FAPI PET versus FDG PET, CT or MRI for Staging Pancreatic-, Gastric- and Cholangiocarcinoma: Systematic Review and Head-to-Head Comparisons of Diagnostic Performances
Abstract
:1. Introduction
2. Materials and Methods
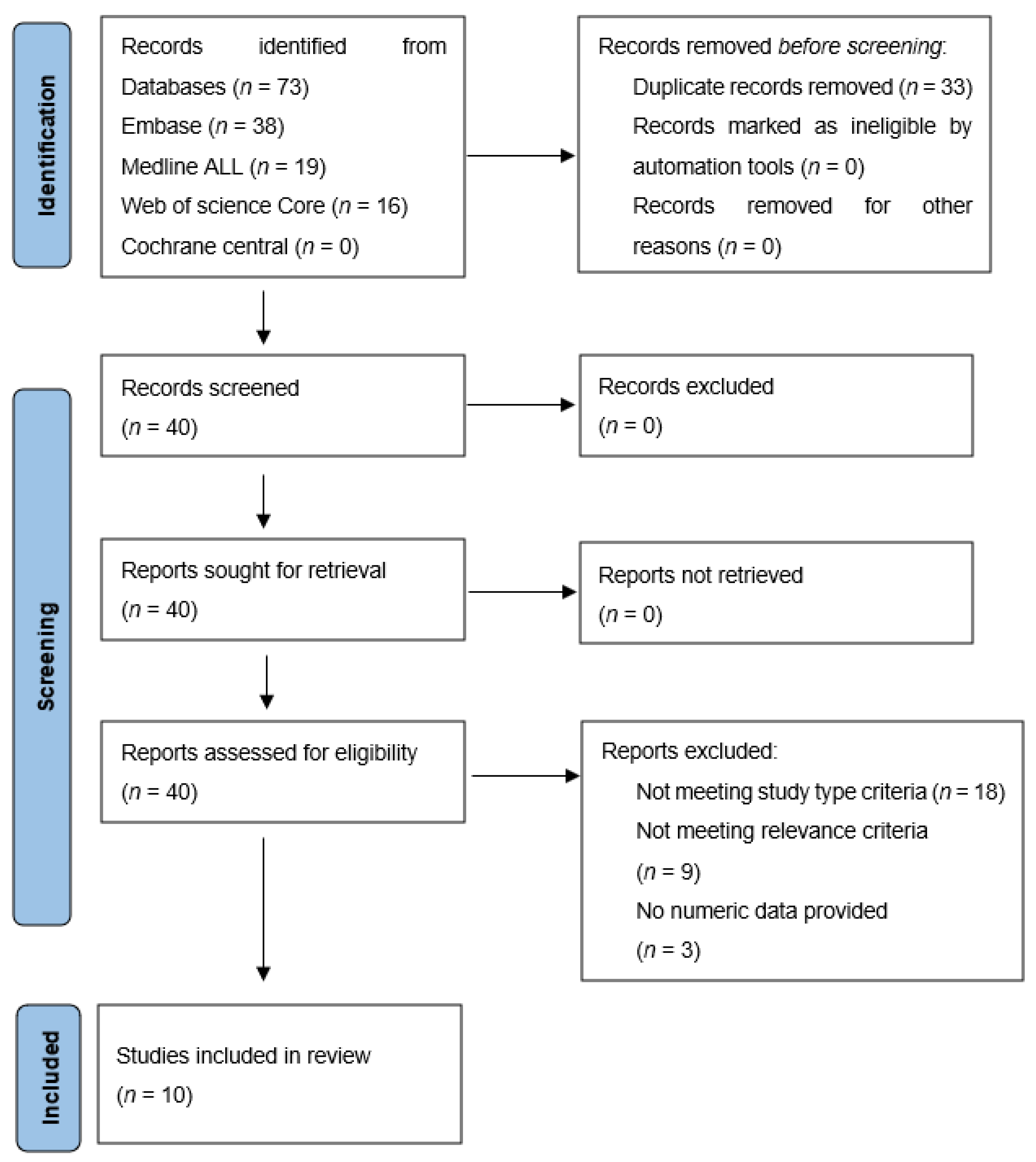
2.1. Search of Literature
2.2. Study Eligibility
2.3. Quality Assessment
2.4. Data Items and Analysis
3. Results
3.1. Study and Patient Characteristics
3.2. Quality Assessment
3.3. Patient-Wise Analysis
3.4. Lesion-Wise Analysis
3.5. Sensitivity of FAPI PET versus FDG PET/CECT/MRI
3.6. Additional Findings—True Positive (i.e., Also Malignant)
3.7. Additional Findings—False Positive (i.e., Avid—Non-Malignant)
3.8. Additional Findings—True Negative (i.e., Non Avid—Non Malignant)
3.9. False-Negative Results of FAPI PET
3.10. Tracer Visualization
4. Discussion
5. Conclusions
Implications for Clinical Practice and Directions for Further Research
Supplementary Materials
Funding
Conflicts of Interest
References
- Bird, N.; Elmasry, M.; Jones, R.; Elniel, M.; Kelly, M.; Palmer, D.; Fenwick, S.; Poston, G.; Malik, H. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br. J. Surg. 2017, 104, 418–425. [Google Scholar]
- Ta, R.; O’Connor, D.B.; Sulistijo, A.; Chung, B.; Conlon, K.C. The Role of Staging Laparoscopy in Resectable and Borderline Resectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Dig. Surg. 2019, 36, 251–260. [Google Scholar]
- Gertsen, E.C.; Brenkman, H.J.F.; van Hillegersberg, R.; van Sandick, J.W.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Lanschot, J.J.B.; Lagarde, S.M.; et al. 18F-Fludeoxyglucose-Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC). JAMA Surg. 2021, 156, e215340. [Google Scholar]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar]
- Hicks, R.J.; Roselt, P.J.; Kallur, K.G.; Tothill, R.W.; Mileshkin, L. FAPI PET/CT: Will It End the Hegemony of (18)F-FDG in Oncology? J. Nucl. Med. 2021, 62, 296–302. [Google Scholar]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar]
- Sideri, S.; Papageorgiou, S.N.; Eliades, T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J. Clin. Epidemiol. 2018, 100, 103–110. [Google Scholar]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C.; et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar]
- Qin, C.; Shao, F.; Gai, Y.; Liu, Q.; Ruan, W.; Liu, F.; Hu, F.; Lan, X. (68)Ga-DOTA-FAPI-04 PET/MR in the Evaluation of Gastric Carcinomas: Comparison with (18)F-FDG PET/CT. J. Nucl. Med. 2022, 63, 81–88. [Google Scholar]
- Röhrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of (68)Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021, 62, 779–786. [Google Scholar]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Congwei, J.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl Med. Mol. Imaging 2021, 48, 1593–1603. [Google Scholar]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Zhang, H.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: A pilot study in patients with suspected hepatic nodules. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 196–203. [Google Scholar]
- Zhao, L.; Pang, Y.; Luo, Z.; Fu, K.; Yang, T.; Zhao, L.; Sun, L.; Wu, H.; Lin, Q.; Chen, H. Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1944–1955. [Google Scholar]
- Pang, Y.; Zhao, L.; Shang, Q.; Meng, T.; Zhao, L.; Feng, L.; Wang, S.; Guo, P.; Wu, X.; Lin, Q.; et al. Positron emission tomography and computed tomography with [(68)Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1322–1337. [Google Scholar]
- Kuten, J.; Levine, C.; Shamni, O.; Pelles, S.; Wolf, I.; Lahat, G.; Mishani, E.; Even-Sapir, E. Head-to-head comparison of [(68)Ga]Ga-FAPI-04 and [(18)F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 743–750. [Google Scholar]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. (68)Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar]
- Lin, R.; Lin, Z.; Zhang, J.; Yao, S.; Miao, W. Increased 68Ga-FAPI-04 Uptake in Schmorl Node in a Patient with Gastric Cancer. Clin. Nucl. Med. 2021, 46, 700–702. [Google Scholar]
- Can, C.; Gündoğan, C.; Güzel, Y.; Kaplan, İ.; Kömek, H. 68Ga-FAPI Uptake of Thyroiditis in a Patient with Breast Cancer. Clin. Nucl. Med. 2021, 46, 683–685. [Google Scholar]
- Liu, H.; Wang, Y.; Zhang, W.; Cai, L.; Chen, Y. Elevated 68Ga-FAPI Activity in Splenic Hemangioma and Pneumonia. Clin. Nucl. Med. 2021, 46, 694–696. [Google Scholar]
- Zhang, Y.; Cai, J.; Wu, Z.; Yao, S.; Miao, W. Intense [(68)Ga]Ga-FAPI-04 uptake in solitary fibrous tumor/hemangiopericytoma of the central nervous system. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4103–4104. [Google Scholar]
- Liu, H.; Chen, Z.; Yang, X.; Fu, W.; Chen, Y. Increased 68Ga-FAPI Uptake in Chronic Cholecystitis and Degenerative Osteophyte. Clin. Nucl. Med. 2021, 46, 601–602. [Google Scholar]
- Zhang, X.; Song, W.; Qin, C.; Liu, F.; Lan, X. Non-malignant findings of focal (68)Ga-FAPI-04 uptake in pancreas. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2635–2641. [Google Scholar]
| Year | Author | Study Design | Period of pt. Inclusion | Cancer Type(s) Matching Our Interest | No. of pts. (Total > per Cancer Type of Interest Specifically) | Gender (M/F) | Median (Range) Age (in Years) | Modalities Studied | Radiopharmaceutical |
|---|---|---|---|---|---|---|---|---|---|
| 2020 | Chen, H. et al. [10] | Pro | July 2019 March 2020 | P/G/Ch | 68 > 1/12/8 | 40/28 | 57 (24–85) | FAPI PET-CT FDG PET-CT | 68Ga-FAPI-04 |
| 2021 | # Guo, W. et al. [11] | Retro | October 2019 June 2020 | Ch | 34 > 12 (8) | 25/9 | 61 (33–75) | FAPI PET-CT FDG PET-CT CECT MRI | 68Ga-FAPI-04 |
| 2021 | # Pang, Y. et al. [12] | Retro | October 2019 June 2020 | G | 35 > 20 (12) | 18/17 | 64 (53–68) | FAPI PET-CT FDG PET-CT | 68Ga-FAPI-04 |
| 2021 | # Zhao, L. et al. [17] | Retro | October 2019 August 2020 | P/ G | 46 > 6 (1)/ 13 (12) | 14/32 | 5 7(32–80) | FAPI PET-CT FDG PET-CT | 68Ga-FAPI-04 |
| 2021 | Qin, C. et al. [13] | Pro | June 2020 June 2020 | G | 20 | 9/11 | 56 (29–70) | FAPI PET MRI FDG PET-CT | 68Ga-FAPI-04 |
| 2021 | Rohrich, M. et al. [14] | Retro | not mentioned | P | 19 | 10/9 | 64 (52–80) | FAPI PET CECT | 68Ga-FAPI-0468 Ga-FAPI-46 |
| 2021 | Shi, X. et al. [15] | Pro | January 2019 June 2020 | Ch | 20 > 3 | 18/2 | 58 (43–78) | FAPI PET-CT FDG PET-CT | 68Ga-FAPI-04 |
| 2021 | Shi, X. et al. [16] | Pro | January 2019 August 2020 | G/Ch | 17 > 1/2 | 13/3 | 63 (47–78) | FAPI PET-CT CECT/MRI | 68GA-FAPI-04 |
| 2021 | Pang, Y. et al. [18] | Retro | June 2020 January 2021 | P | 36 > 26 | 18/17 | 64 (53–68) | FAPI PET-CT FDG PET-CT CECT | 68Ga-FAPI-04 |
| 2021 | Kuten, J. et al. [19] | Pro | July 2020 December 2020 | G | 13 | 6/7 | 70 (35–87) | FAPI PET-CT FDG PET-CT | 68Ga-FAPI-04 |
| Year | Author | Risk of Bias—Participant Selection | Risk of Bias—Index Test | Risk of Bias—Reference Standard | Risk of Bias—Flow and Timing | Applicability Concerns—Participant Selection | Applicability Concerns—Index Test | Applicability Concerns—Reference Standard |
|---|---|---|---|---|---|---|---|---|
| 2020 | Chen, H. et al. [10] | high | low | low | low | low | low | low |
| 2021 | Guo, W. et al. [11] | unclear # | low | low | low | low | low | low |
| 2021 | Pang, Y. et al. [12] | unclear # | low | low | low | low | low | low |
| 2021 | Zhao, L. et al. [17] | unclear # | low | low | low | low | low | low |
| 2021 | Qin, C. et al. [13] | low | low | low (primary tumour), high (metastasis) | low | low | low | low |
| 2021 | Rohrich, M. et al. [14] | low | low | Low (primary tumour), high (metastasis) | low | low | low | low |
| 2021 | Shi, X. et al. [15] | high | low | low (primary tumour) high (metastasis), | low | low | low | low |
| 2021 | Shi, X. et al. [16] | low | low | low | low | low | low | low |
| 2021 | Pang, Y. et al. [18] | low | low | low | low | low | low | low |
| 2021 | Kuten J. et al. [19] | low | low | low | low | low | low | low |
| Year | Author | Primary Tumour | Lymph Node Metastasis | Organ Metastasis | Peritoneal/Omental Metastasis |
|---|---|---|---|---|---|
| 2020 | Chen, H. et al. [10] | Pathology (n = 49/68)/ Imaging FU (n = 19) | Pathology/ Imaging FU | Pathology/ Imaging FU | Pathology/ Imaging FU |
| 2021 | Guo, W. et al. [11] | Pathology (all patients) | Pathology/ Imaging FU | Pathology/ Imaging FU | Pathology/ Imaging FU |
| 2021 | Pang, Y. et al. [12] | Pathology (all patients) | Pathology/ Imaging FU | Pathology/ Imaging FU | Pathology/ Imaging FU |
| 2021 | Zhao, L. et al. [17] | n.p. | n.p. | n.p. | Pathology (n = 27/46)/ Imaging FU (n = 19) |
| 2021 | Qin, C. et al. [13] | Pathology (all patients) | Pathology (n = 5/14) | Pathology (n = 5/14) | Pathology (n = 5/14) |
| 2021 | Rohrich, M. et al. [14] | Pathology (all patients) | - | - | - |
| 2021 | Shi, X. et al. [15] | Pathology (all patients) | - | - | - |
| 2021 | Shi, X. et al. [16] | Pathology (all patients) | n.p. | n.p. | n.p. |
| 2021 | Pang, Y. et al. [18] | Pathology (all patients) | Pathology/ Imaging FU | Pathology/ Imaging FU | Pathology/ Imaging FU |
| 2021 | Kuten J. et al. [19] | Pathology (all patients) | Pathology/ Imaging FU | Pathology/ Imaging FU | Pathology/ Imaging FU |
| Year | Author | Type of Cancer | PATIENT-WISE ANALYSIS Number of Patients with Positive Findings (T/N/M/PC) * on FAPI PET (Confirmed by Reference Standard) | PATIENT-WISE ANALYSIS Number of Patients with Positive Findings (T/N/M/PC) on FDG PET and/or CECT/MRI (Confirmed by Reference Standard) | LESION-WISE ANALYSIS Number of Detected Lesions (N/M/PC/NS) by FAPI PET (Confirmed by Reference Standard) | LESION-WISE ANALYSIS Number of Detected Lesions (N/M/PC/NS) by FDG PET and/or CECT/MRI (Confirmed by Reference Standard) |
|---|---|---|---|---|---|---|
| 2021 | Chen, H. et al. [10] | P | - | - | - / - / - / 15 (1) | - / - / - / 2 (1) |
| G | - | - | - / - / - / 57 (12) | - / - / - / 15 (12) | ||
| Ch | - | - | - / - / - / 10 (4) | - / - / - / 2 (4) | ||
| 2021 | Guo, W. et al. [11] | Ch | 7 (7) / - / - / - | 4 (7) / - / - / - | - | - |
| 2021 | Pang, Y. et al. [12] | G | 11 (11) / - / - / - | 4 (11) / - / - / - | - | - |
| 2021 | Zhao, L. et al. [17] | P G | - / - / - / 6 (6) - / - / - / 13 (13) | - / - / - / 4 (6) - / - / - / 7 (13) | - | - |
| 2021 | Qin, C. et al. [13] | G | 14 (14) / 12 (12) / 13 (13) / 10 (10) | 10 (14) / 10 (12) / 13 (13) / 4 (10) | 45 (12) / 37 (13) / 42 (10) / - / - | 33 (12) / 22 (13) / 14 (10) / - / - |
| 2021 | Rohrich, M. et al. [14] | P | - | n.p. | n.p. | n.p. |
| 2021 | Shi, X. et al. [15] | Ch | 3 (3) / - / - / - / | 3 (3) / - / - / - | 4 (4) / - / - / - / | 4 (4) / - / - / - |
| 2021 | Shi, X. et al. [16] | G Ch | - / - / - / - 2 (2) / - / - / - | n.p. | - / - / - / - - / - / - / - | n.p. |
| 2021 | Pang, Y. et al. [18] | P | 26 (26) / - / - / - | 19 (26) / - / - / - | 45 (15) / 88 (17) / 77 (10) / - | 23 (15) / 22 (17) / 33 (10) / - |
| 2021 | Kuten, J. et al. [19] | G | 10 (10) / 2 (2) / - / 5 (5) | 5 (10) / 2 (2) / - / 0 (5) | 16 (2) / - / - / - | 16 (2) / - / - / - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldhuijzen van Zanten, S.E.M.; Pieterman, K.J.; Wijnhoven, B.P.L.; Pruis, I.J.; Groot Koerkamp, B.; van Driel, L.M.J.W.; Verburg, F.A.; Thomeer, M.G.J. FAPI PET versus FDG PET, CT or MRI for Staging Pancreatic-, Gastric- and Cholangiocarcinoma: Systematic Review and Head-to-Head Comparisons of Diagnostic Performances. Diagnostics 2022, 12, 1958. https://doi.org/10.3390/diagnostics12081958
Veldhuijzen van Zanten SEM, Pieterman KJ, Wijnhoven BPL, Pruis IJ, Groot Koerkamp B, van Driel LMJW, Verburg FA, Thomeer MGJ. FAPI PET versus FDG PET, CT or MRI for Staging Pancreatic-, Gastric- and Cholangiocarcinoma: Systematic Review and Head-to-Head Comparisons of Diagnostic Performances. Diagnostics. 2022; 12(8):1958. https://doi.org/10.3390/diagnostics12081958
Chicago/Turabian StyleVeldhuijzen van Zanten, Sophie E. M., Kay J. Pieterman, Bas P. L. Wijnhoven, Ilanah J. Pruis, Bas Groot Koerkamp, Lydi M. J. W. van Driel, Frederik A. Verburg, and Maarten G. J. Thomeer. 2022. "FAPI PET versus FDG PET, CT or MRI for Staging Pancreatic-, Gastric- and Cholangiocarcinoma: Systematic Review and Head-to-Head Comparisons of Diagnostic Performances" Diagnostics 12, no. 8: 1958. https://doi.org/10.3390/diagnostics12081958
APA StyleVeldhuijzen van Zanten, S. E. M., Pieterman, K. J., Wijnhoven, B. P. L., Pruis, I. J., Groot Koerkamp, B., van Driel, L. M. J. W., Verburg, F. A., & Thomeer, M. G. J. (2022). FAPI PET versus FDG PET, CT or MRI for Staging Pancreatic-, Gastric- and Cholangiocarcinoma: Systematic Review and Head-to-Head Comparisons of Diagnostic Performances. Diagnostics, 12(8), 1958. https://doi.org/10.3390/diagnostics12081958







