Mandelkern, M. FDG-PET in the detection of gastrointestinal metastases in melanoma. Melanoma Res. 2001, 11, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, C.; Juanito, G.; Vander, B.K.; Lawal, I.; Sathekge, M.; Maes, A.; De Spiegeleer, B. Practical Considerations When Interpreting FDG PET/CT Imaging for Staging and Treatment Response Assessment in Melanoma Patients. Semin. Nucl. Med. 2021, 51, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; di Ruffano, L.F.; Takwoingi, Y.; Cheung, S.T.; Nathan, P.; Matin, R.N.; Chuchu, N.; Chan, S.A.; Durack, A.; E Bayliss, S.; et al. Ultrasound, CT, MRI, or PET-CT for staging and re-staging of adults with cutaneous melanoma. Cochrane Database Syst. Rev. 2019, 2019, CD012806. [Google Scholar] [CrossRef] [PubMed]
- Voinea, S.; Sandru, A.; Gherghe, M. Pitfalls in Cutaneous Melanoma Lymphatic Drainage. Chirurgia 2016, 111, 87–89. [Google Scholar]
- Lee, J.H.; Lee, S.M.; Kim, J.E. Prognostic Significance of the Imaging Parameters of Adipose Tissue and Bone Marrow on F-18 Fluorodeoxyglucose PET/CT in Patients with Malignant Melanoma. J. Korean Soc. Radiol. 2019, 80, 1132–1144. [Google Scholar] [CrossRef]
- DeWitt, J.M.; Chappo, J.; Sherman, S. Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Melanoma Metastatic to the Pancreas: Report of Two Cases and Review. Endoscopy 2003, 35, 219–222. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamada, R.; Kaneko, M.; Naota, H.; Fujimura, Y.; Tabata, M.; Kobayashi, K.; Tanaka, K. Isolated pancreatic metastasis from malignant melanoma: A case report and literature review. Clin. J. Gastroenterol. 2019, 12, 626–636. [Google Scholar] [CrossRef]
- Cortellini, F.; Marasco, G.; Renzulli, M.; Vasuri, F.; Ricciardiello, L. Gastric Melanoma of Unknown Primary. J. Gastrointest. Liver Dis. 2021, 30, 14. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Samartzis, E.; Moschetta, M.; Sheriff, M.; Pérez-Fidalgo, J.A.; Pavlidis, N. Melanoma of unknown primary: New perspectives for an old story. Crit. Rev. Oncol. 2021, 158, 103208. [Google Scholar] [CrossRef] [PubMed]
- Opric, D.; Bilanovic, D.; Granic, M.; Randjelovic, T.; Milinic, N.; Opric, S.; Babic, D.; Petrovic, Z.; Radovanovic, D. Visceral metastases of melanoma: Single institution experience an analysis of 15 cases. Acta Chir. Iugosl. 2006, 53, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, A.S.P.; Santacruz, R.S.L. A case report of malignant gastrointestinal melanoma of unknown primary origin. Rev. Colomb. Gastroenterol. 2019, 34, 416–420. [Google Scholar] [CrossRef]
- Wright, J.P.; Geevarghese, S.K. Primary Heptaic Melanoma or Melanoma of Unknown Primary? Am. Surg. 2021, 87, 511–513. [Google Scholar] [CrossRef]
- Serin, G.; Doğanavşargil, B.; Calişkan, C.; Akalin, T.; Sezak, M.; Tunçyürek, M. Colonic malignant melanoma, primary or metastatic? Case report. Turk. J. Gastroenterol. 2010, 21, 45–49. [Google Scholar] [CrossRef]
- Shan, G.-D.; Xu, G.-Q.; Chen, L.-H.; Wang, Z.-M.; Jin, E.-Y.; Hu, F.-L.; Li, Y.-M. Diffuse Liver Infiltration by Melanoma of Unknown Primary Origin: One Case Report and Literature Review. Intern. Med. 2009, 48, 2093–2096. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Lin, Y.-J.; Chiu, S.-H.; Shih, Y.-L. Combined immune checkpoint inhibitors of CTLA4 and PD-1 for hepatic melanoma of unknown primary origin: A case report. World J. Clin. Cases 2021, 9, 2641–2648. [Google Scholar] [CrossRef]
- Karateke, A.; Tug, N.; Sahin, D. Primer odaǧ belli olmayan metastatik over melanomu. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 181–182. [Google Scholar] [CrossRef]
- Ionescu, S.; Nicolescu, A.C.; Madge, O.L.; Marincaş, M.; Mădălina, R.; Simion, L.; Ene, A.; Ceauşu, M. A small bowel metastasis from an achromic melanoma causing intussusception and bowel obstruction—A case presentation. Oncolog-Hematolog.ro 2021, 4, 36. [Google Scholar] [CrossRef]
- Thomas, N.E.; Kricker, A.; Waxweiler, W.T.; Dillon, P.; Busam, K.J.; From, L.; Groben, P.A.; Armstrong, B.K.; Anton-Culver, H.; Gruber, S.B.; et al. Comparison of Clinicopathologic Features and Survival of Histopathologically Amelanotic and Pigmented Melanomas. JAMA Dermatol. 2014, 150, 1306–1314. [Google Scholar] [CrossRef]
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Melanoma of the lower genital tract: Prognostic factors and treatment modalities. Gynecol. Oncol. 2018, 150, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Joste, M.; Dion, L.; Brousse, S.; Timoh, K.N.; Rousseau, C.; Reilhac, A.; Laviolle, B.; Lesimple, T.; Lavoue, V.; Leveque, J. Vulvar and vaginal melanomas: A retrospective study spanning 19 years from a tertiary center. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102091. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Braniff, K. Endometrial metastasis of primary malignant melanoma of the brain. BMJ Case Rep. 2018, 2018, bcr2018224435. [Google Scholar] [CrossRef]
- Samaan, S.; Quddus, M.R.; Matoso, A. “Man in Istanbul” Lesions of the Urinary Tract (Known Entities in an Unusual Context): Melanoma, Carcinoid Tumors, Epithelioid Angiosarcoma. Surg. Pathol. Clin. 2018, 11, 825–836. [Google Scholar] [CrossRef]
- McIntire, P.J.; Kilic, I.; Wojcik, E.M.; Barkan, G.A.; Pambuccian, S.E. The color of urine: Then and now—a comprehensive review of the literature with emphasis on intracytoplasmic pigments encountered in urinary cytology. J. Am. Soc. Cytopathol. 2020, 9, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Addesso, M.; Zeppa, P.; D’Antonio, A. Malignant melanoma of the prostate gland: A systematic review. Pathol. Res. Pr. 2021, 226, 153594. [Google Scholar] [CrossRef] [PubMed]
- Dahm, P.; Gschwend, J.E. Malignant Non-Urothelial Neoplasms of the Urinary Bladder: A Review. Eur. Urol. 2003, 44, 672–681. [Google Scholar] [CrossRef]
- Barillaro, F.; Camilli, M.; Dessanti, P.; Gorji, N.; Chiesa, F.; Villa, A.; Pastorino, A.; Aschele, C.; Conti, E. Primary melanoma of the bladder: Case report and review of the literature. Arch. Ital. Urol. Androl. Organo Uff. Soc. Ital. Ecogr. Urol. E Nefrologica. 2018, 90, 224–226. [Google Scholar] [CrossRef]
- Lange-Welker, U.L.; Papadopoulos, I.; Wacker, H.H. Primary Malignant Melanoma of the Bladder. Urol. Int. 2019, 60, 227–292. [Google Scholar] [CrossRef]
- Quaquarini, E.; Saltalamacchia, G.; Tresoldi, M.M.; Schmid, M.; Villani, L.; Bernardo, A.; Guarneri, C.; Camerota, T.C. Primary melanoma of the bladder: A case report and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5122–5128. [Google Scholar] [CrossRef]
- Bimbatti, D.; Maruzzo, M.; Pierantoni, F.; Diminutto, A.; Dionese, M.; Deppieri, F.M.; Lai, E.; Zagonel, V.; Basso, U. Immune checkpoint inhibitors rechallenge in urological tumors: An extensive review of the literature. Crit. Rev. Oncol. Hematol. 2022, 170, 103579. [Google Scholar] [CrossRef] [PubMed]
- Grilo, I.; Rodrigues, C.; Soares, A.; Grande, E. Facing treatment of non-urothelial bladder cancers in the immunotherapy era. Crit. Rev. Oncol. Hematol. 2020, 153, 103034. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Fukuhara, H.; Homma, Y.; Todo, T. Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int. J. Urol. 2017, 24, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Radhakrishna, N.; A Badhe, B. Malignant melanoma of urethra in a young female—Case report and Literature review. Gulf. J. Oncol. 2021, 1, 92–95. Available online: https://pubmed.ncbi.nlm.nih.gov/33716219/ (accessed on 2 June 2022). [PubMed]
- Shih, M.-H.; Chiang, P.-J.; Meng, E.; Yu, D.-S. Primary melanoma of the vagina with urethral invasion: A case report and literature review. Taiwan. J. Obstet. Gynecol. 2021, 60, 969–970. [Google Scholar] [CrossRef]
- Yang, N.; Lu, J.; Lu, Y.; Guo, J.; Wang, H. Primary malignant melanotic melanoma and hypomelanotic melanoma of the female urethra: Case series and a review of the literature in China. Melanoma Res. 2019, 29, 59–64. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, L.; Huang, Y.; Wen, S.; Liu, T.; Duan, X.; Wang, Y.; Zhang, H.; Fan, B.; Hu, B. Identification of distinct genomic features reveals frequent somatic AHNAK and PTEN mutations predominantly in primary malignant melanoma presenting in the ureter. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef]
- Papalas, J.A.; Selim, M.A. Metastatic vs primary malignant neoplasms affecting the umbilicus: Clinicopathologic features of 77 tumors. Ann. Diagn. Pathol. 2011, 15, 237–242. [Google Scholar] [CrossRef]
- Vulovic, D.; Kozarski, J.; Petrovic, D.; Gregovic, M.; Jovanovic, D.; Radivojcevic, U. Atypical primary melanoma of the umbilicus: A case report. Vojn. Pregl. 2018, 75, 1237–1240. [Google Scholar] [CrossRef]
- Sandru, A.; Bordea, C.I.; Voinea, S.C.; Gherghe, M.; Albert, P.; Condrea, I.; Blidaru, A. Actualităţi în tratamentul chirurgical al melanomului malign cutanat [Latest approaches in the surgical treatment of cutaneous malignant melanoma]. Chirurgia (Bucharest, Romania: 1990) 2011, 106, 301–308. [Google Scholar]
- Kaur, A.; Rayatt, S.; Jagadeesan, J.; Hejmadi, R.; Singh, S. Metastatic melanoma vs lymphoma. Using a sentinel lymph node biopsy a diagnostic tool. J. Surg. Case Rep. 2019, 2019, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kastl, S.; Wutke, R.; Czeczatka, P.; Hohenberger, W.; Horbach, T. Palliation of a primary malignant melanoma of the distal esophagus by stent implantation. Report of a case. Surg. Endosc. 2001, 15, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Pingpank, J.F.; Hoffman, J.P.; Sigurdson, E.R.; Ross, E.; Sasson, A.R.; Eisenberg, B.L. Pancreatic resection for locally advanced primary and metastatic nonpancreatic neoplasms. Am. Surg. 2002, 68, 337–340. Available online: https://pubmed.ncbi.nlm.nih.gov/11952243 (accessed on 17 May 2022).
- Choi, W.K.; Lee, D.H.; Cho, D.H.; Jang, K.Y.; Kim, K.M. Primary malignant melanoma arising from ruptured ovarian mature cystic teratoma with elevated serum CA 19–9: A case report and review of literature. BMC Women’s Health 2019, 19, 1–5. [Google Scholar] [CrossRef]
- Kottakota, V.; Warikoo, V.; Yadav, A.K.; Salunke, A.; Jain, A.; Sharma, M.; Bhatt, S.; Puj, K.; Pandya, S. Clinical and oncological outcomes of surgery in Anorectal melanoma in Asian population: A 15 year analysis at a tertiary cancer institute. Cancer Treat. Res. Commun. 2021, 28, 100415. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Polizzi, M.L.; Beltrame, V.; Moro, M.; Pedrazzoli, S. Pancreatic Resection for Metastatic Melanoma. Case Report and Review of the Literature. J. Gastrointest. Cancer 2011, 42, 302–306. [Google Scholar] [CrossRef]
- Guerra, F.; Marra, U.; Giuliani, G.; Coratti, A. Robotic Extended Ultrasound-Guided Distal Pancreatectomy for Pancreatic Metastases from Uveal Melanoma. Ann. Surg. Oncol. 2022, 29, 2469–2470. [Google Scholar] [CrossRef]
- Zippel, D.; Yalon, T.; Nevo, Y.; Markel, G.; Asher, N.; Schachter, J.; Goitein, D.; Segal, T.A.; Nissan, A.; Hazzan, D. The non-responding adrenal metastasis in melanoma: The case for minimally invasive adrenalectomy in the age of modern therapies. Am. J. Surg. 2020, 220, 349–353. [Google Scholar] [CrossRef]
- Belágyi, T.; Zsoldos, P.; Makay, R.; Issekutz, A.; Oláh, A. Multiorgan resection (including the pancreas) for metastasis of cutaneous malignant melanoma. JOP 2006, 7, 234–240. Available online: https://pubmed.ncbi.nlm.nih.gov/16525211/ (accessed on 17 May 2022).
- Wood, T.F.; DiFronzo, L.A.; Rose, D.M.; Haigh, P.I.; Stern, S.L.; Wanek, L.; Essner, R.; Morton, D.L. Does Complete Resection of Melanoma Metastatic to Solid Intra-Abdominal Organs Improve Survival? Ann. Surg. Oncol. 2001, 8, 658–662. [Google Scholar] [CrossRef]
- Hodgson, R.; Fink, M.A.; Jones, R.M. The Role of Abdominal Resectional Surgery in Metastatic Melanoma. ANZ J. Surg. 2007, 77, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, L.M.; Levine, E.A.; Shen, P.; Votanopoulos, K.I. Role of Surgery for Metastatic Melanoma. Surg. Clin. N. Am. 2020, 100, 127–139. [Google Scholar] [CrossRef]
- Shenoy, S.; Cassim, R. Metastatic melanoma to the gastrointestinal tract: Role of surgery as palliative treatment. West Va. Med. J. 2013, 109, 30–33. [Google Scholar]
- Caputy, G.G.; Donohue, J.H.; Goellner, J.R.; Weaver, A.L. Metastatic melanoma of the gastrointestinal tract. Results of surgical management. Arch. Surg. 1991, 126, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.M.; Panageas, K.S.; Hollmann, T.; Shoushtari, A.N.; Momtaz, P.; Chapman, P.B.; Postow, M.A.; Callahan, M.K.; Wolchok, J.D.; Brady, M.S.; et al. Survival Outcomes After Metastasectomy in Melanoma Patients Categorized by Response to Checkpoint Blockade. Ann. Surg. Oncol. 2020, 27, 1180–1188. [Google Scholar] [CrossRef]
- Wankhede, D.; Grover, S. Outcomes after Curative Metastasectomy for Patients with Malignant Melanoma: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2022, 29, 3709–3723. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, A.; Patel, N.; Chandra, S.R.; Vijayaraghavan, R. Imaging and Laboratory Workup for Melanoma. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 235–250. [Google Scholar] [CrossRef]
- Hintze, J.M.; O’Connor, D.B.; Molony, P.; Neary, P.C. Distant melanoma causing small bowel obstruction. J. Surg. Case Rep. 2017, 2017, 1–3. [Google Scholar] [CrossRef][Green Version]
- Hedayati, A.A.; Bandurski, J.; Lewandowski, A. Bifocal metastasis of melanoma to the small intestine from an unknown primary with intestinal obstruction—Case report. Współczesna Onkologia 2013, 3, 317–320. [Google Scholar] [CrossRef]
- Ben Slama, S.; Bacha, D.; Bayar, R.; Gharbi, L.; Lahmar, A. Pancreatic resection for isolated metastasis from melanoma of unknown primary. Acta Gastro-Enterol. Belg. 2017, 80, 323–324. [Google Scholar]
- Jia, Z.; Jiang, G.; Zhu, C.; Wang, K.; Li, S.; Qin, X. A systematic review of yttrium-90 radioembolization for unresectable liver metastases of melanoma. Eur. J. Radiol. 2017, 92, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Foretová, L.; Navrátilová, M.; Svoboda, M.; Házová, J.; Vašíčková, P.; Hrabincová, E.S.; Fabian, P.; Schneiderová, M.; Macháčková, E. BAP1 Syndrome—Predisposition to Malignant Mesothelioma, Skin and Uveal Melanoma, Renal and Other Cancers. Klin. Onkol. 2019, 32, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Basu, D.; Nikiforova, M.; Hamilton, R.L.; Gehris, R.; Jakacki, R.; Panigrahy, A.; Yatsenko, S.; Reyes-Múgica, M. Amplification of mutated NRAS leading to congenital melanoma in neurocutaneous melanocytosis. Melanoma Res. 2015, 25, 453–460. [Google Scholar] [CrossRef]
- Lynch, H.T.; Brand, R.E.; Hogg, D.; Deters, C.A.; Fusaro, R.M.; Lynch, J.F.; Liu, L.; Knezetic, J.; Lassam, N.J.; Goggins, M.; et al. Phenotypic variation in eight extendedCDKN2A germline mutation: The familial atypical multiple mole melanoma-pancreatic carcinoma-prone families. Cancer 2001, 94, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.; Busam, K.J.; From, L.; Groben, P.A.; Anton-Culver, H.; Cust, A.E.; Begg, C.B.; Dwyer, T.; Gallagher, R.P.; Gruber, S.B.; et al. Inherited Variation at MC1R and Histological Characteristics of Primary Melanoma. PLoS ONE 2015, 10, e0119920. [Google Scholar] [CrossRef]
- Hu, B.; Wei, Q.; Li, X.; Ju, M.; Wang, L.; Zhou, C.; Chen, L.; Li, Z.; Wei, M.; He, M.; et al. Development of an IFNγ response-related signature for predicting the survival of cutaneous melanoma. Cancer Med. 2020, 9, 8186–8201. [Google Scholar] [CrossRef]
- Tate, G.; Tajiri, T.; Suzuki, T.; Mitsuya, T. Mutations of the KIT gene and loss of heterozygosity of the PTEN region in a primary malignant melanoma arising from a mature cystic teratoma of the ovary. Cancer Genet. Cytogenet. 2009, 190, 15–20. [Google Scholar] [CrossRef]
- Inoue, K.; Tsubamoto, H.; Isono-Nakata, R.; Sakata, K.; Nakagomi, N. Itraconazole treatment of primary malignant melanoma of the vagina evaluated using positron emission tomography and tissue cDNA microarray: A case report. BMC Cancer 2018, 18, 630. [Google Scholar] [CrossRef]
- Arnoff, T.E.; El-Deiry, W.S. mdm2/mdm4 amplification and cdkn2a deletion in metastatic melanoma and glioblas-toma multiforme may have implications for targeted therapeutics and immunotherapy. Am. J. Cancer Res. 2022, 12, 2102. [Google Scholar]
- Molina, G.; Kasumova, G.G.; Qadan, M.; Boland, G.M. Use of immunotherapy and surgery for stage IV melanoma. Cancer 2020, 126, 2614–2624. [Google Scholar] [CrossRef]
- Reed, J.A.; Lin, Q.; Chen, D.; Mian, I.S.; Medrano, E.E. SKI pathways inducing progression of human melanoma. Cancer Metastasis Rev. 2005, 24, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Mabaera, R.; Shirai, K. Metastatic uveal melanoma showing durable response to anti-CTLA-4 and anti-PD-1 combination therapy after experiencing progression on anti-PD-1 therapy alone. J. Immunother. Cancer 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Green, M.D.; Huppert, L.A.; Chow, C.; Pierce, R.H.; Daud, A.I. The Liver-Immunity Nexus and Cancer Immunotherapy. Clin. Cancer Res. 2022, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Malissen, N.; Farvacque, G.; Duconseil, P.; Birnbaum, D.J.; Falque, C.; Macagno, N.; Grob, J.-J.; Gaudy-Marqueste, C.; Moutardier, V. Surgery of small bowel melanoma metastases in the era of efficient medical therapies. Melanoma Res. 2021, 31, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ladányi, A.; Hegyi, B.; Balatoni, T.; Liszkay, G.; Rohregger, R.; Waldnig, C.; Dudás, J.; Ferrone, S. HLA Class I Downregulation in Progressing Metastases of Melanoma Patients Treated with Ipilimumab. Pathol. Oncol. Res. 2022, 28, 1610297. [Google Scholar] [CrossRef]
- Genova, P.; Sorce, M.; Cabibi, D.; Genova, G.; Gebbia, V.; Galanti, D.; Ancona, C.; Valerio, M.R. Gastric and Rectal Metastases from Malignant Melanoma Presenting with Hypochromic Anemia and Treated with Immunotherapy. Case Rep. Oncol. Med. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Bonnin, A.; Durot, C.; Barat, M.; Djelouah, M.; Grange, F.; Mulé, S.; Soyer, P.; Hoeffel, C. CT texture analysis as a predictor of favorable response to anti-PD1 monoclonal antibodies in metastatic skin melanoma. Diagn. Interv. Imaging 2022, 103, 97–102. [Google Scholar] [CrossRef]
- Da Silva, I.P.; Long, G.; Quek, C.; Gonzalez, M.; Carlino, M.S.; Long, G.V.; Menzies, A.M. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti–PD-1 therapy. Cancer 2020, 126, 86–97. [Google Scholar] [CrossRef]
- Marletta, S.; Fusco, N.; Munari, E.; Luchini, C.; Cimadamore, A.; Brunelli, M.; Querzoli, G.; Martini, M.; Vigliar, E.; Colombari, R.; et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J. Pers. Med. 2022, 12, 1073. [Google Scholar] [CrossRef]
- Mantas, D.; Tsaparas, P.; Charalampoudis, P.; Gogas, H.; Kouraklis, G. Emergency Surgery for Metastatic Melanoma. Int. J. Surg. Oncol. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Den Uil, S.H.; Thomassen, I.; Vermeulen, E.G.; Vuylsteke, R.J.; Stockmann, H.B.; de Vries, M. Small bowel perforation caused by advanced melanoma. Tumori J. 2014, 100, e140–e143. [Google Scholar] [CrossRef]
- Okamoto, T. Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer. World J. Gastroenterol. 2022, 28, 985–1008. [Google Scholar] [CrossRef] [PubMed]
- Piltcher-da-Silva, R.; Sasaki, V.L.; Hutten, D.O.; Percicote, A.P.; Trippia, C.H.; Junior, R.A.A.; da Costa, M.A.R.; Coelho, J.C. Biliary tract melanoma metastasis mimicking hilar cholangiocarcinoma: A case report. J. Surg. Case Rep. 2021, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M.; Solon, J.; Chang, K.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal metastases from extra-abdominal cancer—A population-based study. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Behranwala, K.A.; Roy, P.; Giblin, V.; A’Hern, R.; Fisher, C.; Thomas, J.M. Intra-abdominal metastases from soft tissue sarcoma. J. Surg. Oncol. 2004, 87, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Gabali, A.M.; Priebe, P.; Ganesan, S. Primary Melanoma of Small Intestine Masquerading as Gastrointestinal Stromal Tumor: A Case Report and Literature Review. Am. Surg. 2008, 74, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Wünsch, P.H. Distribution of neural cell adhesion molecule (NCAM/CD56) in gastrointestinal stromal tumours and their intra-abdominal mesenchymal mimics. J. Clin. Pathol. 2008, 61, 499–503. [Google Scholar] [CrossRef]
- Wong, N.A.C.S.; Melegh, Z. Gastrointestinal stromal tumours can express CD10 and epithelial membrane antigen but not oestrogen receptor or HMB45. Histopathology 2011, 59, 781–785. [Google Scholar] [CrossRef]
- Allanson, B.M.; Weber, M.A.; Jackett, L.A.; Chan, C.; Lau, L.; Ziegler, D.S.; Warby, M.; Mayoh, C.; Cowley, M.J.; Tucker, K.M.; et al. Oral malignant gastrointestinal neuroectodermal tumour with junctional component mimicking mucosal melanoma. Pathology 2018, 50, 648–653. [Google Scholar] [CrossRef]
- De Sá, T.C.; Ferreira, A.I.; Barreto, F.; Santos, M. Jejunal intussusceptions due to metastatic malignant melanoma: A case report. J. Surg. Case Rep. 2021, 2021, 1–3. [Google Scholar] [CrossRef]
- Lasithiotakis, K.; Zoras, O. Metastasectomy in cutaneous melanoma. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Byron, Y.; Nott, L.; Shackleton, M. Case report: Acute tumour lysis syndrome following encorafenib and binimetinib for v600E metastatic melanoma with large intra-abdominal mass. Melanoma Res. 2020, 30, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Ohe, S.; Tanaka, M.; Maniwa, T.; Isei, T. Tumor lysis syndrome induced by BRAF/MEK double blockade in a patient with metastatic melanoma: A first case report. J. Dermatol. 2021, 48, e324–e326. [Google Scholar] [CrossRef] [PubMed]
- Castellani, E.; Covarelli, P.; Boselli, C.; Cirocchi, R.; Rulli, A.; Barberini, F.; Caracappa, D.; Cini, C.; Desiderio, J.; Burini, G.; et al. Spontaneous splenic rupture in patient with metastatic melanoma treated with vemurafenib. World J. Surg. Oncol. 2012, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, T.; Hiramatsu, K.; Nemoto, T.; Saito, Y.; Ozaki, Y.; Takahashi, K.; Naito, T.; Ofuji, K.; Matsuda, H.; Ohtani, M.; et al. Ruptured hepatic metastases of cutaneous melanoma during treatment with vemurafenib: An autopsy case report. BMC Clin. Pathol. 2015, 15, 15. [Google Scholar] [CrossRef]
- Patel, K.R.; Lee, L.Y.; Tripathy, A.; McKean, D. Case of small bowel perforation secondary to nivolumab and ipilimumab related tumour regression. BMJ Case Rep. 2020, 13, e232304. [Google Scholar] [CrossRef]
- Bisschop, C.; Wind, T.T.; Blank, C.U.; Koornstra, R.H.; Kapiteijn, E.; Eertwegh, A.J.V.D.; De Groot, J.W.B.; Jalving, M.; Hospers, G. Association Between Pembrolizumab-related Adverse Events and Treatment Outcome in Advanced Melanoma: Results From the Dutch Expanded Access Program. J. Immunother. 2019, 42, 208–214. [Google Scholar] [CrossRef]
- Olischewsky, A.; De Schrijver, S.; Bankfalvi, A.; Wetter, A.; Zimmer, L.; Livingstone, E.; Schadendorf, D.; Ugurel, S. Dose-dependent toxicity of ipilimumab in metastatic melanoma. Eur. J. Cancer 2018, 95, 104–108. [Google Scholar] [CrossRef]
- Barat, M.; Guegan-Bart, S.; Cottereau, A.-S.; Guillo, E.; Hoeffel, C.; Barret, M.; Gaujoux, S.; Dohan, A.; Soyer, P. CT, MRI and PET/CT features of abdominal manifestations of cutaneous melanoma: A review of current concepts in the era of tumor-specific therapies. Abdom. Radiol. 2021, 46, 2219–2235. [Google Scholar] [CrossRef]
- Pourvaziri, A.; Parakh, A.; Biondetti, P.; Sahani, D.; Kambadakone, A. Abdominal CT manifestations of adverse events to immunotherapy: A primer for radiologists. Abdom. Radiol. 2020, 45, 2624–2636. [Google Scholar] [CrossRef]
- Capaccione, K.M.; Valiplackal, J.P.; Huang, A.; Roa, T.; Fruauff, A.; Liou, C.; Kim, E.; Khurana, S.; Maher, M.; Ma, H.; et al. Checkpoint Inhibitor Immune-Related Adverse Events: A Multimodality Pictorial Review. Acad. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ramelyte, E.; Schindler, S.H.; Dummer, R. The safety of anti PD-1 therapeutics for the treatment of melanoma. Expert Opin. Drug Saf. 2017, 16, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tan, P.; Ai, J.; Zhang, S.; Zheng, X.; Liao, X.; Yang, L.; Wei, Q. Antitumor Activity and Treatment-Related Toxicity Associated with Nivolumab Plus Ipilimumab in Advanced Malignancies: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Wistuba-Hamprecht, K.; Gouttefangeas, C.; Weide, B.; Pawelec, G. Immune Signatures and Survival of Patients with Metastatic Melanoma, Renal Cancer, and Breast Cancer. Front. Immunol. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Gandini, S.; Zanna, I.; De Angelis, S.P.; Cocorocchio, E.; Queirolo, P.; Lee, J.H.; Carlino, M.S.; Mazzarella, L.; Duso, B.A.; Palli, D.; et al. Circulating tumour DNA and melanoma survival: A systematic literature review and meta-analysis. Crit. Rev. Oncol. 2021, 157, 103187. [Google Scholar] [CrossRef]
- Korfiati, A.; Grafanaki, K.; Kyriakopoulos, G.C.; Skeparnias, I.; Georgiou, S.; Sakellaropoulos, G.; Stathopoulos, C. Revisiting miRNA Association with Melanoma Recurrence and Metastasis from a Machine Learning Point of View. Int. J. Mol. Sci. 2022, 23, 1299. [Google Scholar] [CrossRef]

| Research Site/Database | Search Terms | Additional Filters |
|---|---|---|
| www.scopus.com | ”intra-abdominal metastases from malignant melanoma” | “medicine”, “review”, “English” |
| www.scopus.com | ”intra-abdominal malignant melanoma” | 2018–2022, subject area “medicine”, “English” |
| www.scopus.com | ”melanoma of unknown primary”, and “abdominal AND melanoma AND systematic AND review” | “English”, “medicine” and “journal”, “after 2015” |
| www.scopus.com | “melanoma of the urinary tract review” | “medicine”, 2017–2022, “English”, “Journals”, “reviews” |
| www.pubmed.org | “intra-abdominal malignant melanoma” | “English” and “humans” |
| www.sciencedirect.com | “intra-abdominal malignant melanoma” | “subscribed journals”, 2017–2022, review articles, medicine and dentistry |
| www.sciencedirect.com | “amelanotic melanoma of the abdomen”, | “subscribed journal”, 2017–2022, “medicine and dentistry” |
| www.sciencedirect.com | “achromic AND melanoma AND metastases” | |
| www.sciencedirect.com www.pubmed.org http://academic.oup.com | “(urinary OR kidney OR bladder OR urethra OR ureter) AND melanoma” | |
| http://academic.oup.com | “intra-abdominal malignant melanoma” | “journal article”, 2017–2022, medicine and health |
| First Author of the Study and Year of Publication | Type of Model | Principle and Assets |
|---|---|---|
| [7], 2004 | Murine model | It may help in the study of lymph node metastases |
| [8], 2021 | Murine model | It can detect early the appearance of metastases and can monitor the response to immune checkpoint inhibitors |
| [9], 1997 | Murine model | Allows experimental local treatment of the melanoma |
| [10], 2014 | Murine model | It allows the study of the anti-proliferative effect of mushroom mycelia, including in comparison with chemotherapy regimens |
| [11], 2018 | Patient-derived orthotopic mouse models | It may show how melanoma cell lines implanted in the abdominal cavity can form nodules |
| [12], 1992 | Murine model | Radio-immunotargetting-monoclonal antibodies in intraperitoneal malignancy |
| [13], 2014 | Murine model | It allows the study of the pattern of fat loss in cancer |
| [14], 2017 | Murine models | It allows the study of perioperative events that influence cancer recurrence risk |
| [15], 2022 | Review of ultrasound and microbubble-mediated delivery on animal models | The combination of ultrasound and microbubbles is a promising strategy for increasing vascular permeability, thereby enhancing drug delivery to tissues. This combination has also been applied to gene and protein delivery, including immunotherapy cytokines and antigens. |
| Primary Site | Genetic Factors Determining the Anatomic Location | Data on Response to Immunotherapy |
|---|---|---|
| Predisposition to uveal and cutaneous melanoma | BAP1 syndrome | BAP1 syndrome has many facets as a complex cancer syndrome characterized by an increased risk of rare malignant mesothelioma, malignant skin and uveal melanoma, spitzoid-type skin lesions, and other tumors. |
| Detection of this syndrome is crucial for the survival of individuals at high risk [102]. | ||
| Amplification of mutated NRAS | The amplification of mutated NRAS is associated with Congenital melanoma in neurocutaneous melanocytosis [103] | |
| CDKN2A germline mutation | Hereditary pancreatic carcinoma shows extant phenotypic and genotypic heterogeneity as evidenced by its integral association with a variety of hereditary cancer syndromes inclusive of the familial atypical multiple mole melanoma (FAMMM) syndrome in concert with CDKN2A (p16) germline mutations [104]. | |
| Variation in the melanocortin-1receptor MC1R gene | The results of a study by [105] suggest that inherited variation in MC1R may play a significant role in the anatomic site presentation of melanomas and may vary in relation to skin pigmentation phenotype. | |
| Tumor immune microenvironment | IFN response-related gene signature (UBE2L6, PARP14, IFIH1, IRF2, and GBP4) | A novel five-IFN response-related gene signature (UBE2L6, PARP14, IFIH1, IRF2, and GBP4) was developed, which provided a better and more comprehensive understanding of the tumor immune landscape and demonstrated excellent performance in predicting patient outcomes for SKCM (skin cutaneous melanoma) [106]. |
| Mucosal melanomas (respiratory, gastrointestinal, and of the urogenital tract) | MM is genetically distinct from its skin-based counterparts. Common drivers in cutaneous melanoma, such as B-raf proto-oncogene serine/threonine kinase (BRAF), have a lower mutation rate in multiple myeloma (MM), whereas mutations of other genes, such as the KIT proto-oncogene, receptor tyrosine kinase (KIT), and splicing factor 3b subunit 1 gene (SF3B1), are more prevalent The presence of KIT mutations, which are potential targets of tyrosine kinase inhibitors currently in clinical trials (imatinib), as well as SF3B1 mutations, CDK4 amplifications, and CDKN2A gene deletions are being investigated in clinical trials. MM of the ovaries related to KIT gene mutation and loss of heterozygosity of the PTEN region MM of the vagina related to downregulation of the following 4 genes: STATH, EEF1A2, TTR, and CDH2. | Immune checkpoint inhibitors of CTLA4 (ipilimumab) and PD-1 were administered to the patient (pembrolizumab and nivolumab). Research [107] points to the fact that LOH (loss of heterozygosity) of the PTEN region is one of the molecular alterations of an ovarian mature cystic teratoma, and a KIT mutation is an additional event that promotes the oncogenesis of a melanoma arising from an ovarian mature cystic teratoma. The results of this case study suggest that itraconazole may be an effective treatment option for vaginal primary malignant melanoma. In addition, the authors identified potential itraconazole target genes, which could aid in the elucidation of the disease’s underlying mechanism and the development of new therapeutic agents [108]. |
| Primary esophageal | C-KIT, PDGFR, NRAS, KRAS mutations NF1 was the gene most frequently altered. Other mutated genes included SF3B1, KRAS, BRCA2, KIT, and TP53. | |
| Stage IV melanoma | Compared to primary disease, metastatic disease is enriched for MDM2 and MDM4 amplifications, and amplifications are associated with decreased overall survival. Amplifications of MDM2/4 are associated with a higher incidence of brain and liver metastasis. USP7 and PPM1D, two negative regulators of p53, are also altered in metastatic melanoma relative to primary disease. SKI pathways inducing progression of melanoma [109]. Pembrolizumab initially appeared to be significantly less effective in melanoma and non-small cell lung cancer (NSCLC) patients with liver metastases. | [110]: In a study comprising 14,433 patients with stage IV melanoma has found that Immunotherapy was distributed unequally among patients with stage IV melanoma. The rates of surgical resection of metastatic disease for stage IV melanoma did not differ between the checkpoint inhibitor era and the pre-checkpoint inhibitor era across all facilities. Patients with melanoma or GBM and amplifications in MDM2/4 and CDKN2A alterations may benefit from combinations of targeted inhibitors of MDM2/4 and CDK4/6, as well as immunotherapy, according to the authors [109]. SKI plays additional roles both within and without the nucleus. In normal melanocytes and primary non-invasive melanomas, SKI is predominantly nuclear, whereas, in primary invasive melanomas, SKI is both nuclear and cytoplasmic. SKI distribution is intriguingly nuclear and cytoplasmic or predominantly cytoplasmic in metastatic melanoma tumors [111]. Case reports [112,57] showed a durable response to anti CTLA-4 and anti-PD1. Several recent clinical and translational studies [113] have focused on the impact of liver metastases on the effectiveness of immune checkpoint inhibitors in patients with solid-tumor malignancies. A retrospective study on 20 consecutive small bowel melanoma metastases was described. The conclusion was that although medical treatments for metastatic melanoma have dramatically improved survival, surgical control of life-threatening localizations such as small bowel metastases is frequently a prerequisite for long survival [114]. Compared to metastases removed prior to ipilimumab therapy, post-treatment lesions exhibited significantly lower HL class I expression on melanoma cells; HLA class I downregulation was most pronounced in metastases from nonresponding patients that were progressing. The results suggest that HLA class I downregulation may serve as a mechanism of ICI resistance [115]. Case report [116] described immunotherapy with ipilimumab and pembrolizumab. |
| Prediction factors that can influence the response to immunotherapy: 1. CT texture analysis 2. Anatomic location 3. The evaluation of PD-L1 immunohistochemical expression | The conclusion of a study [117] was that patients with metastatic SM may use CT texture analysis-derived tumor skewness and variation of entropy between baseline and first control CT examination as predictors of favorable response to anti-PD1 monoclonal antibodies. In a multivariate analysis, patients with lung metastases had superior ORR and progression-free survival, whereas patients with liver metastases had inferior ORR and progression-free survival, demonstrating that treatment response and, consequently, survival can vary with anatomic location [118]. An atlas of PD-L1 for pathologists has been created [119]. |
| Interventional, phase 2, developed in Rochester, Minn, NCT ID: NCT01143402 |
| Interventional, phase 2, developed in Rochester, Minn, NCT ID: NCT01835145 |
| Interventional, phase 2, developed in Rochester, Minn, NCT ID: NCT00700882 |
| Interventional, Rochester, Minn, Site IRB Rochester, Minnesota: 14-001651 |
| Interventional, phase 2, Scottsdale/Phoenix, Ariz, and Rochester, Minn NCT ID: NCT02301611 |
| Observational NCT ID: NCT02780089 |
| Interventional NCT ID: NCT02320305 |
| Interventional, phase 1, NCT ID: NCT03865212 |
| Interventional, phase 2 NCT ID: NCT02363283 |
| Interventional, phase ½, NCT ID: NCT03325101 |
| Interventional, Sahlgrenska University HospitalGothenburg, Sweden NCT03879395 |
| Interventional, The Affiliated Hospital of Qingdao UniversityQingdao, Shandong, China NCT03966456 |
| Mayo Clinic Hospital in Arizona Phoenix, AZ, USA Mayo Clinic in Arizona Scottsdale, AZ, USA Mayo Clinic in Florida Jacksonville, FL, USA Mayo Clinic in Rochester Rochester, MN, USA NCT02020707 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, S.; Nicolescu, A.C.; Madge, O.-L.; Simion, L.; Marincas, M.; Ceausu, M. Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review. Diagnostics 2022, 12, 2054. https://doi.org/10.3390/diagnostics12092054
Ionescu S, Nicolescu AC, Madge O-L, Simion L, Marincas M, Ceausu M. Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review. Diagnostics. 2022; 12(9):2054. https://doi.org/10.3390/diagnostics12092054
Chicago/Turabian StyleIonescu, Sinziana, Alin Codrut Nicolescu, Octavia-Luciana Madge, Laurentiu Simion, Marian Marincas, and Mihai Ceausu. 2022. "Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review" Diagnostics 12, no. 9: 2054. https://doi.org/10.3390/diagnostics12092054
APA StyleIonescu, S., Nicolescu, A. C., Madge, O.-L., Simion, L., Marincas, M., & Ceausu, M. (2022). Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment—A Literature Review. Diagnostics, 12(9), 2054. https://doi.org/10.3390/diagnostics12092054







