New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement
Abstract
:1. Introduction
2. Methods
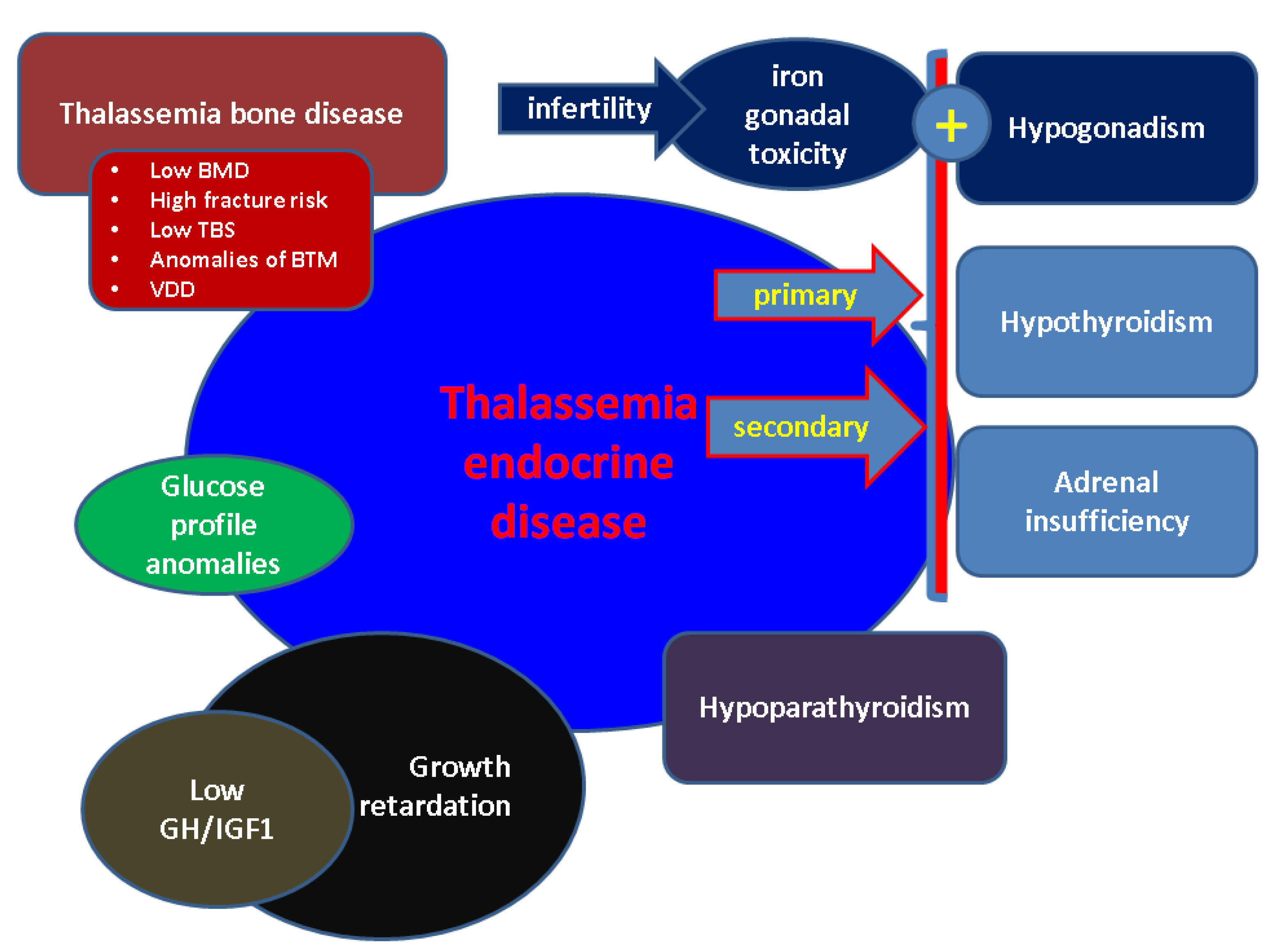
3. Thalassemic Endocrine Disease
3.1. Growth Retardation and GH/IGF1Axis
3.2. Hypogonadism in BTH
3.3. BTH-Related Hypothyroidism
3.4. Major THD-Associated Hypoparathyroidism
3.5. Adrenal Gland Status in Patients with Major BTH
3.6. Glucose Profile in Major BTH
3.7. Thalassemia Bone Disease
3.8. Imaging of Endocrine Glands in BTH
4. Fertility Issues in Females and Males Diagnosed with Major BTH
5. Pregnancy Outcome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic Hormone |
| ART | Assisted Reproductive Technique |
| AMH | Anti-Müllerian Hormone |
| AFC | Antral Follicle Count |
| BMI | Body Mass Index |
| BTH | Beta-Thalassemia |
| BME | Bone Marrow Expansion |
| BMD | Bone Mineral Density |
| CTX | C-terminal Telopeptide |
| DXA | Dual-Energy X-ray Absorptiometry |
| DM | Diabetes Mellitus |
| EH | Extra-Medullary Haematopoiesis |
| ED | Endocrine Diseases |
| FT4 | Free Levothyroxine |
| FSH | Follicle Stimulating Hormone |
| GH | Growth Hormone |
| GHRH | GH Releasing Hormone |
| IGF1 | Insulin-like Growth Factor |
| IO | Iron Overload |
| IGT | Impaired Glucose Tolerance |
| IGF | Impaired Fasting Glucose |
| ICET-A | International Network of Clinicians for Endocrinopathies in Thalassemia and Adolescence Medicine |
| Hb | Haemoglobin |
| LIC | Liver Iron Concentration |
| LH | Luteinizing Hormone |
| MRI | Magnetic Resonance Imaging |
| NGS | Next-Generation Sequencing |
| OGTT | Oral Glucose Tolerance Test |
| OPG | Osteoprotegerin |
| OP | Osteoporosis |
| ROS | Reactive Oxygen Species |
| RANKL | Receptor Activator of Nuclear Factor Kappa-Β Ligand |
| TT | Testicular Tissue |
| TSH | Thyroid Stimulating Hormone |
| TS | Transferrin Saturation |
| TBD | Thalassemia Bone Disease |
| TED | Thalassemic Endocrine Disease |
| TBS | Trabecular Bone Score |
| TA-CVS | Transabdominal Chorionic Villus Sampling |
| TT | Testicular Tissue |
| VDD | Vitamin D Deficiency |
| VF | Vertebral Fracture |
| y | Years |
References
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of β-thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.C.; Batten, S.H.; Sparks, S.K. Alpha- and Beta-thalassemia: Rapid Evidence Review. Am. Fam. Physician 2022, 105, 272–280. [Google Scholar] [PubMed]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Kazmi, S.A.R.; Sadia Irfan, M.; et al. Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genom. Med. 2021, 9, e1788. [Google Scholar] [CrossRef]
- Tahir, A.; Hussain, S.I.; Khan, H.S.; Khalil, S.; Haider, S.Z.; Lodhi, M.A. Efficacy and Tolerability of Oral Iron Chelator, Deferasirox. J. Ayub Med. Coll. Abbottabad 2021, 33, 207–212. [Google Scholar] [PubMed]
- Baghersalimi, A.; Darbandi, B.; Kazemnezhad Leyli, E.; Kamran Mavardiani, Z.; Ahmad Sharbafi, M.; Rezasefat Balesbaneh, A. Evaluation of Self-efficacy in Children and Adolescents with Thalassemia Major. J. Pediatr. Hematol. Oncol. 2021, 43, e754–e758. [Google Scholar] [CrossRef] [PubMed]
- Banjar, H.R.; Zaher, G.F.; Almutiry, H.S.; Alshamarni, A.S.A.; Almouhana, G.I.; Alahwal, H.M.; Bahashwan, S.; Barefah, A.S.; Alnajjar, S.A.; Alharbi, H.M. Web-based expert system with quick response code for beta-thalassemia management. Health Inform. J. 2021, 27, 1460458221989397. [Google Scholar] [CrossRef] [PubMed]
- Amjad, F.; Fatima, T.; Fayyaz, T.; Khan, M.A.; Qadeer, M.I. Novel genetic therapeutic approaches for modulating the severity of β-thalassemia (Review). Biomed. Rep. 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Swain, T.R.; Jena, R.K.; Panigrahi, A.; Debta, N. Effectiveness of Deferasirox in Pediatric Thalassemia Patients: Experience from a Tertiary Care Hospital of Odisha. Indian J. Pharmacol. 2020, 52, 172–178. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Caprari, P.; Massimi, S.; Sorrentino, F.; Maffei, L.; Gabbianelli, M.; Riganò, R. Phenotypical and functional abnormalities of circulating neutrophils in patients with β-thalassemia. Ann. Hematol. 2020, 99, 2265–2277. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Li, X.; Wang, Q.; Zhang, J.; Lin, Y. Cost-Utility Analysis of four Chelation Regimens for β-thalassemia Major: A Chinese Perspective.Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020029. [Google Scholar] [CrossRef]
- Fibach, E.; Rachmilewitz, E.A. Pathophysiology and treatment of patients with beta-thalassemia—An update. F1000Research 2017, 6, 2156. [Google Scholar] [CrossRef] [Green Version]
- Betts, M.; Flight, P.A.; Paramore, L.C.; Tian, L.; Milenković, D.; Sheth, S. Systematic Literature Review of the Burden of Disease and Treatment for Transfusion-dependent β-Thalassemia. Clin. Ther. 2020, 42, 322–337.e2. [Google Scholar] [CrossRef]
- Abd El Hakeem, A.A.; Mousa, S.M.O.; AbdelFattah, M.T.; AbdelAziz, A.O.; Abd El Azeim, S.S. Pulmonary functions in Egyptian children with transfusion-dependent β-thalassemia. Transfus. Med. 2019, 29, 55–60. [Google Scholar] [CrossRef]
- Hoodbhoy, Z.; Ehsan, L.; Alvi, N.; Sajjad, F.; Asghar, A.; Nadeem, O.; Qidwai, A.; Hussain, S.; Hasan, E.; Altaf, S.; et al. Establishment of a thalassaemia major quality improvement collaborative in Pakistan. Arch. Dis. Child. 2020, 105, 487–493. [Google Scholar] [CrossRef]
- Zafar, S.; Saleem, K.; Rashid, A. An Unusual Presentation of a Patient with Leg Ulcers: A Case Report. Cureus 2019, 11, e6293. [Google Scholar] [CrossRef] [Green Version]
- Rattananon, P.; Anurathapan, U.; Bhukhai, K.; Hongeng, S. The Future of Gene Therapy for Transfusion-Dependent Beta-Thalassemia: The Power of the Lentiviral Vector for Genetically Modified Hematopoietic Stem Cells. Front. Pharmacol. 2021, 12, 730873. [Google Scholar] [CrossRef]
- Tang, C.H.; Furnback, W.; Wang, B.C.M.; Tang, J.; Tang, D.; Lu, M.Y.; Huang, V.W.; Musallam, K.M. Relationship between transfusion burden, healthcare resource utilization, and complications in patients with beta-thalassemia in Taiwan: A real-world analysis. Transfusion 2021, 61, 2906–2917. [Google Scholar] [CrossRef]
- Fortin, P.M.; Fisher, S.A.; Madgwick, K.V.; Trivella, M.; Hopewell, S.; Doree, C.; Estcourt, L.J. Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia. Cochrane Database Syst. Rev. 2018, 5, CD012349. [Google Scholar] [CrossRef]
- Horvei, P.; MacKenzie, T.; Kharbanda, S. Advances in the management of α-thalassemia major: Reasons to be optimistic. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 592–599. [Google Scholar] [CrossRef]
- Gagliardi, I.; Mungari, R.; Gamberini, M.R.; Fortini, M.; Dassie, F.; Putti, M.C.; Maffei, P.; Aliberti, L.; Bondanelli, M.; Zatelli, M.C.; et al. GH/IGF-1 axis in a large cohort of ß-thalassemia major adult patients: A cross-sectional study. J. Endocrinol. Invest. 2022, 45, 1439–1445. [Google Scholar] [CrossRef]
- Seow, C.E.; Goh, A.S.; Lim, S.L. High prevalence of central hypothyroidism among patients with transfusion dependent thalassemia in Hospital Pulau Pinang: A cross sectional study. Med. J. Malaysia 2021, 76, 799–803. [Google Scholar]
- Dixit, N.; Shaw, C.K.; Varshney, G.A.; Kumar, R.; Saini, P.A.; Verma, P. Endocrinal Complications in Children and Adolescents with Thalassemia Major in Central India: An Observational Study. Indian J. Pediatr. 2021. [Google Scholar] [CrossRef]
- Atmakusuma, T.D.; Hasibuan, F.D.; Purnamasari, D. The Correlation Between Iron Overload and Endocrine Function in Adult Transfusion-Dependent Beta-Thalassemia Patients with Growth Retardation. J. Blood Med. 2021, 12, 749–753. [Google Scholar] [CrossRef]
- Mahmoud, R.A.; Khodeary, A.; Farhan, M.S. Detection of endocrine disorders in young children with multi-transfused thalassemia major. Ital. J. Pediatr. 2021, 47, 165. [Google Scholar] [CrossRef]
- Arab-Zozani, M.; Kheyrandish, S.; Rastgar, A.; Miri-Moghaddam, E. A Systematic Review and Meta-Analysis of Stature Growth Complications in β-thalassemia Major Patients. Ann. Glob. Health. 2021, 87, 48. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Samaddar, S.; Parakh, N.; Chandra, J.; Seth, A. Pubertal Development and its Determinants in Adolescents with Transfusion-Dependent Thalassemia. Indian Pediatr. 2021, 58, 635–638. [Google Scholar] [CrossRef]
- Nayak, A.M.; Choudhari, A.; Patkar, D.P.; Merchant, R.H. Pituitary Volume and Iron Overload Evaluation by 3T MRI in Thalassemia. Indian J. Pediatr. 2021, 88, 656–662. [Google Scholar] [CrossRef]
- Casale, M.; Forni, G.L.; Cassinerio, E.; Pasquali, D.; Origa, R.; Serra, M.; Campisi, S.; Peluso, A.; Renni, R.; Cattoni, A.; et al. Risk factors for endocrine complications in transfusion-dependent thalassemia patients on chelation therapy with deferasirox: A risk assessment study from a multi-center nation-wide cohort. Haematologica 2022, 107, 467–477. [Google Scholar] [CrossRef]
- Jobanputra, M.; Paramore, C.; Laird, S.G.; McGahan, M.; Telfer, P. Co-morbidities and mortality associated with transfusion-dependent beta-thalassaemia in patients in England: A 10-year retrospective cohort analysis. Br. J. Haematol. 2020, 191, 897–905. [Google Scholar] [CrossRef]
- Karadag, S.I.K.; Karakas, Z.; Yilmaz, Y.; Gul, N.; Demir, A.A.; Bayramoglu, Z.; Darendeliler, F.; Dursun, M. Pituitary Iron Deposition and Endocrine Complications in Patients with β-Thalassemia: From Childhood to Adulthood. Hemoglobin 2020, 44, 344–348. [Google Scholar] [CrossRef]
- Lee, K.T.; Lim, S.L.; Goh, A.S. Prevalence of endocrine complications in transfusion dependent thalassemia in Hospital Pulau Pinang: A pilot study. Med. J. Malaysia 2020, 75, 33–37. [Google Scholar]
- Yassouf, M.Y.; Alquobaili, F.; Kabalan, Y.; Mukhalalaty, Y. Compliance with Deferoxamine Therapy and Thyroid Dysfunction of Patients with β-Thalassemia Major in Syria. Hemoglobin 2019, 43, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, M.; Bozorgi, H.; Saki, F.; Haghpanah, S.; Karimi, M.; Bazrafshan, A.; Zekavat, O.R. Prevalence of endocrine disorders and their associated factors in transfusion-dependent thalassemia patients: A historical cohort study in Southern Iran. J. Endocrinol. Investig. 2019, 42, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- He, L.N.; Chen, W.; Yang, Y.; Xie, Y.J.; Xiong, Z.Y.; Chen, D.Y.; Lu, D.; Liu, N.Q.; Yang, Y.H.; Sun, X.F. Elevated Prevalence of Abnormal Glucose Metabolism and Other Endocrine Disorders in Patients with β-Thalassemia Major: A Meta-Analysis. Biomed. Res. Int. 2019, 2019, 6573497. [Google Scholar] [CrossRef] [Green Version]
- Baghersalimi, A.; Rad, A.H.; Koohmanaee, S.; Darbandi, B.; Mirzaee, M.M.; Aminzadeh, V.; Medghalchi, A.; Dalili, S. The Cutoff of Ferritin for Evaluation of Hypothyroidism in Patients with Thalassemia. J. Pediatr. Hematol. Oncol. 2019, 41, 515–518. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Tzoulis, P.; Daar, S.; Di Maio, S.; Elsedfy, H.; Yassin, M.A.; Filosa, A.; Soliman, N.; et al. An ICET-A survey on occult and emerging endocrine complications in patients with β-thalassemia major: Conclusions and recommendations. Acta Biomed. 2019, 89, 481–489. [Google Scholar] [CrossRef]
- Upadya, S.H.; Rukmini, M.S.; Sundararajan, S.; Baliga, B.S.; Kamath, N. Thyroid Function in Chronically Transfused Children with BetaThalassemia Major: A Cross-Sectional Hospital Based Study. Int. J. Pediatr. 2018, 2018, 9071213. [Google Scholar] [CrossRef] [Green Version]
- Ehsan, L.; Rashid, M.; Alvi, N.; Awais, K.; Nadeem, O.; Asghar, A.; Sajjad, F.; Fatima, M.; Qidwai, A.; Hussain, S.; et al. Clinical utility of endocrine markers predicting myocardial siderosis in transfusion dependent thalassemia major.Pediatr. Blood Cancer 2018, 65, e27285. [Google Scholar] [CrossRef]
- Ambrogio, A.G.; Danesi, L.; Baldini, M.; Radin, R.; Cassinerio, E.; Graziadei, G.; Mirra, N.; D’Angelo, E.; Marcon, A.; Mancarella, M.; et al. Low-dose Synachten test with measurement of salivary cortisol in adult patients with β-thalassemia major. Endocrine 2018, 60, 348–354. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Elsedfy, H.; Karimi, M.; Daar, S.; Rimawi, H.; Christou, S.; Skordis, N.; Tzoulis, P.; et al. An ICET- A survey on Hypoparathyroidism in Patients with Thalassaemia Major and Intermedia: A preliminary report. Acta Biomed. 2018, 88, 435–444. [Google Scholar] [CrossRef]
- Yaghobi, M.; Miri-Moghaddam, E.; Majid, N.; Bazi, A.; Navidian, A.; Kalkali, A. Complications of Transfusion-Dependent β-Thalassemia Patients in Sistan and Baluchistan, South-East of Iran. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 268–272. [Google Scholar]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Putti, M.C.; Gamberini, M.R.; Cuccia, L.; Messina, G.; Massei, F.; Facchini, E.; Righi, R.; et al. Link between Genotype and Multi-Organ Iron and Complications in Children with Transfusion-Dependent Thalassemia. J. Pers. Med. 2022, 12, 400. [Google Scholar] [CrossRef]
- Lambrou, G.I.; Samartzi, A.; Vlachou, E.; Papassotiriou, I.; Geronikolou, S.A.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Kattamis, A. Genotypic and Clinical Analysis of a Thalassemia Major Cohort: An Observational Study. Adv. Exp. Med. Biol. 2021, 1339, 65–76. [Google Scholar] [CrossRef]
- Vetsiou, E.; Mpouras, V.; Nikolaidou, C.; Klonizakis, P.; Mandala, E.; Vamvakis, K.; Psarras, K.; Vlachaki, E. Necrobiosis Lipoidica in a Patient with β-Thalassemia Major: A Case Report and Review of the Literature. Hemoglobin 2020, 44, 221–223. [Google Scholar] [CrossRef]
- Mattia, L.; Samperi, I.; Monti, S.; Toscano, V.; Pugliese, G.; Poggi, M. The Quality of Life of Thalassemic Patients: The Role of Endocrine Defect Compensation. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 2147–2158. [Google Scholar] [CrossRef]
- Bilgin, B.K.; Yozgat, A.K.; Isik, P.; Çulha, V.; Kacar, D.; Kara, A.; Ozbek, N.Y.; Yarali, N. The effect of deferasirox on endocrine complications in children with thalassemia. Pediatr. Hematol. Oncol. 2020, 37, 455–464. [Google Scholar] [CrossRef]
- Ngim, C.F.; Lai, N.M.; Hong, J.Y.; Tan, S.L.; Ramadas, A.; Muthukumarasamy, P.; Thong, M.K. Growth hormone therapy for people with thalassaemia. Cochrane Database Syst. Rev. 2020, 5, CD012284. [Google Scholar] [CrossRef]
- Soliman, A.T.; De Sanctis, V.; Yassin, M.; Adel, A. Growth and Growthhormone—Insulin Like Growth Factor -I (GH-IGF-I) Axis in Chronic Anemias. Acta Biomed. 2017, 88, 101–111. [Google Scholar] [CrossRef]
- Kanbour, I.; Chandra, P.; Soliman, A.; De Sanctis, V.; Nashwan, A.; Abusamaan, S.; Moustafa, A.; Yassin, M.A. Severe Liver Iron Concentrations (LIC) in 24 Patients with β-Thalassemia Major: Correlations with Serum Ferritin, Liver Enzymes and Endocrine Complications.Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018062. [Google Scholar] [CrossRef] [Green Version]
- Yassin, M.A.; Soliman, A.T.; De Sanctis, V.; Yassin, K.S.; Abdulla, M.A. Final Height and Endocrine Complications in Patients with β-Thalassemia Intermedia: Our Experience in Non-Transfused Versus Infrequently Transfused Patients and Correlations with Liver Iron Content.Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019026. [Google Scholar] [CrossRef]
- Vidal, A.; Dhakal, C. Association of Beta-Thalassaemia and Hypogonadotropic Hypogonadism. Case Rep. Obstet. Gynecol. 2022, 2022, 4655249. [Google Scholar] [CrossRef] [PubMed]
- Shahid, Z.; Hassan, S.; Ghazanfar, S.; Kaneez, M.; Khan, M.S.; Tariq, H.T.; Jawad, A.; Shuaib, A.; Bhatti, A.A.; Razzaq, M.T. Investigating the Role of Ferritin in Determining Sexual Underdevelopment in Beta-Thalassemia Major Patients: A Cross-Sectional Analysis From Pakistan. Cureus 2021, 13, e15572. [Google Scholar] [CrossRef] [PubMed]
- Barbero, U.; Ajassa, M.; Gaglioti, C.M.; Piga, A.; Ferrero, G.B.; Longo, F. The Influence of Cardiovascular Risk Factors and Hypogonadism on Cardiac Outcomes in an Aging Population of Beta-Thalassemia Patients. J. Cardiovasc. Dev. Dis. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Yassin, M.A.; Daar, S.; Elsedfy, H.; Di Maio, S.; Raiola, G.; Corrons, J.V.; Kattamis, C. Thyroid Disorders in Homozygous β-Thalassemia: Current Knowledge, Emerging Issues and Open Problems. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019029. [Google Scholar] [CrossRef]
- Sriwichakorn, C.; Nakavachara, P.; Jitpirasakun, S.; Pooliam, J.; Sanpakit, K. Hypothyroidism in children with Hb E/β-thalassemia compared between those who received regular transfusion and those who underwent hematopoietic stem cell transplantation. Pediatr. Hematol. Oncol. 2022, 1–13. [Google Scholar] [CrossRef]
- Ghemigian, A.; Carsote, M.; Petrova, E.; Valea, A.; Dumitru, N.; Cocolos, A. Detection of thyroid nodules by routine ultrasound. Pract. Med. 2017, 12, 224–229. [Google Scholar] [CrossRef]
- Dhouib, N.G.; Ben Khaled, M.; Ouederni, M.; Besbes, H.; Kouki, R.; Mellouli, F.; Bejaoui, M. Growth and Endocrine Function in Tunisian Thalassemia Major Patients.Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018031. [Google Scholar] [CrossRef] [Green Version]
- Saki, F.; Salehifar, A.; Kassaee, S.R.; Omrani, G.R. Association of vitamin D and FGF23 with serum ferritin in hypoparathyroid thalassemia: A case control study. BMC Nephrol. 2020, 21, 482. [Google Scholar] [CrossRef]
- Stefanopoulos, D.; Nasiri-Ansari, N.; Dontas, I.; Vryonidou, A.; Galanos, A.; Psaridi, L.; Fatouros, I.G.; Mastorakos, G.; Papavassiliou, A.G.; Kassi, E.; et al. Fibroblast Growth Factor 23 (FGF23) and Klotho Protein in Beta-Thalassemia. Horm. Metab. Res. 2020, 52, 194–201. [Google Scholar] [CrossRef]
- Kurian, M.E.; Jebasingh, F.K.; Sigamani, E.; Thomas, N. Extramedullary haematopoiesis in the adrenal glands. BMJ Case Rep. 2020, 13, e238916. [Google Scholar] [CrossRef]
- Agarwal, S.; Dey, M.; Yadav, P.; Lal, H. Adrenal extramedullary haematopoiesis: An unusual incidentaloma. BMJ Case Rep. 2021, 14, e238572. [Google Scholar] [CrossRef]
- Georgiou, A.C.; Lisacek-Kiosoglous, A.B.; Mariannis, D.; Christou, S.; Hadjianastassiou, V.G. A rare case of adrenal extramedullary haematopoiesis in a Cypriot woman with β-thalassaemia. Ann. R. Coll. Surg. Engl. 2022. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.; Tzoulis, P.; Daar, S.; Karimi, M.; Yassin, M.A.; Pozzobon, G.; Kattamis, C. The clinical characteristics, biochemical parameters and insulin response to oral glucose tolerance test (OGTT) in 25 transfusion dependent β-thalassemia (TDT) patients recently diagnosed with diabetes mellitus (DM). Acta Biomed. 2022, 92, e2021488. [Google Scholar] [CrossRef]
- Setoodeh, S.; Khorsand, M.; Takhshid, M.A. The effects of iron overload, insulin resistance and oxidative stress on metabolic disorders in patients with β-thalassemiamajor. J. Diabetes Metab. Disord. 2020, 19, 767–774. [Google Scholar] [CrossRef]
- Wankanit, S.; Chuansumrit, A.; Poomthavorn, P.; Khlairit, P.; Pongratanakul, S.; Mahachoklertwattana, P. Acute Effects of Blood Transfusion on Insulin Sensitivity and Pancreatic β-Cell Function in Children with β-Thalassemia/Hemoglobin E Disease. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gomber, S.; Dabas, A.; Bagmar, S.; Madhu, S.V. Glucose Homeostasis and Effect of Chelation on β Cell Function in Children With β-ThalassemiaMajor. J. Pediatr. Hematol. Oncol. 2018, 40, 56–59. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allò, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron With Glucose Metabolism and With Cardiac Complications in ThalassemiaMajor: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef]
- Ansari, A.M.; Bhat, K.G.; Dsa, S.S.; Mahalingam, S.; Joseph, N. Study of Insulin Resistance in Patients With βThalassemiaMajor and Validity of Triglyceride Glucose (TYG) Index. J. Pediatr. Hematol. Oncol. 2018, 40, 128–131. [Google Scholar] [CrossRef]
- Harbi, N.S.; Jawad, A.H.; Alsalman, F.K. Evaluation of Adipokines Concentration in Iraqi Patients with Major and Minor BetaThalassemia. Rep. Biochem. Mol. Biol. 2020, 9, 209–215. [Google Scholar] [CrossRef]
- de Sanctis, V.; Soliman, A.T.; Daar, S.; Tzoulis, P.; Di Maio, S.; Kattamis, C. Long-Term Follow-up of β-Transfusion-Dependent Thalassemia (TDT) Normoglycemic Patients with Reduced Insulin Secretion to Oral Glucose Tolerance Test (OGTT): A Pilot Study. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021021. [Google Scholar] [CrossRef]
- Karadas, N.; Yurekli, B.; Bayraktaroglu, S.; Aydinok, Y. Insulin secretion-sensitivity index-2 could be a novel marker in the identification of the role of pancreatic iron deposition on beta-cell function in thalassemia major. Endocr. J. 2019, 66, 1093–1099. [Google Scholar] [CrossRef]
- El-Samahy, M.H.; Tantawy, A.A.; Adly, A.A.; Abdelmaksoud, A.A.; Ismail, E.A.; Salah, N.Y. Evaluation of continuous glucose monitoring system for detection of alterations in glucose homeostasis in pediatric patients with β-thalassemia major. Pediatr. Diabetes. 2019, 20, 65–72. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, V.; Daar, S.; Soliman, A.T.; Tzoulis, P.; Karimi, M.; Di Maio, S.; Kattamis, C. Screening for glucose dysregulation in β-thalassemiamajor (β-TM): An update of current evidences and personal experience. Acta Biomed. 2022, 93, e2022158. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Z.; Jiang, Z.; Liu, Z.; Hou, L.; Cai, G.; Ou, H.; Huang, S.; Song, Q.; Fang, J.; et al. Indicators of glucose dysregulation and the relationship with iron overload in Chinese children with beta thalassemia major. Pediatr. Diabetes 2021, 23, 562–568. [Google Scholar] [CrossRef]
- Gomber, S.; Bagaria, A.; Madhu, S.V.; Dewan, P. Glucose Homeostasis Markers in Beta-Thalassemia. J. Pediatr. Hematol. Oncol. 2018, 40, 508–510. [Google Scholar] [CrossRef]
- Manolopoulos, P.P.; Lavranos, G.; Mamais, I.; Angouridis, A.; Giannakou, K.; Johnson, E.O. Vitamin D and bone health status in betathalassemia patients-systematic review. Osteoporos. Int. 2021, 32, 1031–1040. [Google Scholar] [CrossRef]
- Stefanopoulos, D.; Papaioannou, N.A.; Papavassiliou, A.G.; Mastorakos, G.; Vryonidou, A.; Michou, A.; Dontas, I.A.; Lyritis, G.; Kassi, E.; Tournis, S. A contemporary therapeutic approach to bone disease in beta-thalassemia—A review. J. FrailtySarcopenia Falls 2018, 3, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Gaudio, A.; Morabito, N.; Catalano, A.; Rapisarda, R.; Xourafa, A.; Lasco, A. Pathogenesis of Thalassemia Major-associated Osteoporosis: A Review with Insights from Clinical Experience. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 110–117. [Google Scholar] [CrossRef]
- Atmakusuma, T.D.; Tenggara, J.B. Correlation of Transferrin Saturation and Serum Ferritin with Bone Mass Density in Adult Transfusion Dependent Beta-Thalassemia Patients. J. Blood Med. 2021, 12, 827–832. [Google Scholar] [CrossRef]
- Kothimira, V.K.; Kumar, A.; Richhele, L.R.; Sood, N.; Gulati, A. An Evaluation of Bone Health Parameters in Regularly Transfused Beta-Thalassemia Major Patients. J. Pediatr. Hematol. Oncol. 2020, 42, 381–385. [Google Scholar] [CrossRef]
- Wanna-Udom, S.; Luesiripong, C.; Sakunrangsit, N.; Metheepakornchai, P.; Intharamonthian, S.; Svasti, S.; Greenblatt, M.B.; Leelahavanichkul, A.; Lotinun, S. High phosphate intake induces bone loss in nephrectomized thalassemic mice. PLoS ONE 2022, 17, e0268732. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Rittiphairoj, T.; Ponvilawan, B. Fracture prevalence in thalassemia: A systematic review and meta-analysis. Arch. Osteoporos. 2021, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Ekbote, V.; Khadilkar, A.; Chauthmal, S.; Padidela, R.; Khadilkar, S.; Mughal, Z.; Crabtree, N. Assessment of Bone Density by DXA in Poorly Controlled Children With β-Thalassemia: Correction for Hepatic Iron Overload by Manual Analysis. J. Clin. Densitom. 2021, 24, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, F.; Zatelli, M.C.; Bondanelli, M.; Carnevale, A.; Cittanti, C.; Fortini, M.; Gamberini, M.R.; Giganti, M.; Ambrosio, M.R. Dual-energy X-ray absorptiometry pitfalls in Thalassemia Major. Endocrine 2019, 65, 469–482. [Google Scholar] [CrossRef]
- Yang, W.P.; Chang, H.H.; Li, H.Y.; Lai, Y.C.; Huang, T.Y.; Tsai, K.S.; Lin, K.H.; Lin, D.T.; Jou, S.T.; Lu, M.Y.; et al. Iron Overload Associated Endocrine Dysfunction Leading to Lower Bone Mineral Density in Thalassemia Major. J. Clin. Endocrinol. Metab. 2020, 105, e1015–e1024. [Google Scholar] [CrossRef]
- Mohseni, F.; Mohajeri-Tehrani, M.R.; Larijani, B.; Hamidi, Z. Correlation between criteria of diagnosis of low bone mineral density in adult and pediatric thalassemic patients: A prospective study. Minerva Pediatr. 2018, 70, 246–251. [Google Scholar] [CrossRef]
- Mohajeri-Tehrani, M.R.; Darvishian, N.; Arab, F.; Salemkar, S.; Mohseni, F.; Larijani, B.; Hamidi, Z. The role of using different reference population in the prevalence of low BMD in the thalassemia patients. J. Diabetes Metab. Disord. 2019, 19, 431–435. [Google Scholar] [CrossRef]
- Karimi, M.; Zarei, T.; Haghpanah, S.; Azarkeivan, A.; Kattamis, C.; Ladis, V.; Kattamis, A.; Kilinc, Y.; Daar, S.; Alyaarubi, S.; et al. Evaluation of endocrine complications in beta-thalassemia intermedia (β-TI): A cross-sectional multicenter study. Endocrine 2020, 69, 220–227. [Google Scholar] [CrossRef]
- Teawtrakul, N.; Chukanhom, S.; Charoensri, S.; Somboonporn, C.; Pongchaiyakul, C. The TrabecularBoneScore as a Predictor for Thalassemia-Induced Vertebral Fractures in Northeastern Thailand. Anemia 2020, 2020, 4634709. [Google Scholar] [CrossRef]
- Osella, G.; Priola, A.M.; Priola, S.M.; Piga, A.; Longo, F.; Ventura, M.; Bentivegna, G.; Angeli, A.; Veltri, A.; Terzolo, M. Dual-Energy X-ray Absorptiometry Predictors of Vertebral Deformities in Beta-Thalassemia Major. J. Clin. Densitom. 2018, 21, 507–516. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J. Med. Life. 2020, 13, 449–453. [Google Scholar]
- Pinto, V.M.; Poggi, M.; Russo, R.; Giusti, A.; Forni, G.L. Management of the aging beta-thalassemia transfusion-dependent population—The Italian experience. Blood Rev. 2019, 38, 100594. [Google Scholar] [CrossRef]
- Motta, I.; Mancarella, M.; Marcon, A.; Vicenzi, M.; Cappellini, M.D. Management of age-associated medical complications in patients with β-thalassemia. Exp. Rev. Hematol. 2020, 13, 85–94. [Google Scholar] [CrossRef]
- Ekbote, V.; Padidela, R.; Khadilkar, V.; Ramanan, V.; Maheshwari, A.; Mughal, Z.; Kariki, E.P.; Crabtree, N.; Khadilkar, A. Increased prevalence of fractures in inadequately transfused and chelated Indian children and young adults with betathalassemia major. Bone 2021, 143, 115649. [Google Scholar] [CrossRef]
- Shah, N.; Khadilkar, A.; Ekbote, V.; Mughal, Z.; Gondhalekar, K.; Khadilkar, S.; Ramanan, V.; Khadilkar, V.; Padidela, R. DXA and pQCT derived parameters in Indian children with betathalassemia major—A case controlled study. Bone 2021, 143, 115730. [Google Scholar] [CrossRef]
- Hamidieh, A.A.; Hamidi, Z.; Behfar, M.; Pajouhi, Z.; Alimoghaddam, K.; Mohseni, F.; Ghavamzadeh, A.; Sobhani, M.; Larijani, B.; Mohajeri Tehrani, M.R. Relationship between endocrine changes and bone markers in pediatric thalassemic patients after hematopoietic stem cell transplantation. Minerva Pediatr. 2021, 73, 414–419. [Google Scholar] [CrossRef]
- Tsartsalis, A.N.; Lambrou, G.I.; Tsartsalis, D.N.; Papassotiriou, I.; Vlachou, E.; Terpos, E.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Kattamis, A. Bone Metabolism Markers in Thalassemia Major-Induced Osteoporosis: Results from a Cross-Sectional Observational Study. Curr. Mol. Med. 2019, 19, 335–341. [Google Scholar] [CrossRef]
- Sapunarova, K.; Goranova-Marinova, V.; Georgiev, P.; Deneva, T.; Tsvetkova, S.; Grudeva-Popova, Z. Associations of serum sclerostin with bone mineral density, markers of bone metabolism and thalassaemia characteristics in adult patients with transfusion-dependent beta-thalassaemia. Ann. Med. 2020, 52, 94–108. [Google Scholar] [CrossRef]
- Youssry, I.; Saad, N.; Madboly, M.; Samy, R.M.; Hamed, S.T.; Tawfik, H.; Elbatrawy, S.R.; Kaddah, N.; Abd Elaziz, D. Bone health in pediatric transfusion-dependent beta-thalassemia: Circulating osteoprotegerin and RANKL system. Pediatr. Blood Cancer 2022, 69, e29377. [Google Scholar] [CrossRef]
- Alfaqih, M.A.; Bashir, N.; Saadeh, R.; Khader, Y.; Barqawi, M.; Alqudah, S. Dysregulation of the RANKL/RANK/OPG axis in thalassemia intermedia patients. BMC Res. Notes 2018, 11, 534. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Moneim, E.S.; Zolaly, M.A.; Al-Hawsawi, Z.M.; Abdelmoneim, A.A.; Abosdera, M.M. Age-related changes in biochemical bone profile in thalassemic children. Pediatr. Neonatol. 2018, 59, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Bajoria, R.; Rekhi, E.; Almusawy, M.; Chatterjee, R. Hepatic Hemosiderosis Contributes to Abnormal Vitamin D-PTH Axis in Thalassemia Major. J. Pediatr. Hematol. Oncol. 2019, 41, e83–e89. [Google Scholar] [CrossRef]
- Baldan, A.; Giusti, A.; Bosi, C.; Malaventura, C.; Forni, G.; Borgna-Pignatti, C. Pseudoxanthoma Elasticum-Like in β-Thalassemia Major, a matter of α-Klotho and ParathyroidHormone? Hemoglobin 2017, 41, 254–259. [Google Scholar] [CrossRef]
- Abbasi, S.; Asl, J.F.; Zadeh, L.M.; Mirdoraghi, M. Measurement bone mineral density (BMD) of patients with betathalassemia. Data Brief 2018, 19, 1021–1024. [Google Scholar] [CrossRef]
- Naithani, R.; Seth, T.; Tandon, N.; Chandra, J.; Choudhry, V.P.; Pati, H.; Saxena, R. Zoledronic Acid for Treatment of Low Bone Mineral Density in Patients with BetaThalassemia Major. Indian J. Hematol. Blood Transfus. 2018, 34, 648–652. [Google Scholar] [CrossRef]
- Voskaridou, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Sonnleitner, L.; Papaefstathiou, A.; Dimopoulou, M.; Missbichler, A.; Kanellias, N.; Repa, K.; Papatheodorou, A.; et al. Denosumab effects on serum levels of the bone morphogenetic proteins antagonist noggin in patients with transfusion-dependent thalassemia and osteoporosis. Hematology 2019, 24, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Tsartsalis, A.N.; Lambrou, G.I.; Tsartsalis, D.; Savvidis, C.; Karantza, M.; Terpos, E.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Kattamis, A. The role of biphosphonates in the management of thalassemia-induced osteoporosis: A systematic review and meta-analysis. Hormones 2018, 17, 153–166. [Google Scholar] [CrossRef]
- Bordbar, M.; Haghpanah, S.; Zekavat, O.R.; Saki, F.; Bazrafshan, A.; Bozorgi, H. Effect of different iron chelation regimens on bone mass in transfusion-dependent thalassemia patients. Exp. Rev. Hematol. 2019, 12, 997–1003. [Google Scholar] [CrossRef]
- Gagliardi, I.; Celico, M.; Gamberini, M.R.; Pontrelli, M.; Fortini, M.; Carnevale, A.; Napoli, N.; Zatelli, M.C.; Ambrosio, M.R. Efficacy and Safety of Teriparatide in Beta-Thalassemia Major Associated Osteoporosis: A Real-Life Experience. Calcif. Tissue Int. 2022, 111, 56–65. [Google Scholar] [CrossRef]
- Hajimoradi, M.; Haseli, S.; Abadi, A.; Chalian, M. Musculoskeletal imaging manifestations of beta-thalassemia. Skeletal Radiol. 2021, 50, 1749–1762. [Google Scholar] [CrossRef]
- Nema, R.; Sengupta, A.; Kumar, A.; Wig, N. Cyclical haematological changes in a case of hypopituitarism. BMJ Case Rep. 2021, 14, e243421. [Google Scholar] [CrossRef] [PubMed]
- Manara, R.; Ponticorvo, S.; Tartaglione, I.; Femina, G.; Elefante, A.; Russo, C.; Carafa, P.A.; Cirillo, M.; Casale, M.; Ciancio, A.; et al. Brain iron content in systemic iron overload: A beta-thalassemia quantitative MRI study. Neuroimage Clin. 2019, 24, 102058. [Google Scholar] [CrossRef] [PubMed]
- Sevimli, C.; Yilmaz, Y.; Bayramoglu, Z.; Comert, R.G.; Gul, N.; Dursun, M.; Karakas, Z. Pancreatic MR imaging and endocrine complications in patients with beta-thalassemia: A single-center experience. Clin. Exp. Med. 2022, 22, 95–101. [Google Scholar] [CrossRef]
- Labranche, R.; Gilbert, G.; Cerny, M.; Vu, K.N.; Soulières, D.; Olivié, D.; Billiard, J.S.; Yokoo, T.; Tang, A. Liver Iron Quantification with MR Imaging: A Primer for Radiologists. Radiographics 2018, 38, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.A.; Soliman, A.T.; De Sanctis, V.; Abdula, M.A.J.; Riaz, L.M.; Ghori, F.F.; Yousaf, A.; Nashwan, A.J.; Abusamaan, S.; Moustafa, A.; et al. Statural Growth and Prevalence of Endocrinopathies in Relation to Liver Iron Content (LIC) in Adult Patients with BetaThalassemia Major (BTM) and Sickle Cell Disease (SCD). Acta Biomed. 2018, 89, 33–40. [Google Scholar] [CrossRef]
- Soliman, A.T.; Yassin, M.A.; De Sanctis, V. Final adult height and endocrine complications in young adults with β-thalassemia major (TM) who received oral iron chelation (OIC) in comparison with those who did not use OIC. Acta Biomed. 2018, 89, 27–32. [Google Scholar] [CrossRef]
- Sood, R.; Rastogi, P.; Bansal, D.; Das, R.; Sharma, P.; Gude, G.; Dhankar, M. An Autopsy Case of β-Thalassemia Major Illuminating the Pathological Spectrum of the Disease. Hemoglobin 2021, 45, 180–185. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Di Maio, S.; Canatan, D.; Soliman, N.; Karimi, M.; Kattamis, C. Gonadal dysfunction in adult male patients with thalassemia major: An update for clinicians caring for thalassemia. Expert Rev. Hematol. 2017, 10, 1095–1106. [Google Scholar] [CrossRef]
- Shafique, F.; Ali, S.; Almansouri, T.; Van Eeden, F.; Shafi, N.; Khalid, M.; Khawaja, S.; Andleeb, S.; Hassan, M.U. Thalassemia, a human blood disorder. Braz. J. Biol. 2021, 83, e246062. [Google Scholar] [CrossRef]
- Carsote, M.; Valea, A. Insights of peak bone mass. ActaMed. Transilv. 2016, 21, 82–85. [Google Scholar]
- Bozdağ, M.; Bayraktaroğlu, S.; Aydınok, Y.; Çallı, M.C. MRI assessment of pituitary iron accumulation by using pituitary-R2 in β-thalassemia patients. Acta Radiol. 2018, 59, 732–739. [Google Scholar] [CrossRef]
- Talaulikar, S.V.; Bajoria, R.; Ehidiamhen, A.J.; Mujawar, E.; Chatterjee, R. A 10-year longitudinal study of evaluation of ovarian reserve in women with transfusion-dependent beta thalassaemia major. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 38–43. [Google Scholar] [CrossRef]
- Uysal, A.; Alkan, G.; Kurtoğlu, A.; Erol, O.; Kurtoğlu, E. Diminished ovarian reserve in women with transfusion-dependent beta-thalassemia major: Is iron gonadotoxic? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 69–73. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Di Maio, S.; Yassin, M.A.; Canatan, D.; Vives Corrons, J.L.; Elsedfy, H.; Kattamis, A.; Kattamis, C. The experience of a tertiary unit on the clinical phenotype and management of hypogonadism in female adolescents and young adults with transfusion dependent thalassemia. Acta Biomed. 2019, 90, 158–167. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Yassin, M.A.; Di Maio, S.; Daar, S.; Elsedfy, H.; Soliman, N.; Kattamis, C. Hypogonadism in male thalassemia major patients: Pathophysiology, diagnosis and treatment. Acta Biomed. 2018, 89, 6–15. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Canatan, D.; Di Maio, S.; Elsedfy, H.; Baioumi, A.; Kattamis, C. Gonadotropin replacement in male thalassemia major patients with arrested puberty and acquired hypogonadotropic hypogonadism (AAH): Preliminary results and potential factors affecting induction of spermatogenesis. Endocrine 2019, 63, 167–170. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Di Maio, S. Adverse events during testosterone replacement therapy in 95 young hypogonadal thalassemic men. Acta Biomed. 2019, 90, 228–232. [Google Scholar] [CrossRef]
- Mamsen, L.S.; Kristensen, S.G.; Pors, S.E.; Bøtkjær, J.A.; Ernst, E.; Macklon, K.T.; Gook, D.; Kumar, A.; Kalra, B.; Andersen, C.Y. Consequences of β-Thalassemia or Sickle Cell Disease for Ovarian Follicle Number and Morphology in Girls Who Had Ovarian Tissue Cryopreserved. Front. Endocrinol. 2021, 11, 593718. [Google Scholar] [CrossRef]
- Matthews, S.J.; Picton, H.; Ernst, E.; Andersen, C.Y. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018, 70, 432–435. [Google Scholar] [CrossRef]
- Elsedfy, H.; De Sanctis, V.; Ahmed, A.Y.; Mohamed, N.R.; Arafa, M.; Elalfy, M.S. A pilot study on sperm DNA damage in β-thalassemia major: Is there a role for antioxidants? Acta Biomed. 2018, 89, 47–54. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Lamb, D.J.; Lipshultz, L.I. Iron and a Man’s Reproductive Health: The Good, the Bad, and the Ugly. Curr. Urol. Rep. 2018, 19, 60. [Google Scholar] [CrossRef]
- Chen, M.J.; Peng, S.S.; Lu, M.Y.; Yang, Y.L.; Jou, S.T.; Chang, H.H.; Chen, S.U.; Lin, D.T.; Lin, K.H. Effect of iron overload on impaired fertility in male patients with transfusion-dependent beta-thalassemia. Pediatr. Res. 2018, 83, 655–661. [Google Scholar] [CrossRef]
- Rostami, T.; Mohammadifard, M.A.; Ansari, S.; Kiumarsi, A.; Maleki, N.; Kasaeian, A.; Aghamahdi, F.; Rad, S.; Ghavamzadeh, A. Indicators of male fertility potential in adult patients with beta-thalassemia major: A comparative study between patients undergone allogeneic stem cell transplantation and transfusion-dependent patients. Fertil. Res. Pract. 2020, 6, 4. [Google Scholar] [CrossRef]
- Delgouffe, E.; Braye, A.; Goossens, E. Testicular Tissue Banking for Fertility Preservation in Young Boys: Which Patients Should Be Included? Front. Endocrinol. 2022, 322, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of Postmeiotic Cells In Vitro from Spermatogonial Cells of Prepubertal Cancer Patients. Stem Cells Dev. 2018, 27, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Santarone, S.; Natale, A.; Olioso, P.; Onofrillo, D.; D’Incecco, C.; Parruti, G.; Bartolomeo, P. Pregnancy outcome following hematopoietic cell transplantation for thalassemia major. Bone Marrow Transplant. 2017, 52, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Origa, R.; Comitini, F. Pregnancy in Thalassemia.Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019019. [Google Scholar] [CrossRef]
- Ghafoor, M.; Sabar, M.F.; Sabir, F. Prevention programmes and prenatal diagnosis for betathalassemia in Pakistan: A narrative review. J. Pak. Med. Assoc. 2021, 71, 326–331. [Google Scholar] [CrossRef]
- Corda, V.; Murgia, F.; Dessolis, F.; Murru, S.; Chervenak, F.A.; McCullough, L.B.; Monni, G. Professionally responsible management of the ethical and social challenges of antenatal screening and diagnosis of β-thalassemia in a high-risk population. J. Perinat. Med. 2021, 49, 847–852. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, H.; Zhong, Z.; Lan, L.; Zeng, M.; Lin, H.; Wang, H.; Zheng, Z.; Su, L.; Guo, W. Molecular prenatal diagnosis of alpha and beta thalassemia in pregnant Hakka women in southern China. J. Clin. Lab. Anal. 2018, 32, e22306. [Google Scholar] [CrossRef]
- Xu, G.; Wang, C.; Wang, J.; Lin, M.; Chang, Z.; Liang, J.; Chen, X.; Zhong, S.; Nong, X.; Wei, W.; et al. Prevalence and molecular characterization of common thalassemia among people of reproductive age in the border area of Guangxi-Yunnan-Guizhou province in Southwestern China. Hematology 2022, 27, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.A.; Mohamadinejad, M.; Shokrgozar, N.; Abbasian, S. Mutations in Thalassemia Carrier Couples: The Importance of Prenatal Diagnostic Tests. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, X.; Wu, C.; Xu, Y.; Ding, C.; Zhang, G.; Xu, Y.; Zhou, C. Eleven healthy live births: A result of simultaneous preimplantation genetic testing of α- and β-double thalassemia and aneuploidy screening. J. Assist. Reprod. Genet. 2020, 37, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.D.; Harton, G.L.; Quang, V.N.; Van, H.N.; Thi, N.H.; Thuy, N.P.; Le Thi, T.H.; Minh, D.N.; Quoc, Q.T. Development and clinical application of a preimplantation genetic testing for monogenic disease (PGT-M) for betathalassemia in Vietnam. J. Assist. Reprod. Genet. 2021, 38, 365–374. [Google Scholar] [CrossRef]
- Sawakwongpra, K.; Tangmansakulchai, K.; Ngonsawan, W.; Promwan, S.; Chanchamroen, S.; Quangkananurug, W.; Sriswasdi, S.; Jantarasaengaram, S.; Ponnikorn, S. Droplet-based digital PCR for non-invasive prenatal genetic diagnosis of α and β-thalassemia. Biomed. Rep. 2021, 15, 82. [Google Scholar] [CrossRef]
- Fazal, Y.; Zohaib, M.; Syed, B.; Ansari, S.H.; Hashim, Z.; Ahmed, A.; Zarina, S. Prenatal diagnosis of maternal serum from mothers carrying β-thalassemic fetus. Pediatr. Int. 2021, 64, e14999. [Google Scholar] [CrossRef] [PubMed]
- Zittersteijn, H.A.; Harteveld, C.L.; Klaver-Flores, S.; Lankester, A.C.; Hoeben, R.C.; Staal, F.J.T.; Gonçalves, M.A.F.V. A Small Key for a Heavy Door: Genetic Therapies for the Treatment of Hemoglobinopathies. Front. Genome Ed. 2021, 2, 617780. [Google Scholar] [CrossRef]
- Monni, G.; Peddes, C.; Iuculano, A.; Ibba, R.M. From prenatal to preimplantation genetic diagnosis of Β-thalassemia. Prevention model in 8748 cases: 40 years of single center experience. J. Clin. Med. 2018, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Kazal, R.K.; Chowdhury, S.A.; Mirza, T.T.; Pervin, H.H.; Noor, F.; Chakma, B.; Aalpona, F.Z. Feasibility and Safety of ChorionicVillusSampling (CVS) for Prenatal Diagnosis of Thalassemia in Bangladesh. Mymensingh Med. J. 2018, 27, 578–584. [Google Scholar]
- Caceres, V.; Murray, T.; Myers, C.; Parbhoo, K. Prenatal Genetic Testing and Screening: A Focused Review. Semin. Pediatr. Neurol. 2022, 42, 100976. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Lan, L.; Zeng, M.; Guo, W.; Zheng, Z.; Zhu, H.; Wu, J.; Zhao, P. Invasive molecular prenatal diagnosis of alpha and betathalassemia among Hakka pregnant women. Medicine 2018, 97, e13557. [Google Scholar] [CrossRef]
- Liang, H.; Pan, L.; Xie, Y.; Fan, J.; Zhai, L.; Liang, S.; Zhang, Z.; Lai, Y. Health-related quality of life in pediatric patients with β-thalassemia major after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022, 57, 1108–1115. [Google Scholar] [CrossRef]
- Mulas, O.; Mola, B.; Caocci, G.; La Nasa, G. Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review. J. Clin. Med. 2022, 11, 907. [Google Scholar] [CrossRef]
- Rahal, I.; Galambrun, C.; Bertrand, Y.; Garnier, N.; Paillard, C.; Frange, P.; Pondarré, C.; Dalle, J.H.; de Latour, R.P.; Michallet, M.; et al. Late effects after hematopoietic stem cell transplantation for β-thalassemia major: The French national experience. Haematologica 2018, 103, 1143–1149. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Zakaria, N.Z.; Arsad, N.; Chew, K.T.; Abu, M.A.; Shafiee, M.N.; Omar, M.H. A case of post-splenectomy transfusion-dependent homozygous beta-thalassemia major complicated with myocardial siderosis and osteoporosis and usage of iron-chelating therapy with deferiprone in pregnancy. Horm. Mol. Biol. Clin. Investig. 2019, 39, 20190005. [Google Scholar] [CrossRef]
- Nourollahpour Shiadeh, M.; Cassinerio, E.; Modarres, M.; Zareiyan, A.; Hamzehgardeshi, Z.; Behboodi Moghadam, Z. Reproductive health issues in female patients with beta-thalassaemia major: A narrative literature review. J. Obstet. Gynecol. 2020, 40, 902–911. [Google Scholar] [CrossRef]
- Aleem, A.; Alsayegh, F.; Keshav, S.; Alfadda, A.; Alfadhli, A.A.; Al-Jebreen, A.; Al-Kasim, F.; Almuhaini, A.; Al-Zahrani, H.; Batwa, F.; et al. Consensus Statement by an Expert Panel on the Diagnosis and Management of Iron Deficiency Anemia in the Gulf Cooperation Council Countries. Med. Princ. Pract. 2020, 29, 371–381. [Google Scholar] [CrossRef]
- Chen, N.; Li, Z.; Huang, Y.; Xiao, C.; Shen, X.; Pan, S.; Su, Q.; Wang, Z. Iron parameters in pregnant women with beta-thalassaemia minor combined with iron deficiency anaemia compared to pregnant women with iron deficiency anaemia alone demonstrate the safety of iron supplementation in beta-thalassaemia minor during pregnancy. Br. J. Haematol. 2022, 196, 390–396. [Google Scholar] [CrossRef]
- Virot, E.; Thuret, I.; Jardel, S.; Herbrecht, R.; Lachenal, F.; Lionnet, F.; Lucchini, M.J.; Machin, J.; Nimubona, S.; Ribeil, J.A.; et al. Pregnancy outcome in women with transfused beta-thalassemia in France. Ann. Hematol. 2022, 101, 289–296. [Google Scholar] [CrossRef]
- Chauhan, A.; Prasad, M. Outcome of Pregnancy with Hemoglobinopathy in a Tertiary Care Center. J. Obstet. Gynecol. 2018, 68, 394–399. [Google Scholar] [CrossRef]
- Fozza, C.; Asara, M.A.; Vacca, N.; Caggiari, S.; Monti, A.; Zaccheddu, F.; Capobianco, G.; Dessole, S.; Dore, F.; Antonucci, R. Pregnancy Outcome among Women with Beta-Thalassemia Major in North Sardinia. Acta Haematol. 2017, 138, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Jin, Y.; Wang, M.; Huang, Y.; Tang, H. Case Report: Abnormally Low Glycosylated Hemoglobin A1c Caused by Clinically Silent Rare β-Thalassemia in a Tujia Chinese Woman. Front. Endocrinol. 2022, 13, 878680. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Fan, Y. Investigating the Reliability of HbA1c Monitoring for Blood Glucose Control During Late Pregnancy in Patients with Gestational Diabetes Mellitus (GDM) with and without β-Thalassemia Minor. Diabetes Ther. 2018, 9, 2305–2313. [Google Scholar] [CrossRef] [Green Version]
- Pepe, J.; Body, J.J.; Hadji, P.; McCloskey, E.; Meier, C.; Obermayer-Pietsch, B.; Palermo, A.; Tsourdi, E.; Zillikens, M.C.; Langdahl, B.; et al. Osteoporosis in Premenopausal Women: A Clinical Narrative Review by the ECTS and the IOF. J. Clin. Endocrinol. Metab. 2020, 105, 2487–2506. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, L.; Yu, L.; Huang, W. Pregnancy- and lactation-associated osteoporosis with vertebral fractures: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 926. [Google Scholar] [CrossRef]
- Lujano-Negrete, A.Y.; Rodríguez-Ruiz, M.C.; Skinner-Taylor, C.M.; Perez-Barbosa, L.; Cardenas de la Garza, J.A.; García-Hernández, P.A.; Espinosa-Banuelos, L.G.; Gutierrez-Leal, L.F.; Jezzini-Martínez, S.; Galarza-Delgado, D.Á. Bone metabolism and osteoporosis during pregnancy and lactation. Arch. Osteoporos. 2022, 17, 36. [Google Scholar] [CrossRef]

| First Author Publication Year Reference Number | Study Design | Results: Endocrine Involvement | Results: Endocrine Parameters Correlations Other Observations |
|---|---|---|---|
| Casale M. 2022 [28] | multi-centre, longitudinal follow-up median of 8 y | N = 426 patients with TST At baseline: 121/425 with 1 ED 187/426 with at least 2 EDs During follow-up: another 104 EDs Overall risk of a new ED = 9.7% within 5 y (95% CI 6.3–13.1). | Age is a positive linear predictor (p = 0.005); so is TSH (p < 0.001) for an ED The number of EDs at baseline is a negative linear predictor for another ED during follow-up (p < 0.001) Deferasirox ↓ risk of ED |
| agliardi I. 2022 [20] | cross-sectional, multi-centre | N = 81 adults with BTH major (44/88 males, mean age of 41 ± 8 y) Evaluation: GHRH + arginine test N1 = 18/81 with GH deficiency N2 = 63 without GH deficiency BMI, cholesterol N1 > N2 (p < 0.05) Liver function similar in N1 versus N2 Low IGF1 in N1: 94.4%; N2: 93.6% | Low IGF1 has multiple mechanisms, not only GH |
| Seow CE. 2021 [21] | cross-sectional, single centre | N = 51 patients with TDT (47% males; 68.6% with major BTH) 21.6% with hypothyroidism (63.6% with central hypothyroidism) | Most often type of hypothyroidism is central Hypothyroidism is not correlated with age, ferritin, splenectomy status, chelation therapy |
| Dixit N. 2021 [22] | observational (transversal) | N = 50 children with major BTH (mean age of 15.98 ± 3.4 y, between 8–18 y) 88% with short stature 71.7% with delayed puberty 16% with hypothyroidism 10% with DM | Ferritin correlates with TSH, glycaemia, and pubertal delay |
| Atmakusuma TD. 2021 [23] | cross-sectional, single centre | N = 58 adults with transfusion-dependent BTH + growth retardation (53.4% males, median age of 21, between 18 and 24 y) 32.7% with subclinical hypothyroidism 79.3% with low IGF1 | TS correlates with FT4, respective IGF1, not with TSH |
| Mahmoud RA. 2021 [24] | cross-sectional | N = 120 children with major BTH (age < 12 y) 70% with malnutrition 23.33% with ED 9.17% with thyroid disease 7.5% with glucose profile anomalies 6.66% with hypoparathyroidism | Most common ED is at thyroid Endocrine involvement risk correlates with high ferritin, and poor compliance to BTH therapy |
| Arab-Zozani M. 2021 [25] | meta-analysis | N = 74 studies (mean age of 14 y) 48.9% = pooled prevalence of short stature (males more affected than females) 26.6% with GH deficiency | Half of patients have different growth anomalies |
| Singh P. 2021 [26] | cross-sectional, single centre | N = 58 patients with TDT (33/58 males, age between 17 and 19 y) 72.4% with normal puberty/delayed onset with spontaneous progression 26.7% with arrested/failure puberty | Multivariate regression identifies serum ferritin to correlate with pubertal failure/arrest |
| Nayak AM. 2021 [27] | cross-sectional (3T MRI) | N1 = 57 patients with major BTH versus 30 controls 56.1% with short stature 23.4% of the pubertal subgroup with hypogonadism | Ferritin negatively correlates with pituitary volume Anterior pituitary volume is lower in subjects with hypogonadism. |
| Jobanputra M. 2020 [29] | retrospective cohort | N = 612 patients with TDT 40% with 1 ED (non-DM) 40% with osteoporosis 34% with DM | 10-y mortality rate of 6.2% |
| Karadag SIK. 2020 [30] | cross-sectional (MRI) | N = 50 patients with TDT 2/3 with at least 1 ED | Hypogonadism and DM do not correlate with pituitary IO. Hypogonadism correlates with cardiac MRI-based IO (p = 0.004) Short stature correlates with hepatic IO (p = 0.05) |
| Lee KT. 2020 [31] | retrospective, single centre | N = 45 adults with TDT (22/45 males; mean age of 28.8 ± 6.9 y; 71.1% with major BTH) 54% with at least 1 ED 38.9% with 2 EDs 11.1% with 3 or more EDs EDs: hypogonadism (most frequent, 22.2%), osteoporosis (20%) hypothyroidism (13.3%) DM (6.7%) hypocortisolism (4.4%) | Ferritin is not correlated with ED |
| Yassouf MY. 2019 [32] | cross-sectional | N = 82 patients with major BTH treated with deferoxamine 29.27% with subclinical hypothyroidism, 1.22% with overt hypothyroidism | Non-compliance to deferoxamine increases the risk of thyroid anomalies by 6.38-foldversus compliant subjects (RR of 6.386; 95% CI 2.4–16.95) |
| Bordbar M. 2019 [33] | cohort | N = 713 patients with TDT (aged between 10 and 62 y) 86.8% with at least 1 ED 72.6% with low BMD 44.5% with hypogonadism 15.9% with DM 13.2% with hypoparathyroidism 10.7% with hypothyroidism | Age, splenectomy status and BMI correlates with ED |
| He LN. 2019 [34] | meta-analysis | N = 44 studies N = 16,605 patients with BTH 6.54% with DM (95% CI 5.3–7.78) 17.21% with IFG (95% CI 8.43–26) 12.46% with IGT (95% CI 5.98–18.94 43.92% with non-DM EDs (95% CI 37.94–49.89) | Highest prevalence of DM (7.9%, 95% CI: 5.75–10) correlates with Middle East region |
| Baghersalimi A. 2019 [35] | cross-sectional | N = 67 patients with BTH (mean age of 15.37 ± 3.73 y) 10.4% with subclinical hypothyroidism | Ferritin positively correlated with TSH (p = 0.008), not with T4 Ferritin is higher in persons with thyroid dysfunction versus normal thyroid function |
| De Sanctis V. 2019 [36] | ICET-A survey | N1 = 3.114 adults with BTH N2 = 202 younger than 18 y with BTH 4.6% and 0.5%, respectively, with hypothyroidism 3% and 4.5%, respectively, with GH deficiency 1.2% and 4.4%, respectively, with latent hypocortisolism | The prevalence of occult EDs varies within different age groups |
| Upadya SH. 2018 [37] | cross-sectional | N = 83 children with major BTH (59% males, age ≥ 3 y) 4.8% subclinical hypothyroidism | TSH is not correlated with serum ferritin, oral chelation and transfusions |
| Ehsan L. 2018 [38] | cross-sectional | N = 280 with TDT 82% with hypogonadism 69% with stunting 40% with hypoparathyroidism 30% with hypothyroidism | The sensitivity of hypogonadism to predict severe myocardial siderosis is 90% |
| Ambrogio AG. 2018 [39] | cross-sectional | N = 72 adults with major BTH 20% adrenal dysfunction based on short Synacthen stimulation test | |
| De Sanctis V. 2018 [40] | ICET-A survey | N1 = 3023 patients with major BTH N2 = 739 patients with intermedia BTH 6.8% and 4.4%, respectively, with hypoparathyroidism onset age between 10.5 and 57 y, respectively, between 20 and 54 y | Hypoparathyroidism is associated mostly with growth retardation and hypogonadism in major BTH (53% and 67%, respectively, of cases) |
| Yaghobi M. 2017 [41] | cross-sectional | N = 613 patients with TDT (54.3% males, mean age of 13.3 ± 7.7 y) 46.8% with hypogonadism 22% with hypoparathyroidism 8.3% with hypothyroidism 7.3% with DM | Hypogonadism is the most frequent complication below the age of 15, and cardiac events are, for people older than 15 y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsote, M.; Vasiliu, C.; Trandafir, A.I.; Albu, S.E.; Dumitrascu, M.-C.; Popa, A.; Mehedintu, C.; Petca, R.-C.; Petca, A.; Sandru, F. New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics 2022, 12, 1921. https://doi.org/10.3390/diagnostics12081921
Carsote M, Vasiliu C, Trandafir AI, Albu SE, Dumitrascu M-C, Popa A, Mehedintu C, Petca R-C, Petca A, Sandru F. New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics. 2022; 12(8):1921. https://doi.org/10.3390/diagnostics12081921
Chicago/Turabian StyleCarsote, Mara, Cristina Vasiliu, Alexandra Ioana Trandafir, Simona Elena Albu, Mihai-Cristian Dumitrascu, Adelina Popa, Claudia Mehedintu, Razvan-Cosmin Petca, Aida Petca, and Florica Sandru. 2022. "New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement" Diagnostics 12, no. 8: 1921. https://doi.org/10.3390/diagnostics12081921
APA StyleCarsote, M., Vasiliu, C., Trandafir, A. I., Albu, S. E., Dumitrascu, M.-C., Popa, A., Mehedintu, C., Petca, R.-C., Petca, A., & Sandru, F. (2022). New Entity—Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics, 12(8), 1921. https://doi.org/10.3390/diagnostics12081921








