Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples Collection
2.2. Rapid Immunochromatographic Assay
2.3. Analysis of Results Using the Measurement Application
2.4. Rapid Immunochromatographic Clinical Performance Assessment
2.5. Serologic Antibody ELISA Assay
2.6. Statistical Analysis
3. Results
3.1. Clinical Study of RapiSureTM (EDGCTM) COVID-19 S1 RBD IgG/Neutralizing Ab Test
3.2. Analyzing Descriptive Statistics for Different Vaccinations
3.3. Analysis of Factors for Generating General Antibodies and Neutralizing Antibody after Vaccination
3.4. Analysis of Neutralizing Antibody Production Rate According to Various Factors
3.5. Verification of Antibody Production Rate through Multiple Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- WHO Health Emergency Dashboard. 2021. Available online: https://covid19.who.int/ (accessed on 1 March 2020).
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.; Tsang, O.T.; Hui, D.S.; Kwan, M.Y.; Chan, W.H.; Chiu, S.S.; Ko, R.L.; Chan, K.H.; Cheng, S.; Perera, R.A.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.; Roberts, J.; Bond, K.; Tran, T.; Kostecki, R.; Yoga, Y.; Naughton, W.; Taiaroa, G.; Seemann, T.; et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med. J. Aust. 2021, 212, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Davide, F.R.; Christian, G.; Frauke, M.; Julio, C.C.; Lorenzi, J.C.; Zijun, W.; Alice, C.; Marianna, A.; Christopher, O.B.; Anna, G.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2021, 584, 437–442. [Google Scholar]
- Sacristan, M.S.; Collazos-Blanco, A.; Cintas, M.I.Z.; García, A.S.; de Villavicencio, C.Y.; Maestre, M.M. Comparison of various serological assays for novel SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 963–968. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Kathryn, J.A.S.; Hemmings, O.; Neophytos Kouphou, A.O.; Galao, R.P.; Betancor, G.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody Resistance of SARS-CoV-2 Variants, B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- David, S.K.; Deborah, C.; Arnold, R.; Timothy, E.S.; Adam, K.W.; Jennifer, A.J.; Kanta, S.; Stephen, J.K.; James, A.T.; Davenport, M.P. What level of neutralising antibody protects from COVID-19? Nat. Med. 2021, 27, 1205–1211. [Google Scholar]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptorbinding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef]
- Thomson, E.C.; Rosen, L.E.; Shepherd, J.G.; Spreafico, R.; da Silva Filipe, A.; Wojcechowskyj, J.A.; Davis, C.; Piccoli, L.; Pascall, D.J.; Dillen, J.; et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading anti-body-mediated immunity. Cell 2021, 184, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and envi-ronmental factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive mapping of mutations to the SARS-CoV-2 receptor-binding domain that affect recognition by polyclonal human serum antibodies. Cell Host Microbe 2021, 29, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P.; Girardin, R.C.; Rathod, B.; et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef] [PubMed]
- Ashlesha, D.; Bethany, D.H.; Luis, M.S.; James, J.K.; Mark, R.W. Epitope Classification and RBD Binding Properties of Neu-tralizing antibody Against SARS-CoV-2 Variants of Concern. Front. Immunol. 2021, 12, 691715. [Google Scholar]
- Walker, S.N.; Chokkalingam, N.; Reuschel, E.L.; Purwar, M.; Xu, Z.; Gary, E.N.; Kim, K.Y.; Helble, M.; Schultheis, K.; Walters, J.; et al. SARS-CoV-2 Assays To Detect Functional Antibody Responses That Block ACE2 recognition in vaccinated animals and infected patients. J. Clin. Microbiol. 2020, 58, e01533–e20. [Google Scholar] [CrossRef]
- Lake, D.F.; Roeder, A.J.; Kaleta, E.; Jasbi, P.; Pfeffer, K.; Koelbela, C.; Periasamy, S.; Kuzmina, N.; Bukreyev, A.; Grys, T.E.; et al. Development of a rapid point-of-care test that measures neutralizing antibody to SARS-CoV-2. J. Clin. Virol. 2021, 145, 105024. [Google Scholar] [CrossRef] [PubMed]
- Muchi, S.; Tayler, N.; Hoang, T.; Ballard, S.A.; Graham, M.; Rojek, A.; Kwong, J.C.; Trubiano, J.A.; Smibert, O.; Drewett, G.; et al. Multi-site assessment of rapid, point-of-care antigen testing for the diagnosis of SARS-CoV-2 infection in a low-prevalence setting: A validation and implementation study. Lancet Reg. Health-West. Pac. 2021, 9, 100115. [Google Scholar]
- Kim, Y.J.; Bae, J.Y.; Bae, S.; Hwang, S.; Kwon, K.T.; Chang, H.H.; Lee, W.K.; Cui, C.; Lee, G.E.; Kim, S.W.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in Korean Patients Who Have Recovered from COVID-19. Yonsei Med. J. 2021, 62, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.Y.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn. Microbiol. Infect. Dis. 2021, 99, 115294. [Google Scholar] [CrossRef]
- Gordon, M. Gmisc: Descriptive Statistics, Transition Plots, and More. 2016. Available online: https://CRAN.R-project.org/package=Gmisc (accessed on 3 January 2022).
- Kalinga, A.K.; Mwanziva, C.; Chiduo, S.; Mswanya, C.; Ishengoma, D.I.; Francis, F.; Temu, L.; Mahikwano, L.; Mgata, S.; Amoo, G.; et al. Comparison of visual and automated Deki Reader interpretation of malaria rapid diagnostic tests in rural Tanzanian military health facilities. Malar. J. 2018, 17, 214. [Google Scholar] [CrossRef]
- Visser, T.; Ramachandra, S.; Pothin, E.; Jacobs, J.; Cunningham, J.; Menach, A.L.; Gatton, M.L.; Dos Santos Souza, S.; Nelson, S.; Rooney, L.; et al. A comparative evaluation of mobile medical APPS (MMAS) for reading and interpreting malaria rapid di-agnostic tests. Malar. J. 2021, 20, 39. [Google Scholar] [CrossRef]
- Papenburg, J.; Cheng, M.P.; Corsini, R.; Caya, C.; Mendoza, E.; Manguiat, K.; Lindsay, L.R.; Wood, H.; Drebot, M.A.; Dibernardo, A.; et al. Evaluation of a Commercial Culture-Free Neutralization Antibody Detection Kit for Severe Acute Respiratory Syndrome-Related Coronavirus-2 and Comparison With an Antireceptor-Binding Domain Enzyme-Linked Immunosorbent Assay. Open Forum Infect. Dis. 2021, 8, ofab220. [Google Scholar] [CrossRef]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. 2020, 92, 2243–2247. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, Y.; Hu, Z.; Xu, Z.; Li, M.; Chen, X.; Yang, C.; Liu, J. Recent Progress on Rapid Lateral Flow Assay-Based Early Diagnosis of COVID-19. Front. Bioeng. Biotechnol. 2022, 10, 866368. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Timothy, E.S.; Adam, K.W.; Jennifer, A.J.; Kanta, S.; Stephen, J.K.; James, A.T.; Miles, P.D. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Timothy, A.B.; Hans, C.L.; Zoe, L.L.; James, R.G.; Marcel, E.C.; William, B.M.; Fikadu, G.T. Age-Dependent Neutralization of SARS-CoV-2 and P.1 Variant by Vaccine Immune Serum Samples. JAMA 2021, 326, 868–869. [Google Scholar]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2020, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Chvatal-Medina, M.; Mendez-Cortina, Y.; Patiño, P.J.; Velilla, P.A.; Rugeles, M.T. Antibody Responses in COVID-19: A Review. Front. Immunol. 2021, 12, 633184. [Google Scholar] [CrossRef]
- Dunn-Walters, D.K.; Stewart, A.T.; Sinclair, E.L.; Serangeli, I. Age-Related Changes in B Cells Relevant to Vaccine Responses. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Pope, A.; Marriott, D.; Chapman, B.; Bak, N.; Daveson, K.; Sorrell, T.C.; European Antimicrobial Resistance Genes Surveillance Network EURGen-Net Capacity Survey Group. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Eurosurveillance 2019, 26, 2100096. [Google Scholar] [CrossRef]
- Markmann, A.J.; Giallourou, N.; Bhowmik, D.R.; Hou, Y.J.; Lerner, A.; Martinez, D.R.; Premkumar, L.; Root, H.; van Duin, D.; Napravnik, S.; et al. Sex Disparities and Neutralizing-Antibody Durability to SARS-CoV-2 Infection in Convalescent Individuals. mSphere 2021, 6, e0027521. [Google Scholar] [CrossRef]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M.; et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2022, 24, 160–165. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Coggins, S.A.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse Effects and Antibody Titers in Response to the BNT162b2 mRNA COVID-19 Vaccine in a Prospective Study of Healthcare Workers. Open Forum Infect. Dis. 2021, 9, ofab575. [Google Scholar] [CrossRef]
| Total | AZD1222 | AZD1222 + BNT162b2 | BNT162b2 | mRNA-1273 | Ad26.COV2.S | p-Value | |
|---|---|---|---|---|---|---|---|
| Sex | <0.0001 | ||||||
| F | 433 (58.8%) | 137 (66.8%) | 52 (55.9%) | 216 (61.2%) | 18 (47.4%) | 10 (21.3%) | |
| M | 303 (41.2%) | 68 (33.2%) | 41 (44.1%) | 137 (38.8%) | 20 (52.6%) | 37 (78.7%) | |
| AGE | 51.5 (±15.2) | 63.4 (±7.4) | 46.0 (±9.7) | 47.9 (±16.7) | 41.4 (±10.8) | 45.7 (±10.9) | <0.0001 |
| Age Group | <0.0001 | ||||||
| 20’s | 74 (10.1%) | 0 (0.0%) | 0 (0.0%) | 67 (19.0%) | 7 (18.4%) | 0 (0.0%) | |
| 30’s | 105 (14.3%) | 3 (1.5%) | 22 (23.7%) | 51 (14.4%) | 9 (23.7%) | 20 (42.6%) | |
| 40’s | 137 (18.6%) | 6 (2.9%) | 44 (47.3%) | 65 (18.4%) | 13 (34.2%) | 9 (19.1%) | |
| 50’s | 171 (23.2%) | 35 (17.1%) | 18 (19.4%) | 99 (28.0%) | 7 (18.4%) | 12 (25.5%) | |
| 60’s | 162 (22.0%) | 124 (60.5%) | 6 (6.5%) | 26 (7.4%) | 2 (5.3%) | 4 (8.5%) | |
| 70’s | 72 (9.8%) | 37 (18.0%) | 3 (3.2%) | 30 (8.5%) | 0 (0.0%) | 2 (4.3%) | |
| Over 80’s | 15 (2.0%) | 0 (0.0%) | 0 (0.0%) | 15 (4.2%) | 0 (0.0%) | 0 (0.0%) | |
| Bloodtype | 0.69 | ||||||
| A | 282 (38.3%) | 79 (38.5%) | 33 (35.5%) | 141 (39.9%) | 13 (34.2%) | 16 (34.0%) | |
| B | 180 (24.5%) | 54 (26.3%) | 22 (23.7%) | 76 (21.5%) | 14 (36.8%) | 14 (29.8%) | |
| O | 207 (28.1%) | 54 (26.3%) | 27 (29.0%) | 107 (30.3%) | 8 (21.1%) | 11 (23.4%) | |
| AB | 67 (9.1%) | 18 (8.8%) | 11 (11.8%) | 29 (8.2%) | 3 (7.9%) | 6 (12.8%) | |
| BMI | 23.1 (±3.4) | 23.2 (±2.7) | 22.9 (±3.4) | 23.0 (±3.6) | 22.6 (±3.4) | 24.4 (±4.0) | 0.075 |
| BMI Group | 0.001 | ||||||
| Underweight | 37 (5.0%) | 5 (2.4%) | 7 (7.5%) | 21 (5.9%) | 4 (10.5%) | 0 (0.0%) | |
| Normal | 343 (46.6%) | 89 (43.4%) | 44 (47.3%) | 172 (48.7%) | 16 (42.1%) | 22 (46.8%) | |
| Overweight | 161 (21.9%) | 63 (30.7%) | 14 (15.1%) | 72 (20.4%) | 8 (21.1%) | 4 (8.5%) | |
| Obesity | 168 (22.8%) | 44 (21.5%) | 27 (29.0%) | 71 (20.1%) | 9 (23.7%) | 17 (36.2%) | |
| High obesity | 27 (3.7%) | 4 (2.0%) | 1 (1.1%) | 17 (4.8%) | 1 (2.6%) | 4 (8.5%) | |
| HBP | 0.0002 | ||||||
| NO | 611 (83.0%) | 151 (73.7%) | 86 (92.5%) | 297 (84.1%) | 35 (92.1%) | 42 (89.4%) | |
| YES | 125 (17.0%) | 54 (26.3%) | 7 (7.5%) | 56 (15.9%) | 3 (7.9%) | 5 (10.6%) | |
| Diabetes | 0.074 | ||||||
| NO | 688 (93.5%) | 183 (89.3%) | 90 (96.8%) | 334 (94.6%) | 37 (97.4%) | 44 (93.6%) | |
| YES | 48 (6.5%) | 22 (10.7%) | 3 (3.2%) | 19 (5.4%) | 1 (2.6%) | 3 (6.4%) | |
| Hyperlipidemia | <0.0001 | ||||||
| NO | 586 (79.6%) | 134 (65.4%) | 78 (83.9%) | 296 (83.9%) | 34 (89.5%) | 44 (93.6%) | |
| YES | 150 (20.4%) | 71 (34.6%) | 15 (16.1%) | 57 (16.1%) | 4 (10.5%) | 3 (6.4%) | |
| History of Cancer | 0.14 | ||||||
| NO | 688 (93.5%) | 188 (91.7%) | 90 (96.8%) | 328 (92.9%) | 35 (92.1%) | 47 (100.0%) | |
| YES | 48 (6.5%) | 17 (8.3%) | 3 (3.2%) | 25 (7.1%) | 3 (7.9%) | 0 (0.0%) | |
| Chronic Fatigue | 0.13 | ||||||
| NO | 693 (94.2%) | 194 (94.6%) | 83 (89.2%) | 333 (94.3%) | 36 (94.7%) | 47 (100.0%) | |
| YES | 43 (5.8%) | 11 (5.4%) | 10 (10.8%) | 20 (5.7%) | 2 (5.3%) | 0 (0.0%) | |
| Insomnia | 0.048 | ||||||
| NO | 662 (89.9%) | 173 (84.4%) | 86 (92.5%) | 323 (91.5%) | 35 (92.1%) | 45 (95.7%) | |
| YES | 74 (10.1%) | 32 (15.6%) | 7 (7.5%) | 30 (8.5%) | 3 (7.9%) | 2 (4.3%) | |
| IBS | 0.80 | ||||||
| NO | 692 (94.0%) | 190 (92.7%) | 89 (95.7%) | 333 (94.3%) | 35 (92.1%) | 45 (95.7%) | |
| YES | 44 (6.0%) | 15 (7.3%) | 4 (4.3%) | 20 (5.7%) | 3 (7.9%) | 2 (4.3%) | |
| Weakened Immunity | 0.69 | ||||||
| NO | 695 (94.4%) | 193 (94.1%) | 89 (95.7%) | 334 (94.6%) | 34 (89.5%) | 45 (95.7%) | |
| YES | 41 (5.6%) | 12 (5.9%) | 4 (4.3%) | 19 (5.4%) | 4 (10.5%) | 2 (4.3%) | |
| Degree of Pain after Vaccination | 1.6 (±0.7) | 1.5 (±0.6) | 1.8 (±0.6) | 1.6 (±0.7) | 2.1 (±0.8) | 1.7 (±0.9) | <0.0001 |
| Degree of Pain after Vaccination (Ordinal Data) | <0.0001 | ||||||
| 1 | 321 (43.6%) | 106 (51.7%) | 21 (22.6%) | 162 (45.9%) | 6 (15.8%) | 26 (55.3%) | |
| 1.5 | 112 (15.2%) | 39 (19.0%) | 17 (18.3%) | 50 (14.2%) | 6 (15.8%) | 0 (0.0%) | |
| 2 | 174 (23.6%) | 34 (16.6%) | 34 (36.6%) | 84 (23.8%) | 10 (26.3%) | 12 (25.5%) | |
| 2.5 | 77 (10.5%) | 16 (7.8%) | 15 (16.1%) | 37 (10.5%) | 9 (23.7%) | 0 (0.0%) | |
| 3 | 36 (4.9%) | 7 (3.4%) | 3 (3.2%) | 16 (4.5%) | 4 (10.5%) | 6 (12.8%) | |
| 3.5 | 7 (1.0%) | 1 (0.5%) | 2 (2.2%) | 3 (0.8%) | 1 (2.6%) | 0 (0.0%) | |
| 4 | 9 (1.2%) | 2 (1.0%) | 1 (1.1%) | 1 (0.3%) | 2 (5.3%) | 3 (6.4%) | |
| Days after Completion of Vaccination | 77.1 (±41.9) | 76.4 (±31.8) | 100.8 (±32.4) | 67.7 (±43.0) | 41.8 (±29.6) | 132.4 (±24.2) | <0.0001 |
| Days after Completion of Vaccination (Ordinal Data) | <0.0001 | ||||||
| ~30 days | 74 (10.1%) | 5 (2.4%) | 1 (1.1%) | 54 (15.3%) | 14 (36.8%) | 0 (0.0%) | |
| 30~90 days | 420 (57.1%) | 140 (68.3%) | 36 (38.7%) | 220 (62.3%) | 22 (57.9%) | 2 (4.3%) | |
| After 90 days | 242 (32.9%) | 60 (29.3%) | 56 (60.2%) | 79 (22.4%) | 2 (5.3%) | 45 (95.7%) | |
| Neutralizing Antibody | S1 RBD IgG Antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Negative | Positive | p-Value | Total | Negative | Positive | p-Value | |
| Sex | ||||||||
| F | 433 (58.8%) | 139 (32.1%) | 294 (67.9%) | 0.21 | 433 (58.8%) | 37 (8.5%) | 396 (91.5%) | <0.0001 |
| M | 303 (41.2%) | 111 (36.6%) | 192 (63.4%) | 303 (41.2%) | 63 (20.8%) | 240 (79.2%) | ||
| Age | 51.5 (±15.2) | 59.6 (±13.4) | 47.3 (±14.4) | <0.0001 | 51.5 (±15.2) | 58.0 (±13.7) | 50.5 (±15.2) | <0.0001 |
| Age Group | ||||||||
| 20’s | 74 (10.1%) | 1 (1.4%) | 73 (98.6%) | <0.0001 | 74 (10.1%) | 0 (0.0%) | 74 (100.0%) | <0.0001 |
| 30’s | 105 (14.3%) | 30 (28.6%) | 75 (71.4%) | 105 (14.3%) | 18 (17.1%) | 87 (82.9%) | ||
| 40’s | 137 (18.6%) | 28 (20.4%) | 109 (79.6%) | 137 (18.6%) | 10 (7.3%) | 127 (92.7%) | ||
| 50’s | 171 (23.2%) | 41 (24.0%) | 130 (76.0%) | 171 (23.2%) | 16 (9.4%) | 155 (90.6%) | ||
| 60’s | 162 (22.0%) | 93 (57.4%) | 69 (42.6%) | 162 (22.0%) | 38 (23.5%) | 124 (76.5%) | ||
| 70’s | 72 (9.8%) | 47 (65.3%) | 25 (34.7%) | 72 (9.8%) | 14 (19.4%) | 58 (80.6%) | ||
| Over 80’s | 15 (2.0%) | 10 (66.7%) | 5 (33.3%) | 15 (2.0%) | 4 (26.7%) | 11 (73.3%) | ||
| Bloodtype | ||||||||
| A | 282 (38.3%) | 96 (34.0%) | 186 (66.0%) | 0.95 | 282 (38.3%) | 33 (11.7%) | 249 (88.3%) | 0.36 |
| B | 180 (24.5%) | 64 (35.6%) | 116 (64.4%) | 180 (24.5%) | 29 (16.1%) | 151 (83.9%) | ||
| O | 207 (28.1%) | 68 (32.9%) | 139 (67.1%) | 207 (28.1%) | 26 (12.6%) | 181 (87.4%) | ||
| AB | 67 (9.1%) | 22 (32.8%) | 45 (67.2%) | 67 (9.1%) | 12 (17.9%) | 55 (82.1%) | ||
| BMI | 23.1 (±3.4) | 23.3 (±3.0) | 23.0 (±3.6) | 0.046 | 23.1 (±3.4) | 23.7 (±3.1) | 23.0 (±3.4) | 0.013 |
| BMI Group | ||||||||
| Underweight | 37 (5.0%) | 11 (29.7%) | 26 (70.3%) | 0.17 | 37 (5.0%) | 5 (13.5%) | 32 (86.5%) | 0.25 |
| Normal | 343 (46.6%) | 106 (30.9%) | 237 (69.1%) | 343 (46.6%) | 40 (11.7%) | 303 (88.3%) | ||
| Overweight | 161 (21.9%) | 62 (38.5%) | 99 (61.5%) | 161 (21.9%) | 20 (12.4%) | 141 (87.6%) | ||
| Obesity | 168 (22.8%) | 65 (38.7%) | 103 (61.3%) | 168 (22.8%) | 32 (19.0%) | 136 (81.0%) | ||
| High obesity | 27 (3.7%) | 6 (22.2%) | 21 (77.8%) | 27 (3.7%) | 3 (11.1%) | 24 (88.9%) | ||
| HBP | ||||||||
| No | 611 (83.0%) | 191 (31.3%) | 420 (68.7%) | 0.0009 | 611 (83.0%) | 80 (13.1%) | 531 (86.9%) | 0.39 |
| Yes | 125 (17.0%) | 59 (47.2%) | 66 (52.8%) | 125 (17.0%) | 20 (16.0%) | 105 (84.0%) | ||
| Diabetes | ||||||||
| No | 688 (93.5%) | 220 (32.0%) | 468 (68.0%) | <0.0001 | 688 (93.5%) | 87 ( 12.6%) | 601 (87.4%) | 0.008 |
| Yes | 48 (6.5%) | 30 (62.5%) | 18 (37.5%) | 48 (6.5%) | 13 (27.1%) | 35 (72.9%) | ||
| Hyperlipidemia | ||||||||
| No | 586 (79.6%) | 180 (30.7%) | 406 (69.3%) | 0.0003 | 586 (79.6%) | 76 (13.0%) | 510 (87.0%) | 0.35 |
| Yes | 150 (20.4%) | 70 (46.7%) | 80 (53.3%) | 150 (20.4%) | 24 (16.0%) | 126 (84.0%) | ||
| History of cancer | ||||||||
| No | 688 (93.5%) | 227 (33.0%) | 461 (67.0%) | 0.041 | 688 (93.5%) | 92 (13.4%) | 596 (86.6%) | 0.51 |
| Yes | 48 (6.5%) | 23 (47.9%) | 25 (52.1%) | 48 (6.5%) | 8 (16.7%) | 40 (83.3%) | ||
| Chronic fatigue | ||||||||
| No | 693 (94.2%) | 243 (35.1%) | 450 (64.9%) | 0.012 | 693 (94.2%) | 97 (14.0%) | 596 (86.0%) | 0.25 |
| Yes | 43 ( 5.8%) | 7 (16.3%) | 36 (83.7%) | 43 (5.8%) | 3 (7.0%) | 40 (93.0%) | ||
| Insomnia | ||||||||
| No | 662 (89.9%) | 219 (33.1%) | 443 (66.9%) | 0.15 | 662 (89.9%) | 93 (14.0%) | 569 (86.0%) | 0.37 |
| Yes | 74 (10.1%) | 31 (41.9%) | 43 (58.1%) | 74 (10.1%) | 7 (9.5%) | 67 (90.5%) | ||
| IBS | ||||||||
| No | 692 (94.0%) | 237 (34.2%) | 455 (65.8%) | 0.62 | 692 (94.0%) | 91 (13.2%) | 601 (86.8%) | 0.17 |
| Yes | 44 (6.0%) | 13 (29.5%) | 31 (70.5%) | 44 (6.0%) | 9 ( 20.5%) | 35 (79.5%) | ||
| Weakened immunity | ||||||||
| No | 695 (94.4%) | 234 (33.7%) | 461 (66.3%) | 0.5 | 695 (94.4%) | 95 (13.7%) | 600 (86.3%) | 1 |
| Yes | 41 (5.6%) | 16 (39.0%) | 25 (61.0%) | 41 (5.6%) | 5 (12.2%) | 36 (87.8%) | ||
| Degree of Pain after Vaccination | 1.6 (±0.7) | 1.5 (±0.7) | 1.7 (±0.7) | <0.0001 | 1.6 (±0.7) | 1.4 (±0.7) | 1.7 (±0.7) | <0.0001 |
| Degree of Pain after Vaccination | ||||||||
| 1 | 321 (43.6%) | 144 (44.9%) | 177 (55.1%) | <0.0001 | 321 (43.6%) | 64 (19.9%) | 257 (80.1%) | <0.0001 |
| 1.5 | 112 (15.2%) | 38 (33.9%) | 74 (66.1%) | 112 (15.2%) | 11 (9.8%) | 101 (90.2%) | ||
| 2 | 174 (23.6%) | 41 (23.6%) | 133 (76.4%) | 174 (23.6%) | 15 (8.6%) | 159 (91.4%) | ||
| 2.5 | 77 (10.5%) | 8 (10.4%) | 69 (89.6%) | 77 (10.5%) | 1 (1.3%) | 76 (98.7%) | ||
| 3 | 36 (4.9%) | 15 (41.7%) | 21 (58.3%) | 36 (4.9%) | 6 (16.7%) | 30 (83.3%) | ||
| 3.5 | 7 (1.0%) | 0 (0.0%) | 7 (100.0%) | 7 (1.0%) | 0 (0.0%) | 7 (100.0%) | ||
| 4 | 9 (1.2%) | 4 (44.4%) | 5 (55.6%) | 9 (1.2%) | 3 (33.3%) | 6 (66.7%) | ||
| Days after completion of Vaccination | 77.1 (±41.9) | 99.3 (±40.1) | 65.7 (±38.0) | <0.0001 | 77.1 (±41.9) | 104.9 (±42.6) | 72.7 (±40.0) | <0.0001 |
| Days after completion of Vaccination | ||||||||
| ~30 days | 74 (10.1%) | 2 (2.7%) | 72 (97.3%) | <0.0001 | 74 (10.1%) | 2 (2.7%) | 72 (97.3%) | <0.0001 |
| 30~90 days | 420 (57.1%) | 110 (26.2%) | 310 (73.8%) | 420 (57.1%) | 38 (9.0%) | 382 (91.0%) | ||
| After 90 days | 242 (32.9%) | 138 (57.0%) | 104 (43.0%) | 242 (32.9%) | 60 (24.8%) | 182 (75.2%) | ||
| Classification | ||||||||
| AZD1222 | 205 (27.9%) | 135 (65.9%) | 70 (34.1%) | <0.0001 | 205 (27.9%) | 59 (28.8%) | 146 (71.2%) | <0.0001 |
| AZD1222 + BNT162b2 | 93 (12.6%) | 24 (25.8%) | 69 (74.2%) | 93 (12.6%) | 3 (3.2%) | 90 (96.8%) | ||
| BNT162b2 | 353 (48.0%) | 49 (13.9%) | 304 (86.1%) | 353 (48.0%) | 8 (2.0%) | 345 (97.7%) | ||
| mRNA-1273 | 38 (5.2%) | 0 (0.0%) | 38 (100.0%) | 38 (5.2%) | 0 (0.0%) | 38 (100.0%) | ||
| Ad26.COV2.S | 47 (6.4%) | 42 (89.4%) | 5 (10.6%) | 47 (6.4%) | 30 (63.8%) | 17 (36.2%) | ||
| Count | PRNT (IU/mL) Mean (SD) | ||
|---|---|---|---|
| Sex | Total | 675 | 1167.1 (±816.7) |
| F | 404 | 1216.2 (±780.5) | |
| M | 271 | 1093.9 (±864.1) | |
| p-value | 0.021 | ||
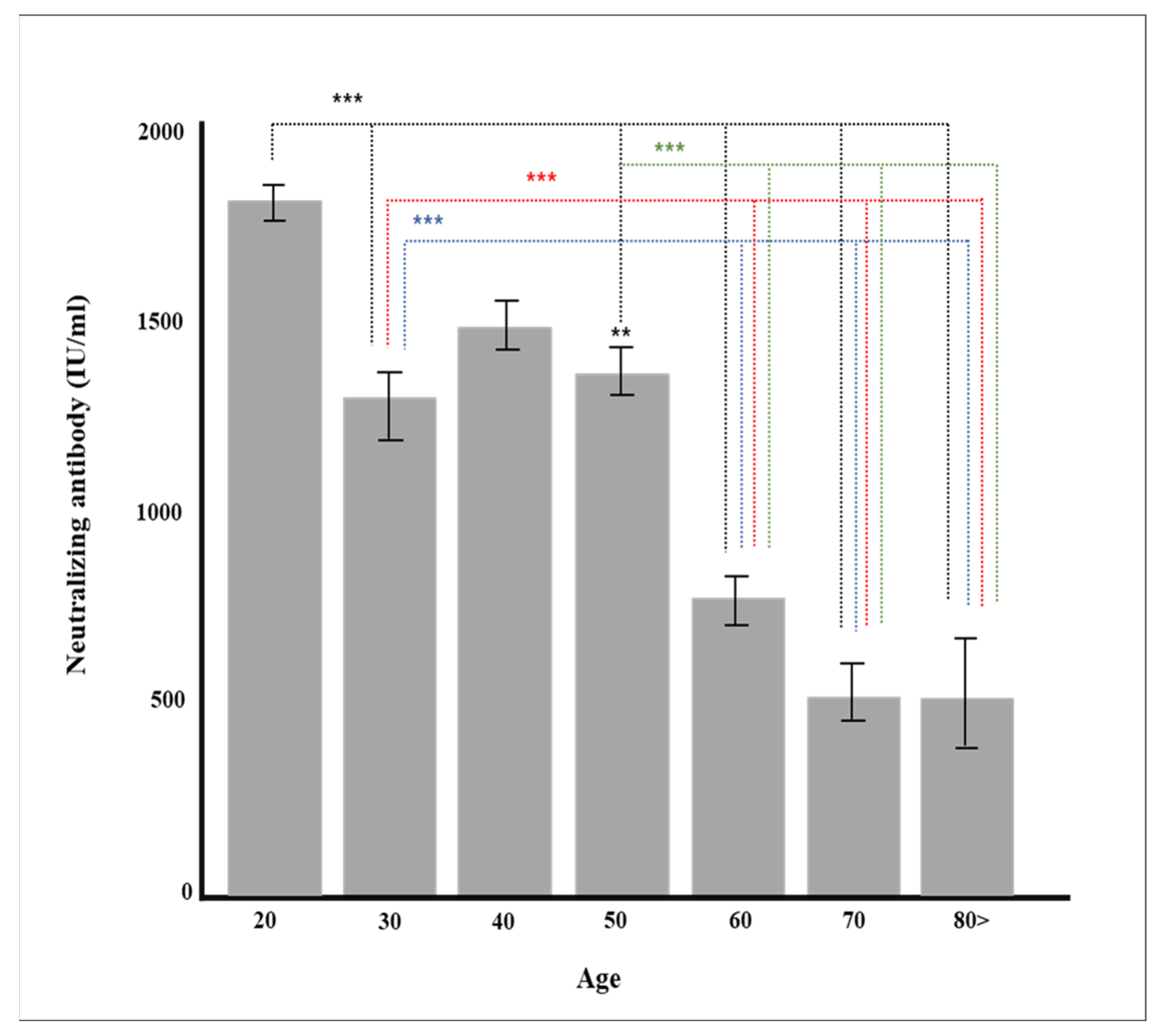
| Age Group | Total | 675 | 1167.1 (±816.7) |
| 20’s | 59 | 1807.7 (±351.0) | |
| 30’s | 91 | 1272.2 (±849.4) | |
| 40’s | 120 | 1489.5 (±718.7) | |
| 50’s | 161 | 1368.5 (±775.3) | |
| 60’s | 157 | 768.2 (±730.2) | |
| 70’s | 72 | 525.6 (±619.4) | |
| Over 80’s | 15 | 523.3 (±557.7) | |
| p-value | <0.0001 | ||
| Blood Type | Total | 675 | 1167.1 (±816.7) |
| A | 258 | 1191.3 (±817.9) | |
| B | 157 | 1084.3 (±812.1) | |
| O | 196 | 1212.0 (±815.5) | |
| AB | 64 | 1134.9 (±829.6) | |
| p-value | 0.47 | ||
| BMI Group | Total | 675 | 1167.1 (±816.7) |
| Underweight | 31 | 1347.4 (±779.6) | |
| Normal | 322 | 1227.3 (±777.6) | |
| Overweight | 150 | 1096.6 (±815.9) | |
| Obesity | 147 | 1046.0 (±892.9) | |
| High obesity | 25 | 1303.4 (±817.9) | |
| p-value | 0.121 | ||
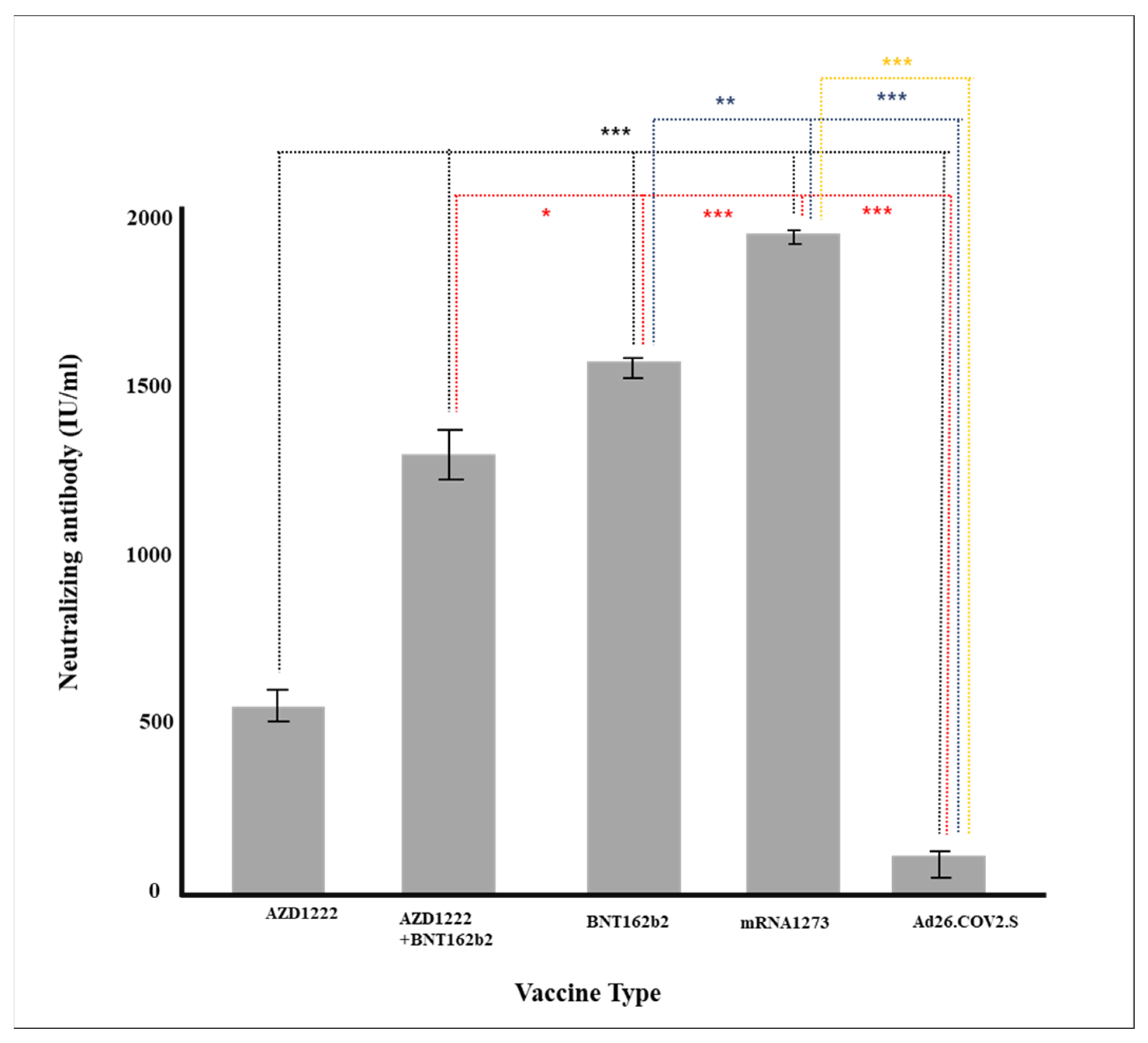
| Classification | Total | 675 | 1167.1 (±816.7) |
| AZD1222 | 198 | 572.9 (±629.3) | |
| AZD1222 + BNT162b2 | 89 | 1339.4 (±706.4) | |
| BNT162b2 | 310 | 1566.2 (±639.1) | |
| mRNA-1273 | 33 | 992.2 (±103.7) | |
| Ad26.COV2.S | 45 | 86.2 (±304.5) | |
| p-value | <0.0001 | ||
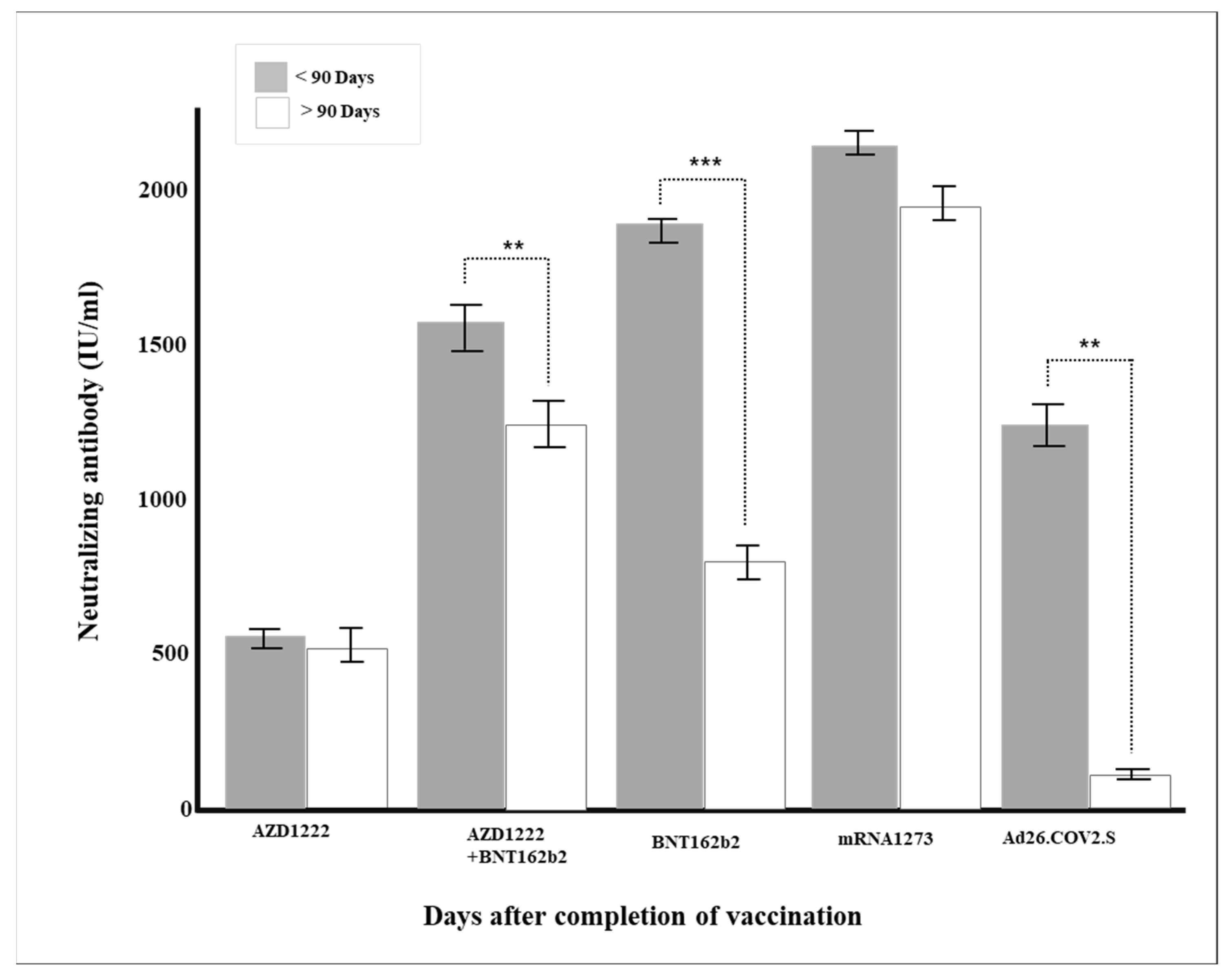
| Days after Completion of Vaccination | Total | 675 | 1167.1 (±816.7) |
| ~30 DAYS | 71 | 1861.2 (±475.8) | |
| 30~90 DAYS | 383 | 1324.4 (±769.5) | |
| 90~ DAYS | 221 | 671.4 (±705.6) | |
| p-value | <0.0001 | ||
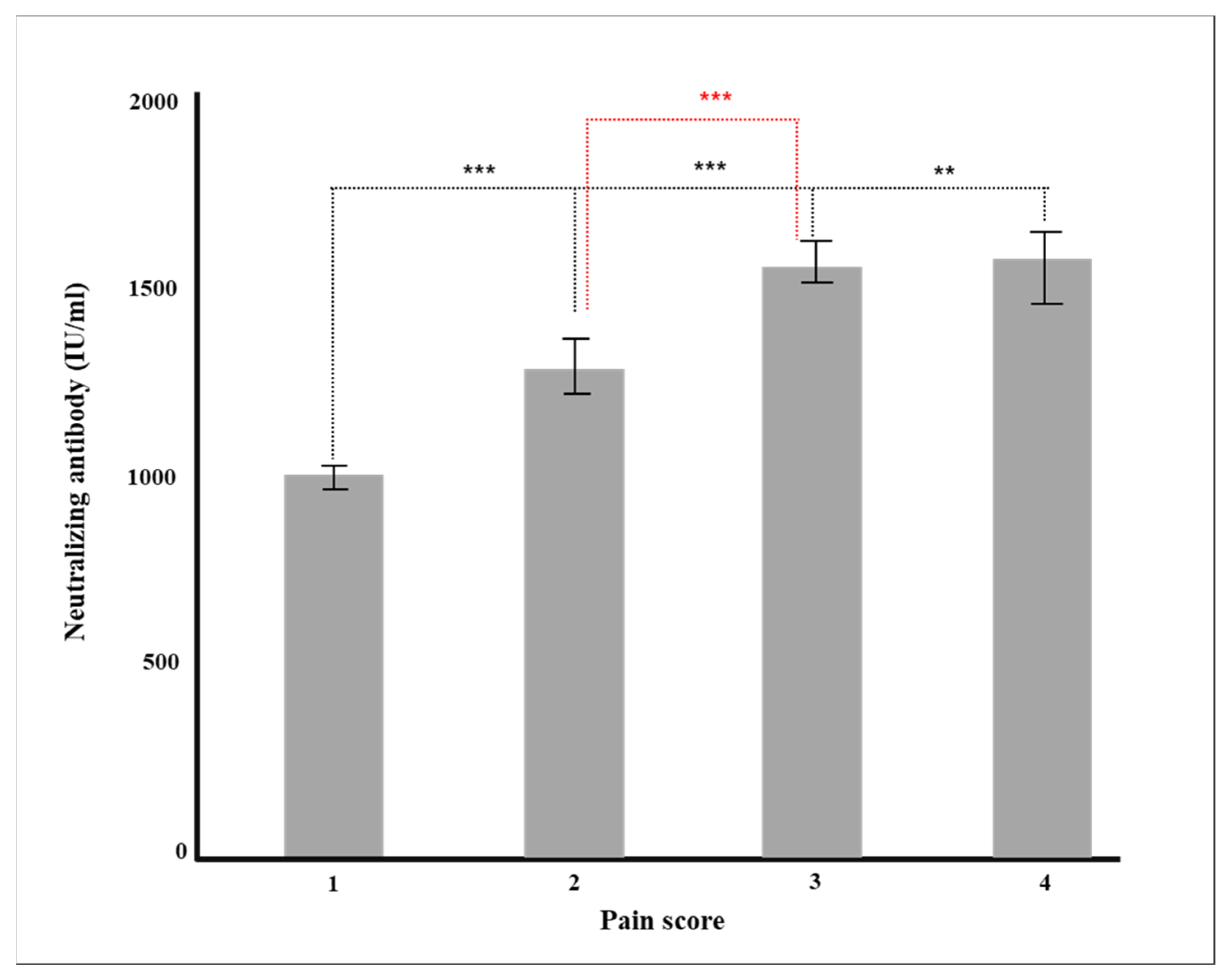
| Degree of Pain after Vaccination | Total | 675 | 1167.1 (±816.7) |
| 1 | 296 | 948.3 (±822.4) | |
| 1.5 | 104 | 1231.4 (±795.7) | |
| 2 | 162 | 1326.1 (±784.3) | |
| 2.5 | 66 | 1600.5 (±588.3) | |
| 3 | 33 | 1202.4 (±827.0) | |
| 3.5 | 6 | 1878.1 (±175.6) | |
| 4 | 8 | 950.9 (±980.9) | |
| p-value | <0.0001 | ||
| Diabetes | Total | 675 | 1167.1 (±816.7) |
| NO | 628 | 1212.0 (±805.4) | |
| YES | 47 | 566.9 (±731.8) | |
| p-value | <0.0001 | ||
| HBP | Total | 675 | 1167.1 (±816.7) |
| NO | 528 | 1218.9 (±803.8) | |
| YES | 147 | 981.1 (±838.0) | |
| p-value | 0.002 | ||
| History of Cancer | Total | 675 | 1167.1 (±816.7) |
| NO | 629 | 1178.1 (±815.2) | |
| YES | 46 | 1017.3 (±830.9) | |
| p-value | 0.21 | ||
| Chronic Fatigue | Total | 675 | 1167.1 (±816.7) |
| NO | 635 | 1148.1 (±820.5) | |
| YES | 40 | 1468.1 (±694.8) | |
| p-value | 0.018 | ||
| Insomnia | Total | 675 | 1167.1 (±816.7) |
| NO | 603 | 1186.2 (±822.4) | |
| YES | 72 | 1006.8 (±753.6) | |
| p-value | 0.062 | ||
| IBS | Total | 675 | 1167.1 (±816.7) |
| NO | 635 | 1163.8 (±816.8) | |
| YES | 40 | 1220.0 (±823.2) | |
| p-value | 0.677 | ||
| Weakened Immunity | Total | 675 | 1167.1 (±816.7) |
| NO | 635 | 1162.1 (±815.8) | |
| YES | 40 | 1246.2 (±837.1) | |
| p-value | 0.54 |
| PRNT (IU/mL) | |||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 1380.67 | 979.52–1781.81 | <0.001 |
| Sex (M, F = ref.) | −63.44 | −157.10–30.22 | 0.184 |
| Age | −7.94 | −11.55–−4.33 | <0.001 |
| Classification (AZD1222 = ref.) | |||
| AZD1222 + BNT162b2 | 730.48 | 578.47–882.48 | <0.001 |
| BNT162b2 | 814.33 | 709.45–919.21 | <0.001 |
| mRNA-1273 | 978.87 | 769.62–1188.13 | <0.001 |
| Ad26.COV2.S | −286.57 | −488.00–−85.14 | 0.005 |
| Blood Type (A = ref.) | |||
| B | 21.06 | −85.19–127.31 | 0.697 |
| O | −19.00 | −118.92–80.92 | 0.709 |
| AB | −30.93 | −176.70–114.84 | 0.677 |
| BMI | 3.42 | −10.57–17.42 | 0.631 |
| HBP (YES, NO = ref.) | −36.39 | −157.71–84.93 | 0.556 |
| Diabetes (YES, NO = ref.) | −283.29 | −452.61–−113.97 | 0.001 |
| Hyperlipidemia (YES, NO = ref.) | 24.11 | −81.76–129.98 | 0.655 |
| History of Cancer (YES, NO = ref.) | −13.15 | −178.13–151.82 | 0.876 |
| Chronic Fatigue (YES, NO = ref.) | 94.08 | −93.21–281.37 | 0.324 |
| Insomnia (YES) (YES, NO = ref.) | −30.86 | −170.24–108.52 | 0.664 |
| IBS (YES, NO = ref.) | 46.92 | −125.92–219.77 | 0.594 |
| Weakened Immunity (YES, NO = ref.) | −98.44 | −282.78–85.91 | 0.295 |
| Degree of Pain after vaccination | 97.15 | 34.63–159.66 | 0.002 |
| Days after Completion OF Vaccination | −6.24 | −7.40–−5.08 | <0.001 |
| Observations | 675 | ||
| R2/R2 adjusted | 0.597/0.585 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.W.; Shin, J.h.; Shin, S.C.; Lee, H.J.; So, K.S.; Lee, S.Y.; Jun, J.W.; Seo, J.K.; Lee, H.S.; Lee, S.Y.; et al. Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics 2022, 12, 1924. https://doi.org/10.3390/diagnostics12081924
Shim HW, Shin Jh, Shin SC, Lee HJ, So KS, Lee SY, Jun JW, Seo JK, Lee HS, Lee SY, et al. Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics. 2022; 12(8):1924. https://doi.org/10.3390/diagnostics12081924
Chicago/Turabian StyleShim, Hyeon Woo, Jae hang Shin, Shang Cheol Shin, Hwa Jung Lee, Kyung Soon So, So Young Lee, Jae Woo Jun, Jeong Ku Seo, Hwa Seop Lee, Suk Young Lee, and et al. 2022. "Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test" Diagnostics 12, no. 8: 1924. https://doi.org/10.3390/diagnostics12081924
APA StyleShim, H. W., Shin, J. h., Shin, S. C., Lee, H. J., So, K. S., Lee, S. Y., Jun, J. W., Seo, J. K., Lee, H. S., Lee, S. Y., Kim, S. H., Kim, S. J., Kim, K.-C., & Ryu, G. H. (2022). Analysis of Factors Affecting Neutralizing Antibody Production after COVID-19 Vaccination Using Newly Developed Rapid Point-of-Care Test. Diagnostics, 12(8), 1924. https://doi.org/10.3390/diagnostics12081924






