Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies
Abstract
1. Introduction
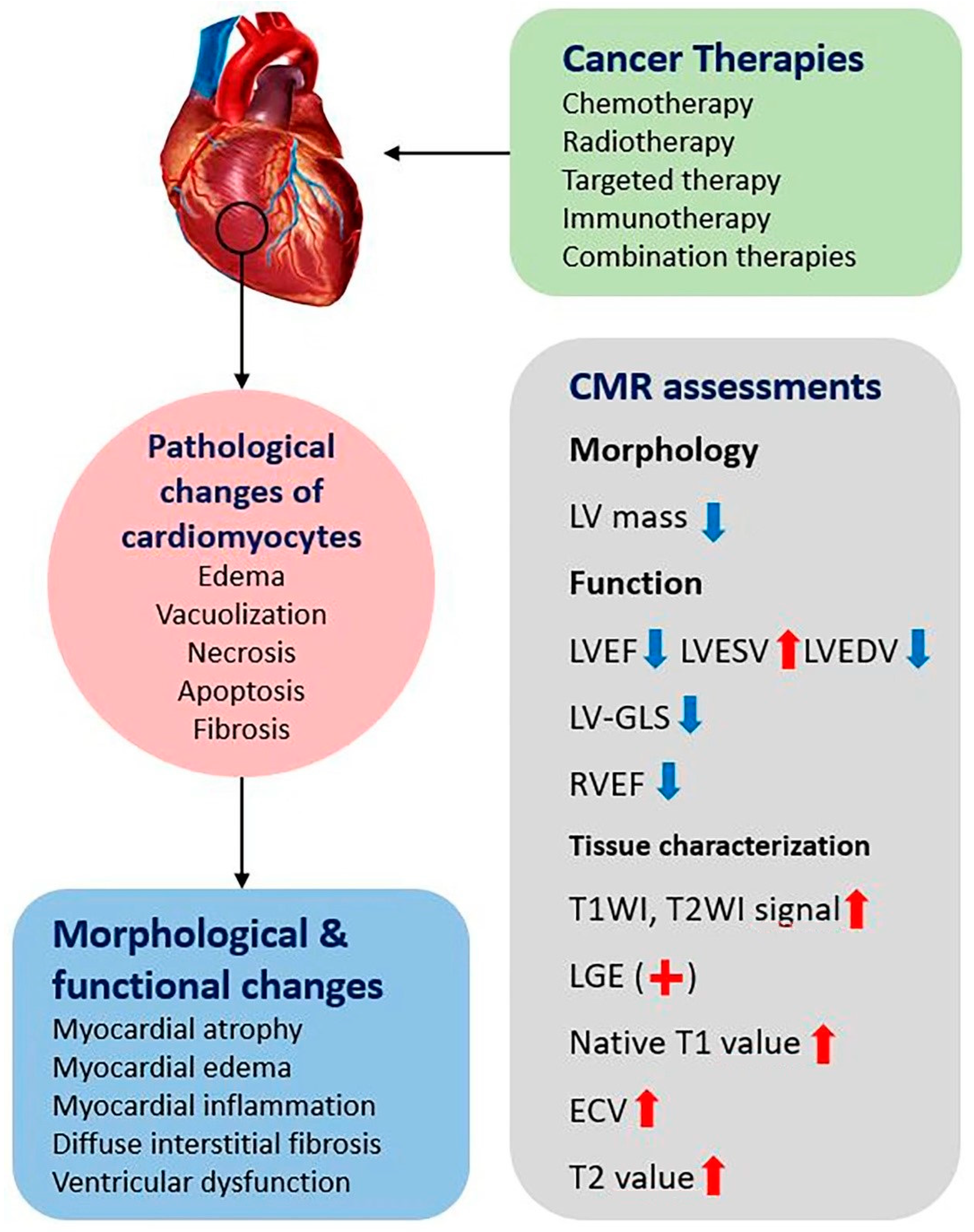
2. Clinical Types and Pathophysiology of Cardiotoxicity
3. CMR Imaging Biomarkers in the Assessment of Cardiotoxicity
3.1. Left Ventricular Morphology and Function
3.1.1. LV Mass
3.1.2. LVEF
3.1.3. Left Ventricular Strain
3.1.4. Right Ventricular Function
3.2. Myocardial Tissue Characterization
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef]
- Smith, S.M.; Wachter, K.; Burris, H.A., 3rd; Schilsky, R.L.; George, D.J.; Peterson, D.E.; Johnson, M.L.; Markham, M.J.; Mileham, K.F.; Beg, M.S.; et al. Clinical cancer advances 2021: Asco’s report on progress against cancer. Am. J. Clin. Oncol. 2021, 39, 1165–1184. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Lefrak, E.A.; Piťha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef]
- Gottlieb, J.; Lefrak, E.; O’Bryan, R.; Burgess, M. Fatal adriamycin cardiomyopathy: Prevention by dose limitation. Proc. Am. Assoc. Cancer Res. 1973, 14, 352. [Google Scholar]
- Von Hoff, D.D.; Rozencweig, M.; Layard, M.; Slavik, M.; Muggia, F.M. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am. J. Med. 1977, 62, 200–208. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.; Lyon, A.R. 2016 esc position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the esc committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the european society of cardiology (esc). Eur. J. Heart Fail. 2016, 74, 1193. [Google Scholar] [CrossRef]
- Groarke, J.D.; Nguyen, P.L.; Nohria, A.; Ferrari, R.; Cheng, S.; Moslehi, J. Cardiovascular complications of radiation therapy for thoracic malignancies: The role for non-invasive imaging for detection of cardiovascular disease. Eur. Heart J. 2014, 35, 612–623. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef]
- Chung, R.; Ghosh, A.K.; Banerjee, A. Cardiotoxicity: Precision medicine with imprecise definitions. Open Heart 2018, 5, e000774. [Google Scholar] [CrossRef]
- Billingham, M.E.; Mason, J.W.; Bristow, M.R.; Daniels, J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978, 62, 865–872. [Google Scholar]
- Ahmed, T.; Goyal, A. Endomyocardial biopsy. In Statpearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: Esmo consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the american society of echocardiography and the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An international cardio-oncology society (ic-os) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Khan, A.A.; Ashraf, A.; Singh, R.; Rahim, A.; Rostom, W.; Hussain, M.; Renner, I.; Collins, N.J. Incidence, time of occurrence and response to heart failure therapy in patients with anthracycline cardiotoxicity. Intern. Med. J. 2017, 47, 104–109. [Google Scholar] [CrossRef]
- Fei, H.-w.; Ali, M.T.; Tan, T.C.; Cheng, K.-H.; Salama, L.; Hua, L.; Zeng, X.; Halpern, E.F.; Taghian, A.; MacDonald, S.M.; et al. Left ventricular global longitudinal strain in her-2 + breast cancer patients treated with anthracyclines and trastuzumab who develop cardiotoxicity is associated with subsequent recovery of left ventricular ejection fraction. Echocardiography 2016, 33, 519–526. [Google Scholar] [CrossRef]
- Barac, A.; Murtagh, G.; Carver, J.R.; Chen, M.H.; Freeman, A.M.; Herrmann, J.; Iliescu, C.; Ky, B.; Mayer, E.L.; Okwuosa, T.M.; et al. Cardiovascular health of patients with cancer and cancer survivors: A roadmap to the next level. J. Am. Coll. Cardiol. 2015, 65, 2739–2746. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Ewer, M.S.; Lippman, S.M. Type ii chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900–2902. [Google Scholar] [CrossRef]
- Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Taylor, C.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the childhood cancer survivor study cohort. BMJ (Clin. Res. Ed.) 2009, 339, b4606. [Google Scholar] [CrossRef]
- Jensen, B.V.; Skovsgaard, T.; Nielsen, S.L. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002, 13, 699–709. [Google Scholar] [CrossRef]
- van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.; Krol, A.D.; Petersen, E.J.; Raemaekers, J.M.; Kok, W.E.; Aleman, B.M.; van Leeuwen, F.E. Cardiovascular disease after hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Fanous, I.; Dillon, P. Cancer treatment-related cardiac toxicity: Prevention, assessment and management. Med. Oncol. 2016, 33, 84. [Google Scholar] [CrossRef]
- Nolan, M.T.; Lowenthal, R.M.; Venn, A.; Marwick, T.H. Chemotherapy-related cardiomyopathy: A neglected aspect of cancer survivorship. Intern. Med. J 2014, 44, 939–950. [Google Scholar] [CrossRef]
- Vejpongsa, P.; Yeh, E.T. Prevention of anthracycline-induced cardiotoxicity: Challenges and opportunities. J. Am. Coll. Cardiol. 2014, 64, 938–945. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Kerrigan, J.E.; Lin, C.P.; Azarova, A.M.; Tsai, Y.C.; Ban, Y.; Liu, L.F. Topoisomerase iibeta mediated DNA double-strand breaks: Implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007, 67, 8839–8846. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic effects of polyphenols in cardiovascular system. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef]
- Olson, H.M.; Capen, C.C. Chronic cardiotoxicity of doxorubicin (adriamycin) in the rat: Morphologic and biochemical investigations. Toxicol. Appl. Pharmacol. 1978, 44, 605–616. [Google Scholar] [CrossRef]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef]
- Anghel, N.; Herman, H.; Balta, C.; Rosu, M.; Stan, M.S.; Nita, D.; Ivan, A.; Galajda, Z.; Ardelean, A.; Dinischiotu, A.; et al. Acute cardiotoxicity induced by doxorubicin in right ventricle is associated with increase of oxidative stress and apoptosis in rats. Histol. Histopathol. 2018, 33, 365–378. [Google Scholar] [CrossRef]
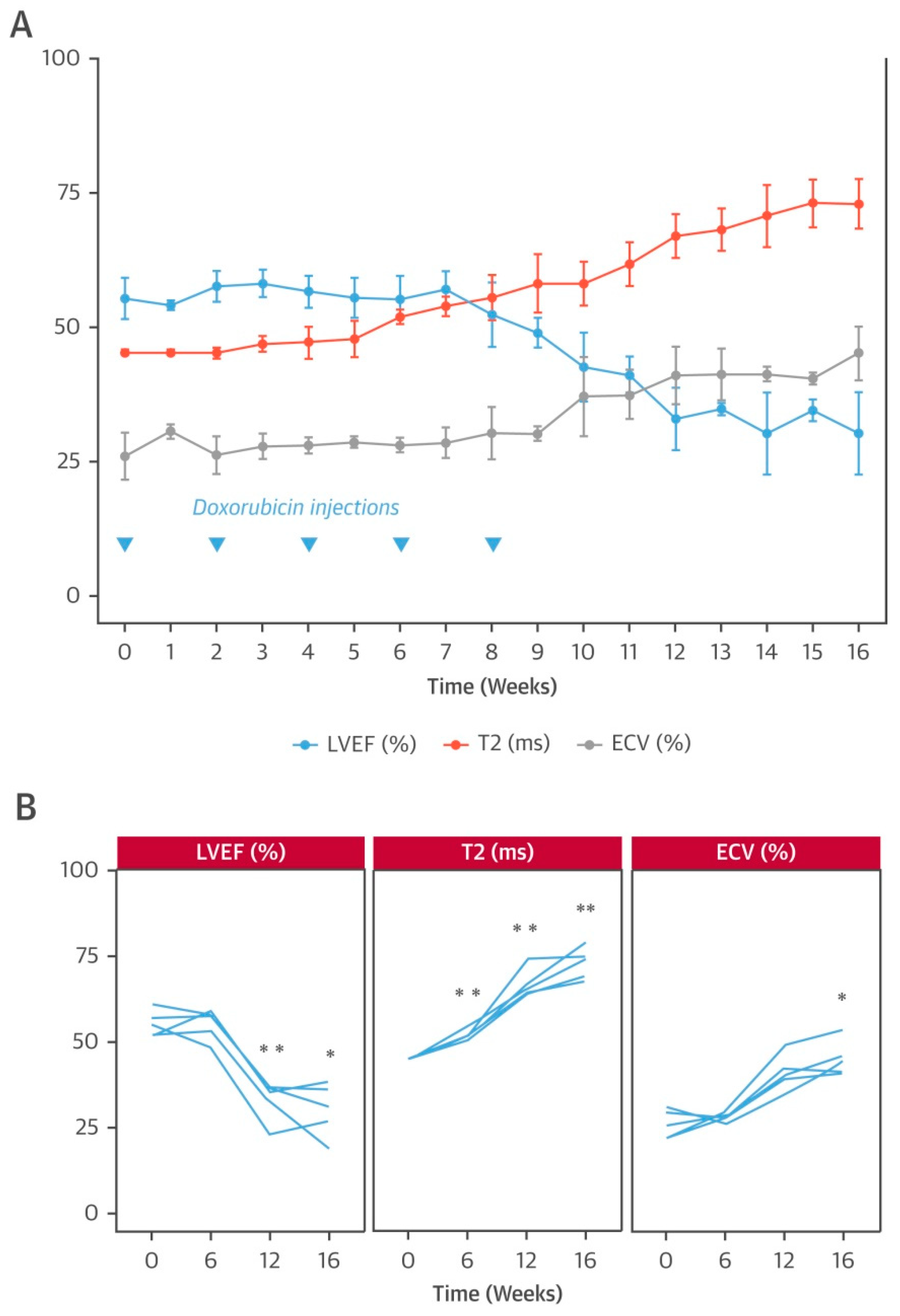
- Galán-Arriola, C.; Lobo, M.; Vílchez-Tschischke, J.P.; López, G.J.; de Molina-Iracheta, A.; Pérez-Martínez, C.; Agüero, J.; Fernández-Jiménez, R.; Martín-García, A.; Oliver, E.; et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 2019, 73, 779–791. [Google Scholar] [CrossRef]
- Lightfoot, J.C.; D’Agostino, R.B., Jr.; Hamilton, C.A.; Jordan, J.; Torti, F.M.; Kock, N.D.; Jordan, J.; Workman, S.; Hundley, W.G. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ. Cardiovasc. Imaging 2010, 3, 550–558. [Google Scholar] [CrossRef]
- Haslbauer, J.D.; Lindner, S.; Valbuena-Lopez, S.; Zainal, H.; Zhou, H.; D’Angelo, T.; Pathan, F.; Arendt, C.A.; Bug, G.; Serve, H.; et al. Cmr imaging biosignature of cardiac involvement due to cancer-related treatment by t1 and t2 mapping. Int. J. Cardiol. 2019, 275, 179–186. [Google Scholar] [CrossRef]
- Ferreira de Souza, T.; Quinaglia, A.C.S.T.; Osorio Costa, F.; Shah, R.; Neilan, T.G.; Velloso, L.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R.; et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. JACC Cardiovasc. Imaging 2018, 11, 1045–1055. [Google Scholar] [CrossRef]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Neilan, T.G.; Coelho-Filho, O.R.; Pena-Herrera, D.; Shah, R.V.; Jerosch-Herold, M.; Francis, S.A.; Moslehi, J.; Kwong, R.Y. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am. J. Cardiol. 2012, 110, 1679–1686. [Google Scholar] [CrossRef]
- Santoro, C.; Arpino, G.; Esposito, R.; Lembo, M.; Paciolla, I.; Cardalesi, C.; de Simone, G.; Trimarco, B.; De Placido, S.; Galderisi, M. 2d and 3d strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: A balance with feasibility. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 930–936. [Google Scholar] [CrossRef]
- van Royen, N.; Jaffe, C.C.; Krumholz, H.M.; Johnson, K.M.; Lynch, P.J.; Natale, D.; Atkinson, P.; Deman, P.; Wackers, F.J. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am. J. Cardiol. 1996, 77, 843–850. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Plana, J.C.; Zhang, N.; Srivastava, D.; Green, D.M.; Ness, K.K.; Daniel Donovan, F.; Metzger, M.L.; Arevalo, A.; Durand, J.B.; et al. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J. Clin. Oncol. 2012, 30, 2876–2884. [Google Scholar] [CrossRef]
- Alexander, J.; Dainiak, N.; Berger, H.J.; Goldman, L.; Johnstone, D.; Reduto, L.; Duffy, T.; Schwartz, P.; Gottschalk, A.; Zaret, B.L. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N. Engl. J. Med. 1979, 300, 278–283. [Google Scholar] [CrossRef]
- Altena, R.; Perik, P.J.; van Veldhuisen, D.J.; de Vries, E.G.; Gietema, J.A. Cardiovascular toxicity caused by cancer treatment: Strategies for early detection. Lancet Oncol. 2009, 10, 391–399. [Google Scholar] [CrossRef]
- Choi, B.W.; Berger, H.J.; Schwartz, P.E.; Alexander, J.; Wackers, F.J.; Gottschalk, A.; Zaret, B.L. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. Am. Heart J. 1983, 106, 638–643. [Google Scholar] [CrossRef]
- Huang, H.; Nijjar, P.S.; Misialek, J.R.; Blaes, A.; Derrico, N.P.; Kazmirczak, F.; Klem, I.; Farzaneh-Far, A.; Shenoy, C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: Comparison with cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2017, 19, 34. [Google Scholar] [CrossRef]
- Makavos, G.; Ikonomidis, I.; Palios, J.; Rigopoulos, A.; Katogiannis, K.; Parissis, J.; Paraskevaidis, I.; Noutsias, M. Cardiac imaging in cardiotoxicity: A focus on clinical practice. Heart Fail. Rev. 2021, 26, 1175–1187. [Google Scholar] [CrossRef]
- Kolla, B.C.; Roy, S.S.; Duval, S.; Weisdorf, D.; Valeti, U.; Blaes, A. Cardiac imaging methods for chemotherapy-related cardiotoxicity screening and related radiation exposure: Current practice and trends. Anticancer Res. 2017, 37, 2445–2449. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Finn, J.P.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Ho, V.B.; Jerosch-Herold, M.; Kramer, C.M.; Manning, W.J.; et al. Accf/acr/aha/nasci/scmr 2010 expert consensus document on cardiovascular magnetic resonance: A report of the american college of cardiology foundation task force on expert consensus documents. J. Am. Coll. Cardiol. 2010, 55, 2614–2662. [Google Scholar] [CrossRef]
- Constantine, G.; Shan, K.; Flamm, S.D.; Sivananthan, M.U. Role of mri in clinical cardiology. Lancet 2004, 363, 2162–2171. [Google Scholar] [CrossRef]
- Jordan, J.H.; D’Agostino, R.B., Jr.; Hamilton, C.A.; Vasu, S.; Hall, M.E.; Kitzman, D.W.; Thohan, V.; Lawrence, J.A.; Ellis, L.R.; Lash, T.L.; et al. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using t1-weighted and t2-weighted cardiovascular magnetic resonance. Circ. Cardiovasc. Imaging 2014, 7, 872–879. [Google Scholar] [CrossRef]
- Drafts, B.C.; Twomley, K.M.; D’Agostino, R., Jr.; Lawrence, J.; Avis, N.; Ellis, L.R.; Thohan, V.; Jordan, J.; Melin, S.A.; Torti, F.M.; et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc. Imaging 2013, 6, 877–885. [Google Scholar] [CrossRef]
- Jordan, J.H.; Todd, R.M.; Vasu, S.; Hundley, W.G. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc. Imaging 2018, 11, 1150–1172. [Google Scholar] [CrossRef]
- Meléndez, G.C.; Sukpraphrute, B.; D’Agostino, R.B., Jr.; Jordan, J.H.; Klepin, H.D.; Ellis, L.; Lamar, Z.; Vasu, S.; Lesser, G.; Burke, G.L.; et al. Frequency of left ventricular end-diastolic volume-mediated declines in ejection fraction in patients receiving potentially cardiotoxic cancer treatment. Am. J. Cardiol. 2017, 119, 1637–1642. [Google Scholar] [CrossRef]
- Stokke, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, O.A.; Edvardsen, T.; Remme, E.W. Geometry as a confounder when assessing ventricular systolic function: Comparison between ejection fraction and strain. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef]
- Ewer, M.S.; Lenihan, D.J. Left ventricular ejection fraction and cardiotoxicity: Is our ear really to the ground? J. Clin. Oncol. 2008, 26, 1201–1203. [Google Scholar] [CrossRef]
- Ewer, M.S.; Ali, M.K.; Mackay, B.; Wallace, S.; Valdivieso, M.; Legha, S.S.; Benjamin, R.S.; Haynie, T.P. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving adriamycin. J. Clin. Oncol. 1984, 2, 112–117. [Google Scholar] [CrossRef]
- Greenberg, N.L.; Firstenberg, M.S.; Castro, P.L.; Main, M.; Travaglini, A.; Odabashian, J.A.; Drinko, J.K.; Rodriguez, L.L.; Thomas, J.D.; Garcia, M.J. Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 2002, 105, 99–105. [Google Scholar] [CrossRef]
- Toro-Salazar, O.H.; Gillan, E.; O’Loughlin, M.T.; Burke, G.S.; Ferranti, J.; Stainsby, J.; Liang, B.; Mazur, W.; Raman, S.V.; Hor, K.N. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ. Cardiovasc. Imaging 2013, 6, 873–880. [Google Scholar] [CrossRef]
- Ong, G.; Brezden-Masley, C.; Dhir, V.; Deva, D.P.; Chan, K.K.W.; Chow, C.M.; Thavendiranathan, D.; Haq, R.; Barfett, J.J.; Petrella, T.M.; et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int. J. Cardiol. 2018, 261, 228–233. [Google Scholar] [CrossRef]
- Nakano, S.; Takahashi, M.; Kimura, F.; Senoo, T.; Saeki, T.; Ueda, S.; Tanno, J.; Senbonmatsu, T.; Kasai, T.; Nishimura, S. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: A pilot study to evaluate the feasibility of the method. Cardiol. J. 2016, 23, 270–280. [Google Scholar] [CrossRef]
- Poterucha, J.T.; Kutty, S.; Lindquist, R.K.; Li, L.; Eidem, B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 733–740. [Google Scholar] [CrossRef]
- MacIver, D.H.; Adeniran, I.; Zhang, H. Left ventricular ejection fraction is determined by both global myocardial strain and wall thickness. Int. J. Cardiol. Heart Vasc. 2015, 7, 113–118. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-guided management of potentially cardiotoxic cancer therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef]
- Ali, M.T.; Yucel, E.; Bouras, S.; Wang, L.; Fei, H.W.; Halpern, E.F.; Scherrer-Crosbie, M. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J. Am. Soc. Echocardiogr. 2016, 29, 522–527.e3. [Google Scholar] [CrossRef]
- Mousavi, N.; Tan, T.C.; Ali, M.; Halpern, E.F.; Wang, L.; Scherrer-Crosbie, M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50-59% treated with anthracyclines. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 977–984. [Google Scholar] [CrossRef][Green Version]
- Narayan, H.K.; French, B.; Khan, A.M.; Plappert, T.; Hyman, D.; Bajulaiye, A.; Domchek, S.; DeMichele, A.; Clark, A.; Matro, J.; et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1131–1141. [Google Scholar] [CrossRef]
- Jolly, M.P.; Jordan, J.H.; Meléndez, G.C.; McNeal, G.R.; D’Agostino, R.B., Jr.; Hundley, W.G. Automated assessments of circumferential strain from cine cmr correlate with lvef declines in cancer patients early after receipt of cardio-toxic chemotherapy. J. Cardiovasc. Magn. Reson. 2017, 19, 59. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The eacvi/ase inter-vendor comparison study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
- Grover, S.; Leong, D.P.; Chakrabarty, A.; Joerg, L.; Kotasek, D.; Cheong, K.; Joshi, R.; Joseph, M.X.; DePasquale, C.; Koczwara, B.; et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: A prospective study using novel cardiac imaging and biochemical markers. Int. J. Cardiol. 2013, 168, 5465–5467. [Google Scholar] [CrossRef]
- Ylänen, K.; Poutanen, T.; Savikurki-Heikkilä, P.; Rinta-Kiikka, I.; Eerola, A.; Vettenranta, K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J. Am. Coll. Cardiol. 2013, 61, 1539–1547. [Google Scholar] [CrossRef]
- Murbraech, K.; Holte, E.; Broch, K.; Smeland, K.B.; Holte, H.; Rösner, A.; Lund, M.B.; Dalen, H.; Kiserud, C.; Aakhus, S. Impaired right ventricular function in long-term lymphoma survivors. J. Am. Soc. Echocardiogr. 2016, 29, 528–536. [Google Scholar] [CrossRef]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef]
- Cummings, K.W.; Bhalla, S.; Javidan-Nejad, C.; Bierhals, A.J.; Gutierrez, F.R.; Woodard, P.K. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at mr imaging. Radiographics 2009, 29, 89–103. [Google Scholar] [CrossRef]
- Cottin, Y.; Ribuot, C.; Maupoil, V.; Godin, D.; Arnould, L.; Brunotte, F.; Rochette, L. Early incidence of adriamycin treatment on cardiac parameters in the rat. Can. J. Physiol. Pharmacol. 1994, 72, 140–145. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A jacc white paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Pepe, A.; Pizzino, F.; Gargiulo, P.; Perrone-Filardi, P.; Cadeddu, C.; Mele, D.; Monte, I.; Novo, G.; Zito, C.; Di Bella, G. Cardiovascular imaging in the diagnosis and monitoring of cardiotoxicity: Cardiovascular magnetic resonance and nuclear cardiology. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), e45–e54. [Google Scholar] [CrossRef]
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976. [Google Scholar] [CrossRef]
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S.; Bittencourt, M.S.; Murthy, V.L.; Kwong, R.; Di Carli, M.F.; et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: A systematic review and meta-analysis. Circ. Cardiovasc. Imaging 2016, 9, e005001. [Google Scholar] [CrossRef]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical risk prediction in patients with left ventricular myocardial noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef]
- Lunning, M.A.; Kutty, S.; Rome, E.T.; Li, L.; Padiyath, A.; Loberiza, F.; Bociek, R.G.; Bierman, P.J.; Vose, J.M.; Armitage, J.O.; et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am. J. Clin. Oncol. 2015, 38, 377–381. [Google Scholar] [CrossRef]
- Oyakawa, T.; Iida, K.; Kusuhara, M.; Kenmotsu, H.; Sugino, T. Chemotherapy-induced cardiomyopathy caused by pemetrexed. Investig. New Drugs 2018, 36, 147–150. [Google Scholar] [CrossRef]
- Muehlberg, F.; Funk, S.; Zange, L.; von Knobelsdorff-Brenkenhoff, F.; Blaszczyk, E.; Schulz, A.; Ghani, S.; Reichardt, A.; Reichardt, P.; Schulz-Menger, J. Native myocardial t1 time can predict development of subsequent anthracycline-induced cardiomyopathy. ESC Heart Fail. 2018, 5, 620–629. [Google Scholar] [CrossRef]
- Xu, J.; Zhuang, B.; Sirajuddin, A.; Li, S.; Huang, J.; Yin, G.; Song, L.; Jiang, Y.; Zhao, S.; Lu, M. Mri t1 mapping in hypertrophic cardiomyopathy: Evaluation in patients without late gadolinium enhancement and hemodynamic obstruction. Radiology 2020, 294, 275–286. [Google Scholar] [CrossRef]
- Pattanayak, P.; Bluemke, D.A. Cardiac mr imaging to probe tissue composition of the heart by using t1 mapping. Radiology 2014, 271, 320–322. [Google Scholar] [CrossRef]
- Sibley, C.T.; Noureldin, R.A.; Gai, N.; Nacif, M.S.; Liu, S.; Turkbey, E.B.; Mudd, J.O.; Geest, R.J.v.d.; Lima, J.A.C.; Halushka, M.K.; et al. T1 mapping in cardiomyopathy at cardiac mr: Comparison with endomyocardial biopsy. Radiology 2012, 265, 724–732. [Google Scholar] [CrossRef]
- Kellman, P.; Wilson, J.R.; Xue, H.; Bandettini, W.P.; Shanbhag, S.M.; Druey, K.M.; Ugander, M.; Arai, A.E. Extracellular volume fraction mapping in the myocardium, part 2: Initial clinical experience. J. Cardiovasc. Magn. Reson. 2012, 14, 64. [Google Scholar] [CrossRef]
- Park, C.H.; Choi, E.Y.; Kwon, H.M.; Hong, B.K.; Lee, B.K.; Yoon, Y.W.; Min, P.K.; Greiser, A.; Paek, M.Y.; Yu, W.; et al. Quantitative t2 mapping for detecting myocardial edema after reperfusion of myocardial infarction: Validation and comparison with t2-weighted images. Int. J. Cardiovasc. Imaging 2013, 29 (Suppl. 1), 65–72. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Walls, M.; Giri, S.; Verhaert, D.; Rajagopalan, S.; Moore, S.; Simonetti, O.P.; Raman, S.V. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using t2 mapping. Circ. Cardiovasc. Imaging 2012, 5, 102–110. [Google Scholar] [CrossRef]
- Wassmuth, R.; Prothmann, M.; Utz, W.; Dieringer, M.; von Knobelsdorff-Brenkenhoff, F.; Greiser, A.; Schulz-Menger, J. Variability and homogeneity of cardiovascular magnetic resonance myocardial t2-mapping in volunteers compared to patients with edema. J. Cardiovasc. Magn. Reson. 2013, 15, 27. [Google Scholar] [CrossRef]
- Ugander, M.; Bagi, P.S.; Oki, A.J.; Chen, B.; Hsu, L.Y.; Aletras, A.H.; Shah, S.; Greiser, A.; Kellman, P.; Arai, A.E. Myocardial edema as detected by pre-contrast t1 and t2 cmr delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Tham, E.B.; Haykowsky, M.J.; Chow, K.; Spavor, M.; Kaneko, S.; Khoo, N.S.; Pagano, J.J.; Mackie, A.S.; Thompson, R.B. Diffuse myocardial fibrosis by t1-mapping in children with subclinical anthracycline cardiotoxicity: Relationship to exercise capacity, cumulative dose and remodeling. J. Cardiovasc. Magn. Reson. 2013, 15, 48. [Google Scholar] [CrossRef]
- Hundley, W.G.; Jordan, J.H. When left ventricular extracellular volume fraction changes after anthracyclines: Is it due to a change in the numerator, denominator, or both? JACC Cardiovasc. Imaging 2018, 11, 1056–1058. [Google Scholar] [CrossRef]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native t1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Neilan, T.G.; Coelho-Filho, O.R.; Shah, R.V.; Feng, J.H.; Pena-Herrera, D.; Mandry, D.; Pierre-Mongeon, F.; Heydari, B.; Francis, S.A.; Moslehi, J.; et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am. J. Cardiol. 2013, 111, 717–722. [Google Scholar] [CrossRef]
- Jordan, J.H.; Vasu, S.; Morgan, T.M.; D’Agostino, R.B., Jr.; Meléndez, G.C.; Hamilton, C.A.; Arai, A.E.; Liu, S.; Liu, C.Y.; Lima, J.A.; et al. Anthracycline-associated t1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ. Cardiovasc. Imaging 2016, 9, e004325. [Google Scholar] [CrossRef]
- Meléndez, G.C.; Jordan, J.H.; D’Agostino, R.B., Jr.; Vasu, S.; Hamilton, C.A.; Hundley, W.G. Progressive 3-month increase in lv myocardial ecv after anthracycline-based chemotherapy. JACC Cardiovasc. Imaging 2017, 10, 708–709. [Google Scholar] [CrossRef]
- Hong, Y.J.; Park, H.S.; Park, J.K.; Han, K.; Park, C.H.; Kim, T.K.; Yoo, S.J.; Lee, J.Y.; Kim, P.K.; Hur, J.; et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using t1-mapping cardiac magnetic resonance imaging: An animal study. Sci. Rep. 2017, 7, 2663. [Google Scholar] [CrossRef]
- Farhad, H.; Staziaki, P.V.; Addison, D.; Coelho-Filho, O.R.; Shah, R.V.; Mitchell, R.N.; Szilveszter, B.; Abbasi, S.A.; Kwong, R.Y.; Scherrer-Crosbie, M.; et al. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ. Cardiovasc. Imaging 2016, 9, e003584. [Google Scholar] [CrossRef]
- Yang, E.Y.; Ghosn, M.G.; Khan, M.A.; Gramze, N.L.; Brunner, G.; Nabi, F.; Nambi, V.; Nagueh, S.F.; Nguyen, D.T.; Graviss, E.A.; et al. Myocardial extracellular volume fraction adds prognostic information beyond myocardial replacement fibrosis. Circ. Cardiovasc. Imaging 2019, 12, e009535. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of t1, t2, t2* and extracellular volume: A consensus statement by the society for cardiovascular magnetic resonance (scmr) endorsed by the european association for cardiovascular imaging (eacvi). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef]
- van Ewijk, P.A.; Schrauwen-Hinderling, V.B.; Bekkers, S.C.; Glatz, J.F.; Wildberger, J.E.; Kooi, M.E. Mrs: A noninvasive window into cardiac metabolism. NMR Biomed. 2015, 28, 747–766. [Google Scholar] [CrossRef]
- Stoll, V.M.; Clarke, W.T.; Levelt, E.; Liu, A.; Myerson, S.G.; Robson, M.D.; Neubauer, S.; Rodgers, C.T. Dilated cardiomyopathy: Phosphorus 31 mr spectroscopy at 7 t. Radiology 2016, 281, 409–417. [Google Scholar] [CrossRef]
- Peterzan, M.A.; Lewis, A.J.M.; Neubauer, S.; Rider, O.J. Non-invasive investigation of myocardial energetics in cardiac disease using (31)p magnetic resonance spectroscopy. Cardiovasc. Diagn. Ther. 2020, 10, 625–635. [Google Scholar] [CrossRef]
- Gupta, A.; Rohlfsen, C.; Leppo, M.K.; Chacko, V.P.; Wang, Y.; Steenbergen, C.; Weiss, R.G. Creatine kinase-overexpression improves myocardial energetics, contractile dysfunction and survival in murine doxorubicin cardiotoxicity. PLoS ONE 2013, 8, e74675. [Google Scholar] [CrossRef]
- Macnaught, G.; Oikonomidou, O.; Rodgers, C.T.; Clarke, W.; Cooper, A.; McVicars, H.; Hayward, L.; Mirsadraee, S.; Semple, S.; Denvir, M.A. Cardiac energetics before, during, and after anthracycline-based chemotherapy in breast cancer patients using (31)p magnetic resonance spectroscopy: A pilot study. Front. Cardiovasc. Med. 2021, 8, 653648. [Google Scholar] [CrossRef]
- Nguyen, C.; Lu, M.; Fan, Z.; Bi, X.; Kellman, P.; Zhao, S.; Li, D. Contrast-free detection of myocardial fibrosis in hypertrophic cardiomyopathy patients with diffusion-weighted cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 107. [Google Scholar] [CrossRef]
- Wu, L.M.; Chen, B.H.; Yao, Q.Y.; Ou, Y.R.; Wu, R.; Jiang, M.; Hu, J.; An, D.A.; Xu, J.R. Quantitative diffusion-weighted magnetic resonance imaging in the assessment of myocardial fibrosis in hypertrophic cardiomyopathy compared with t1 mapping. Int. J. Cardiovasc. Imaging 2016, 32, 1289–1297. [Google Scholar] [CrossRef]
- Das, A.; Kelly, C.; Teh, I.; Stoeck, C.T.; Kozerke, S.; Chowdhary, A.; Brown, L.A.E.; Saunderson, C.E.D.; Craven, T.P.; Chew, P.G.; et al. Acute microstructural changes after st-segment elevation myocardial infarction assessed with diffusion tensor imaging. Radiology 2021, 299, 86–96. [Google Scholar] [CrossRef]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. Scmr position paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]

| Society | Diagnostic Criteria of Cardiotoxicity | Year of Publication |
|---|---|---|
| ASE/EACVI | Decrease in LVEF of >10%, to LVEF < 53% Relative drop in GLS > 15% from baseline suggests subclinical LV dysfunction | 2014 [14] |
| ESC | Decrease in LVEF of >10% from baseline, to LVEF < 50% Decrease in GLS of >15% from baseline may suggest risk of cardiotoxicity | 2016 [7] |
| ESMO | LVEF drop by ≥10–15%, or to <50% Symptomatic heart failure regardless of LVEF | 2020 [13] |
| IC-OS | For asymptomatic patients: Mild: LVEF ≥ 50% and new relative decrease in GLS by >15% from baseline, and/or new rise in cardiac biomarkers (cardiac troponin I/T > 99th percentile, BNP > 35 pg/mL, NT-proBNP ≥ 125 pg/mL). New reduction in LVEF by ≥10% or <10%, to absolute 40% < LVEF < 50%, and new relative decrease in GLS by >15% from baseline, and/or new rise in cardiac biomarkers. Severe: new LVEF reduction to <40%. For symptomatic patients: mild heart failure symptoms or more. | 2021 [15] |
| Imaging Techniques | Monitoring Index or Characteristic | Advantages | Limitations |
|---|---|---|---|
| MUGA | LVEF | Reproducibility | Radiation exposure Limited morphological and functional information of other cardiac chambers and extra-cardiac structures |
| Echocardiography | LVEF RVEF Strain (GLS, GCS, GRS) LV mass | Wide availability High portability No radiation Morphological and functional information Valvular function Low cost | Suboptimal acoustic window High operator dependency High variability GLS: inter-vendor variability and technical requirements |
| CMR | LVEF RVEF Strain (GLS, GCS, GRS) LV mass Edema Inflammation Fibrosis | Reproducibility Accuracy No radiation Morphological and functional information Valvular function Tissue characterization | Limited availability High cost Technical requirements Patient adaptation (contraindications for CMR: difficulty in holding breath or lying flat) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Lin, L.; Zhang, G.; Zhou, X. Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies. Diagnostics 2022, 12, 1846. https://doi.org/10.3390/diagnostics12081846
Wei X, Lin L, Zhang G, Zhou X. Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies. Diagnostics. 2022; 12(8):1846. https://doi.org/10.3390/diagnostics12081846
Chicago/Turabian StyleWei, Xiaoting, Ling Lin, Guizhi Zhang, and Xuhui Zhou. 2022. "Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies" Diagnostics 12, no. 8: 1846. https://doi.org/10.3390/diagnostics12081846
APA StyleWei, X., Lin, L., Zhang, G., & Zhou, X. (2022). Cardiovascular Magnetic Resonance Imaging in the Early Detection of Cardiotoxicity Induced by Cancer Therapies. Diagnostics, 12(8), 1846. https://doi.org/10.3390/diagnostics12081846






