Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs
Abstract
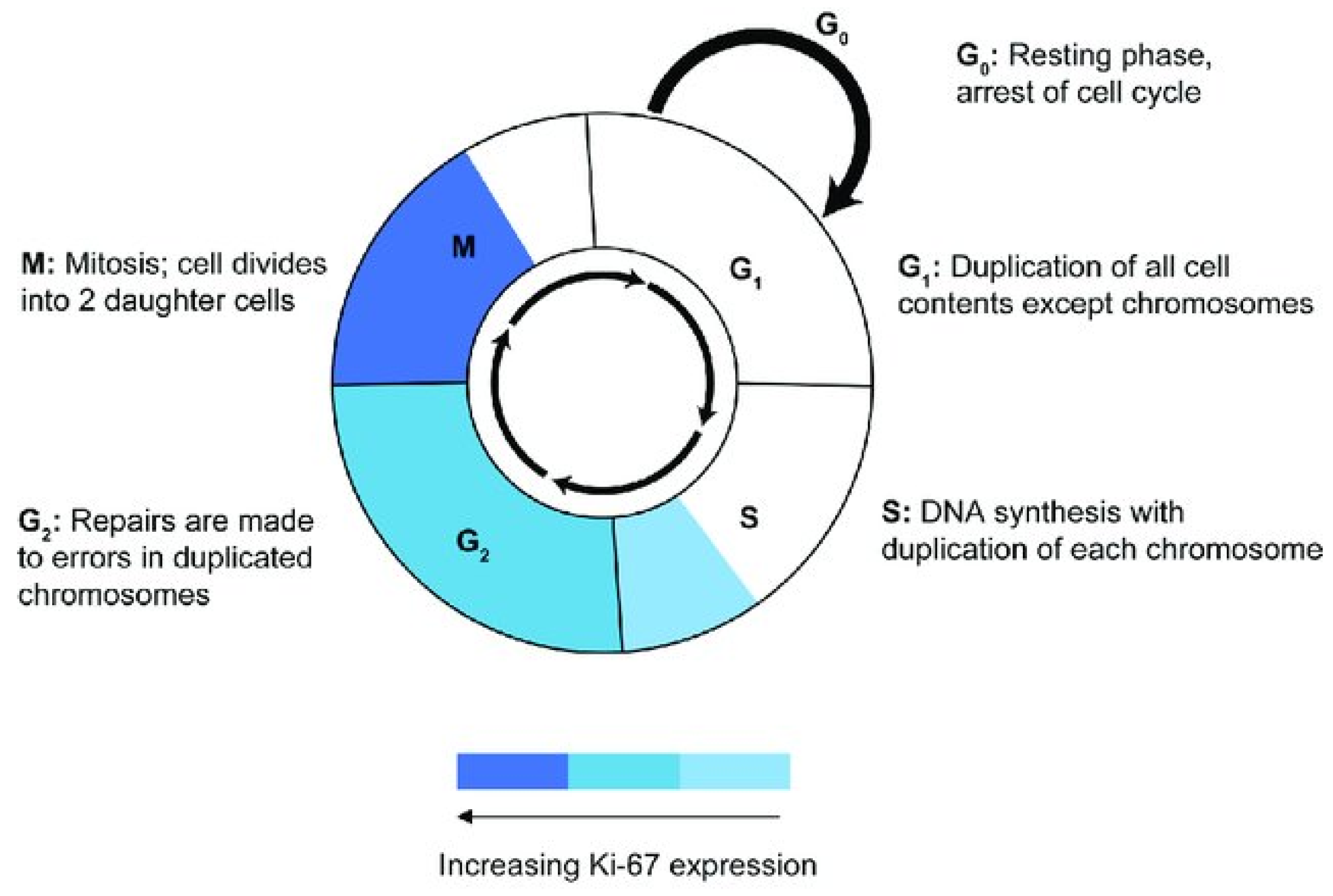
:1. Introduction
2. Materials and Methods
2.1. Patients and Classification
2.2. Statement of Ethics
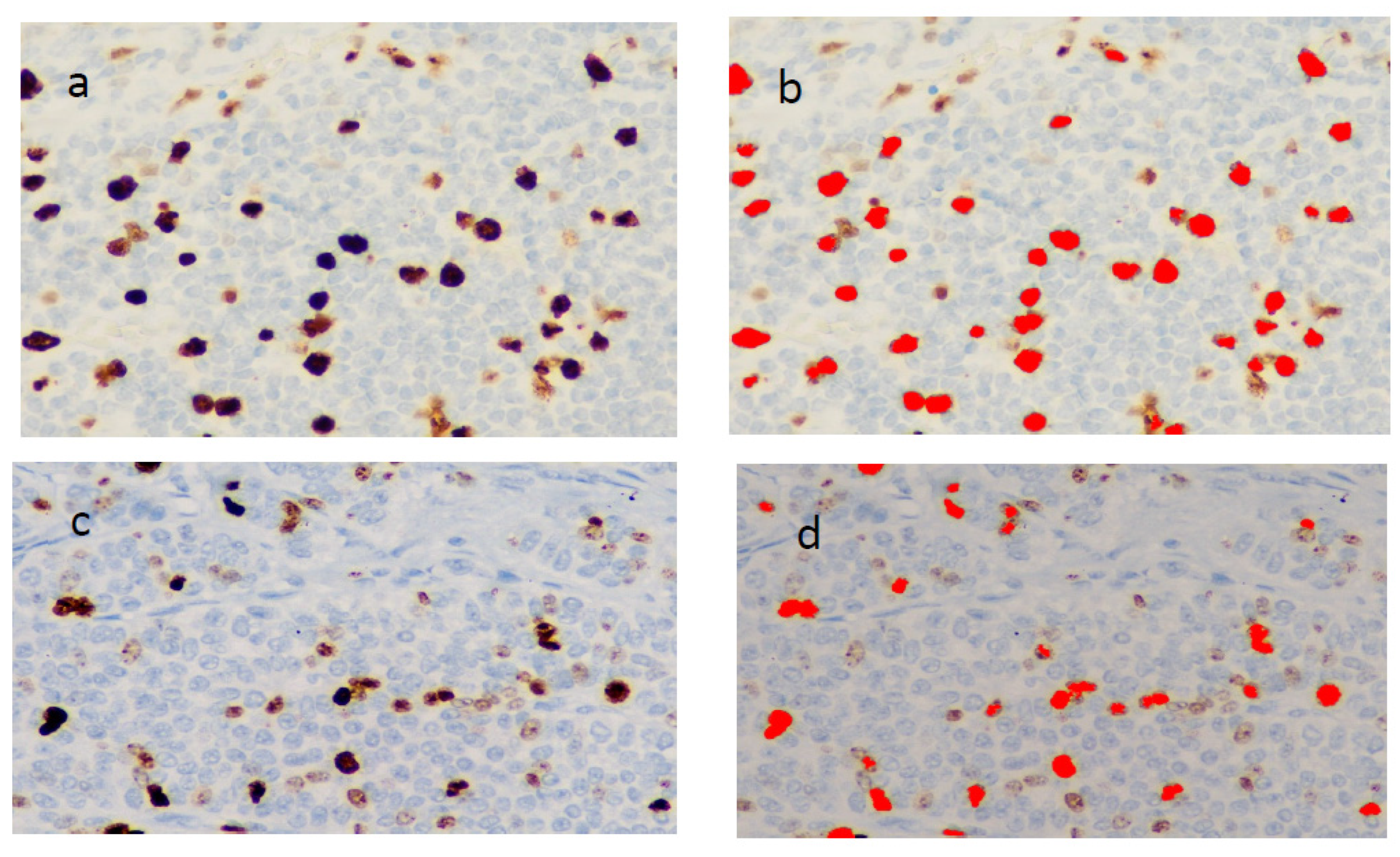
2.3. Ki-67 Immunohistochemistry
2.4. Mitotic Count (MC)
2.5. Statistical Analysis
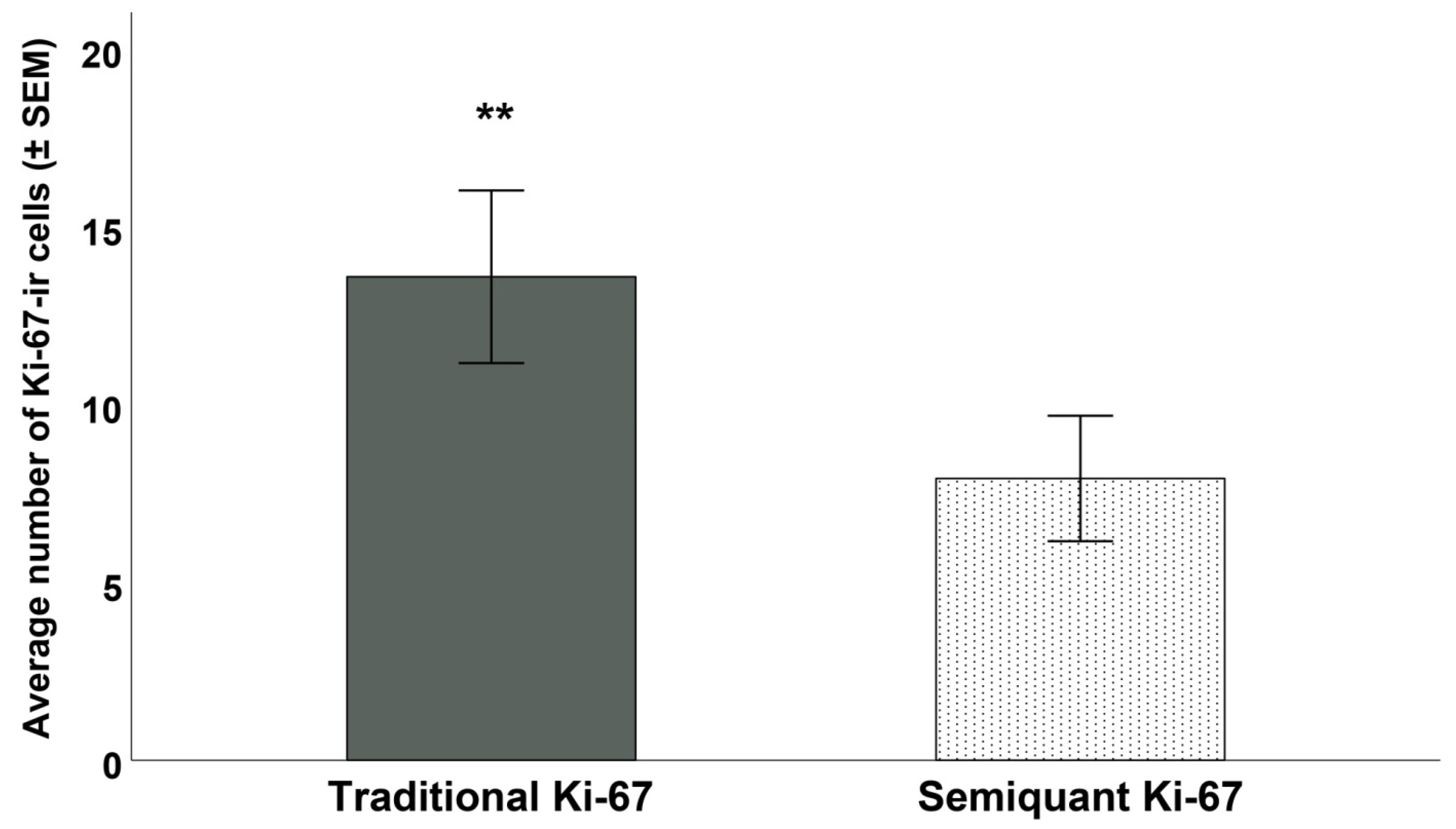
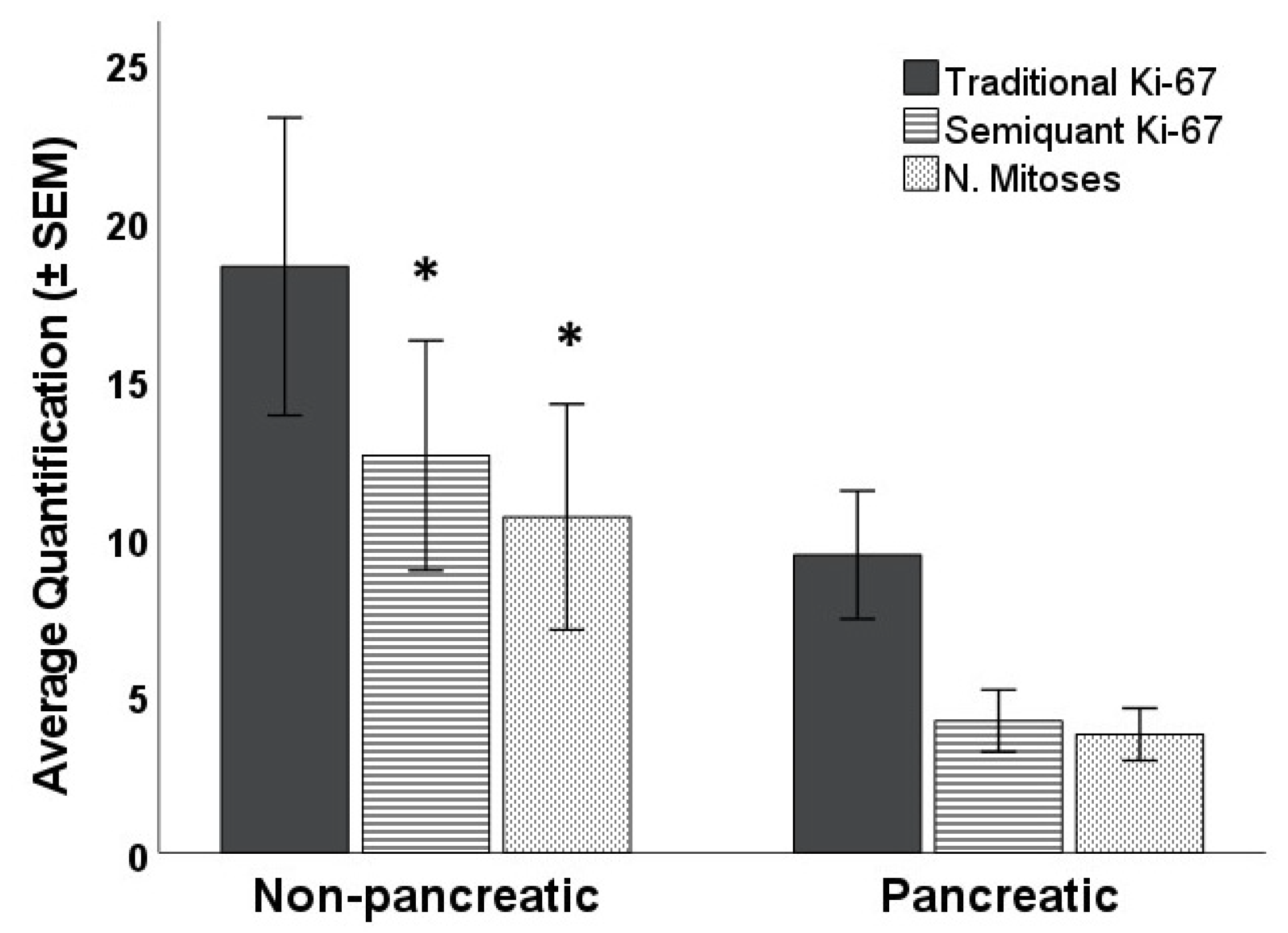
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.S.; Luong, T.V.; Watkins, J.; Toumpanakis, C.; Caplin, M.E.; Meyer, T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br. J. Cancer 2013, 108, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Endl, E.; Kausch, I.; Baack, M.; Knippers, R.; Gerdes, J.; Scholzen, T. The expression of Ki-67, MCM3, and p27 defines distinct subsets of proliferating, resting, and differentiated cells. J. Pathol. 2001, 195, 457–462. [Google Scholar] [CrossRef]
- McCall, C.M.; Shi, C.; Cornish, T.C.; Klimstra, D.S.; Tang, L.H.; Basturk, O.; Mun, L.J.; Ellison, T.A.; Wolfgang, C.L.; Choti, M.A.; et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am. J. Surg. Pathol. 2013, 37, 1671–1677. [Google Scholar] [CrossRef]
- Reid, M.D.; Bagci, P.; Ohike, N.; Saka, B.; Erbarut Seven, I.; Dursun, N.; Balci, S.; Gucer, H.; Jang, K.T.; Tajiri, T.; et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: A comparative analysis of four counting methodologies. Mod. Pathol. 2015, 28, 686–694, Erratum in Mod. Pathol. 2016, 29, 93. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Pucci, E.; Giordano, T.J.; Lu, H.; Duregon, E.; Volante, M.; Papotti, M.; Lloyd, R.V.; Tischler, A.S.; van Nederveen, F.H.; et al. An International Ki67 Reproducibility Study in Adrenal Cortical Carcinoma. Am. J. Surg. Pathol. 2016, 40, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Inzani, F.; Rindi, G. Introduction to neuroendocrine neoplasms of the digestive system: Definition and classification. Pathologica 2021, 113, 1–4. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Couvelard, A.; Komminoth, P.; Körner, M.; Lopes, J.M.; McNicol, A.M.; Nilsson, O.; Perren, A.; Scarpa, A.; et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2007, 451, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Weynand, B.; Borbath, I.; Bernard, V.; Sempoux, C.; Gigot, J.F.; Hubert, C.; Lannoy, V.; Deprez, P.H.; Jouret-Mourin, A. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: High reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 2014, 25, 389–395. [Google Scholar] [CrossRef]
- Tang, L.H.; Gonen, M.; Hedvat, C.; Modlin, I.M.; Klimstra, D.S. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: A comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012, 36, 1761–1770. [Google Scholar] [CrossRef]
- Sysel, A.M.; Valli, V.E.; Bauer, J.A. Immunohistochemical quantification of the cobalamin transport protein, cell surface receptor and Ki-67 in naturally occurring canine and feline malignant tumors and in adjacent normal tissues. Oncotarget. 2015, 6, 2331–2348. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017, 77, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; La Rosa, S. Ki67 labeling index: Assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018, 472, 341–349, Erratum in Virchows Arch. 2017, 472, 515. [Google Scholar] [CrossRef] [PubMed]
- van Velthuysen, M.L.; Groen, E.J.; van der Noort, V.; van de Pol, A.; Tesselaar, M.E.; Korse, C.M. Grading of neuroendocrine neoplasms: Mitoses and Ki-67 are both essential. Neuroendocrinology 2014, 100, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Sasaki, K.; Murakami, T.; Kawasaki, M.; Takahashi, M. The cell cycle associated change of the Ki-67 reactive nuclear antigen expression. J. Cell. Physiol. 1987, 133, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Matsumura, K.; Murakami, T.; Shinozaki, F.; Takahashi, M. Intranuclear localization of the Ki-67 reactive antigen in HeLa cells. Flow cytometric analysis. Biol. Cell 1990, 68, 129–132. [Google Scholar] [CrossRef]
- Kill, I.R. Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J. Cell Sci. 1996, 109 Pt 6, 1253–1263. [Google Scholar] [CrossRef]
- Matheson, T.D.; Kaufman, P.D. The p150N domain of chromatin assembly factor-1 regulates Ki-67 accumulation on the mitotic perichromosomal layer. Mol. Biol. Cell 2017, 28, 21–29. [Google Scholar] [CrossRef]
- Verheijen, R.; Kuijpers, H.J.; Schlingemann, R.O.; Boehmer, A.L.; van Driel, R.; Brakenhoff, G.J.; Ramaekers, F.C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J. Cell Sci. 1989, 92 Pt 1, 123–130. [Google Scholar] [CrossRef]
- van Dierendonck, J.H.; Keijzer, R.; van de Velde, C.J.; Cornelisse, C.J. Nuclear distribution of the Ki-67 antigen during the cell cycle: Comparison with growth fraction in human breast cancer cells. Cancer Res. 1989, 49, 2999–3006. [Google Scholar]
- Endl, E.; Gerdes, J. The Ki-67 protein: Fascinating forms and an unknown function. Exp. Cell Res. 2000, 257, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Starborg, M.; Gell, K.; Brundell, E.; Höög, C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J. Cell Sci. 1996, 109 Pt 1, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.T.; Wang, C.; Gjidoda, A.; Henry, R.W.; Huang, S. The perinucleolar compartment is directly associated with DNA. J. Biol. Chem. 2009, 284, 4090–4101. [Google Scholar] [CrossRef] [PubMed]
- van Koningsbruggen, S.; Gierlinski, M.; Schofield, P.; Martin, D.; Barton, G.J.; Ariyurek, Y.; den Dunnen, J.T.; Lamond, A.I. High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol. Biol. Cell 2010, 21, 3735–3748. [Google Scholar] [CrossRef]
- Cuylen, S.; Blaukopf, C.; Politi, A.Z.; Müller-Reichert, T.; Neumann, B.; Poser, I.; Ellenberg, J.; Hyman, A.A.; Gerlich, D.W. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 2016, 535, 308–312. [Google Scholar] [CrossRef]
- Braun, N.; Papadopoulos, T.; Müller-Hermelink, H.K. Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1988, 56, 25–33. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Dias, E.P.; Oliveira, N.S.C.; Serra-Campos, A.O.; da Silva, A.K.F.; da Silva, L.E.; Cunha, K.S. A novel evaluation method for Ki-67 immunostaining in paraffin-embedded tissues. Virchows Arch. 2021, 479, 121–131. [Google Scholar] [CrossRef]
- du Manoir, S.; Guillaud, P.; Camus, E.; Seigneurin, D.; Brugal, G. Ki-67 labeling in postmitotic cells defines different Ki-67 pathways within the 2c compartment. Cytometry 1991, 12, 455–463. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Pan, D.; Wei, K.; Ling, Y.; Su, S.; Zhu, M.; Chen, G. The prognostic role of Ki-67/MIB-1 in cervical cancer: A systematic review with meta-analysis. Med. Sci. Monit. 2015, 21, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.; Mrklić, I.; Razumović, J.J.; Jonjić, N.; Šarčević, B.; Blažičević, V.; Jurković, I.; Vrbičić, B.; Lisica Šikic, N.; Peteh, L.L.; et al. Inter-laboratory comparison of Ki-67 proliferating index detected by visual assessment and automated digital image analysis. Breast Dis. 2019, 38, 73–79. [Google Scholar] [CrossRef]
- Yano, S.; Tazawa, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. FUCCI Real-Time Cell-Cycle Imaging as a Guide for Designing Improved Cancer Therapy: A Review of Innovative Strategies to Target Quiescent Chemo-Resistant Cancer Cells. Cancers 2020, 12, 2655. [Google Scholar] [CrossRef]
- Ferro, A.; Mestre, T.; Carneiro, P.; Sahumbaiev, I.; Seruca, R.; Sanches, J.M. Blue intensity matters for cell cycle profiling in fluorescence DAPI-stained images. Lab. Investig. 2017, 97, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Clyde, R.G.; Bown, J.L.; Hupp, T.R.; Zhelev, N.; Crawford, J.W. The role of modelling in identifying drug targets for diseases of the cell cycle. J. R. Soc. Interface 2006, 3, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Dökümcü, K.; Farahani, R.M. Evolution of Resistance in Cancer: A Cell Cycle Perspective. Front. Oncol. 2019, 9, 376. [Google Scholar] [CrossRef]
- Booth, D.G.; Takagi, M.; Sanchez-Pulido, L.; Petfalski, E.; Vargiu, G.; Samejima, K.; Imamoto, N.; Ponting, C.P.; Tollervey, D.; Earnshaw, W.C.; et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife 2014, 3, e01641. [Google Scholar] [CrossRef]
- Bridger, J.M.; Kill, I.R.; Lichter, P. Association of pKi-67 with satellite DNA of the human genome in early G1 cells. Chromosome Res. 1998, 6, 13–24. [Google Scholar] [CrossRef]
- O’Toole, D.; Kianmanesh, R.; Caplin, M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology 2016, 103, 117–118. [Google Scholar] [CrossRef]
- Sadot, E.; Reidy-Lagunes, D.L.; Tang, L.H.; Do, R.K.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Groot Koerkamp, B.; Untch, B.R.; et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann. Surg. Oncol. 2016, 23, 1361–1370. [Google Scholar] [CrossRef]
- Weidner, N.; Moore, D.H., 2nd; Vartanian, R. Correlation of Ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum. Pathol. 1994, 25, 337–342. [Google Scholar] [CrossRef]
- Rudolph, P.; Peters, J.; Lorenz, D.; Schmidt, D.; Parwaresch, R. Correlation between mitotic and Ki-67 labeling indices in paraffin-embedded carcinoma specimens. Hum. Pathol. 1998, 29, 1216–1222. [Google Scholar] [CrossRef]
- Huang, W.; Nebiolo, C.; Esbona, K.; Hu, R.; Lloyd, R. Ki67 index and mitotic count: Correlation and variables affecting the accuracy of the quantification in endocrine/neuroendocrine tumors. Ann. Diagn. Pathol. 2020, 48, 151586, Erratum in Ann. Diagn. Pathol. 2022, 56, 151656. [Google Scholar] [CrossRef] [PubMed]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef] [PubMed]
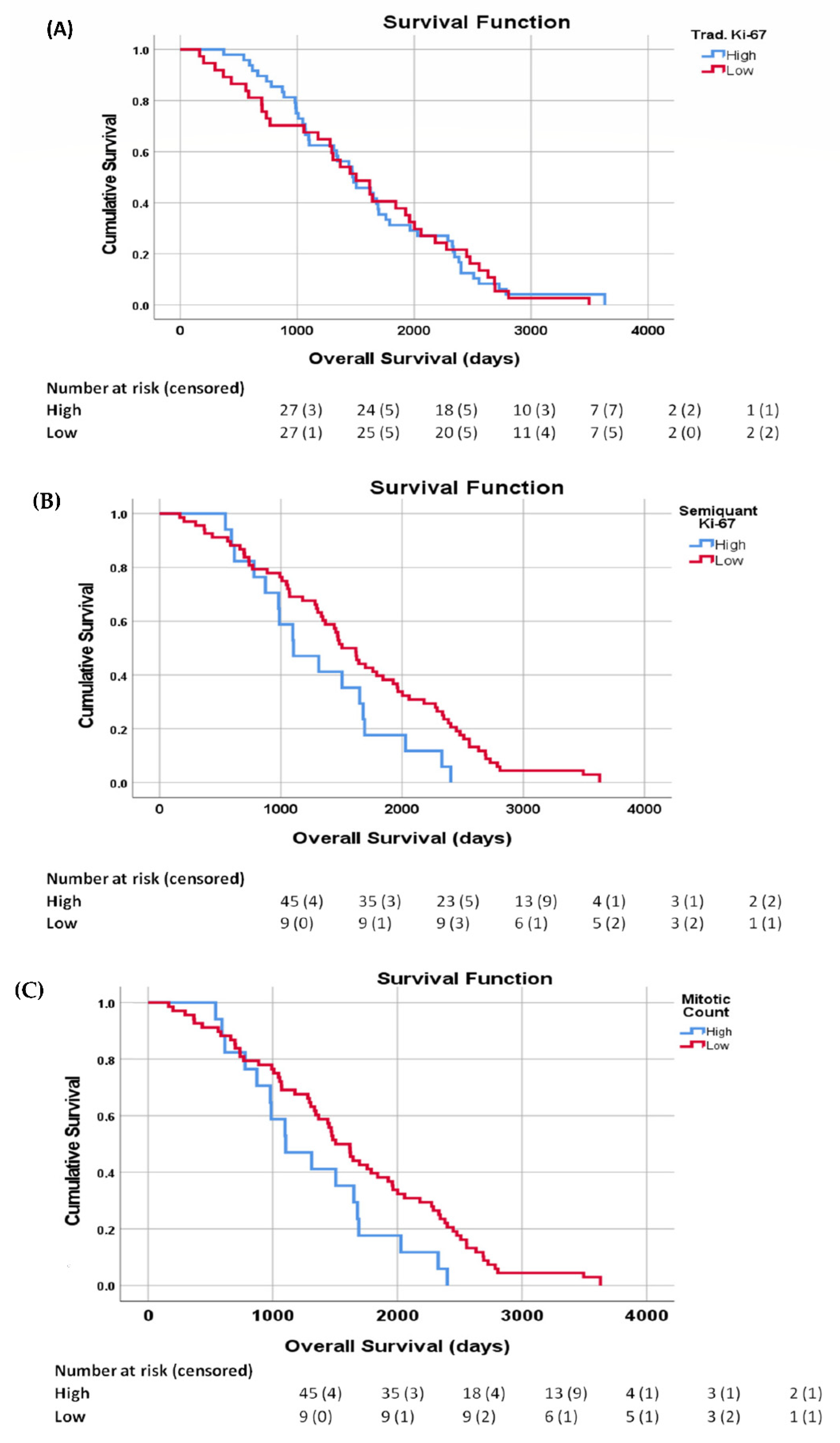
| All Patients (n = 87) | G1 (N. Patients) | G2 (N. Patients) | G3 NET (N. Patients) | |
|---|---|---|---|---|
| Age-Median (Range) | 55 years (27–94 years) | |||
| <49 | n = 33 | 10 | 16 | 7 |
| 50–69 | n = 32 | 3 | 23 | 6 |
| >70 | n = 22 | 3 | 19 | 0 |
| Sex | ||||
| Female | n = 44 | 9 | 29 | 6 |
| Male | n = 43 | 7 | 29 | 7 |
| Location | ||||
| Pancreatic | n = 48 | 4 | 40 | 4 |
| Non-Pancreatic | n = 39 | 12 | 18 | 9 |
| DFI-Median (Range) | 4.03 years (0.2–9.4 years) |
| Semiquant Ki-67 | n° Mitosi | ||
|---|---|---|---|
| Traditional Ki-67 | Pearson Correlation | 0.925 ** | 0.824 ** |
| Sig. (2-tailed) | <0.001 | <0.001 | |
| Semiquant Ki-67 | Pearson Correlation | 0.703 ** | |
| Sig. (2-tailed) | <0.001 |
| Traditional Ki-67 | Semiquant Ki-67 | n° Mitosi | ||
|---|---|---|---|---|
| Age | Pearson Correlation | 0.151 | 0.118 | 0.138 |
| Sig. (2-tailed) | 0.164 | 0.279 | 0.203 | |
| Time since Diagnosis | Pearson Correlation | −0.016 | −0.054 | −0.001 |
| Sig. (2-tailed) | 0.887 | 0.619 | 0.980 |
| Traditional Ki-67 | |||||
|---|---|---|---|---|---|
| N. of Patients | Percent | Valid Percent | Cumulative Percent | ||
| Valid | G1 | 16 | 18.4 | 18.4 | 18.4 |
| G2 | 58 | 66.7 | 66.7 | 85.1 | |
| G3 | 13 | 14.9 | 14.9 | 100.0 | |
| Total | 87 | 100.0 | 100.0 | ||
| Semiquent Ki-67 | |||||
| N. of Patients | Percent | Valid Percent | Cumulative Percent | ||
| Valid | G1 | 53 | 60.9 | 60.9 | 60.9 |
| G2 | 26 | 29.9 | 29.9 | 90.8 | |
| G3 | 8 | 9.2 | 9.2 | 100.0 | |
| Total | 87 | 100.0 | 100.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faviana, P.; Boldrini, L.; Gentile, C.; Erba, P.A.; Sammarco, E.; Bartoli, F.; Esposito, E.; Galli, L.; Lippolis, P.V.; Bardi, M. Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs. Diagnostics 2022, 12, 1960. https://doi.org/10.3390/diagnostics12081960
Faviana P, Boldrini L, Gentile C, Erba PA, Sammarco E, Bartoli F, Esposito E, Galli L, Lippolis PV, Bardi M. Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs. Diagnostics. 2022; 12(8):1960. https://doi.org/10.3390/diagnostics12081960
Chicago/Turabian StyleFaviana, Pinuccia, Laura Boldrini, Carlo Gentile, Paola Anna Erba, Enrico Sammarco, Francesco Bartoli, Enrica Esposito, Luca Galli, Piero Vincenzo Lippolis, and Massimo Bardi. 2022. "Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs" Diagnostics 12, no. 8: 1960. https://doi.org/10.3390/diagnostics12081960
APA StyleFaviana, P., Boldrini, L., Gentile, C., Erba, P. A., Sammarco, E., Bartoli, F., Esposito, E., Galli, L., Lippolis, P. V., & Bardi, M. (2022). Proposal for a New Diagnostic Histopathological Approach in the Evaluation of Ki-67 in GEP-NETs. Diagnostics, 12(8), 1960. https://doi.org/10.3390/diagnostics12081960






