Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Lung Ultrasound Examination
2.2.1. Equipment
2.2.2. LUS Examination Method
2.2.3. Observation Indexes
2.2.4. Statistical Analysis
3. Results
3.1. Clinical Manifestations of Fungal Pneumonia
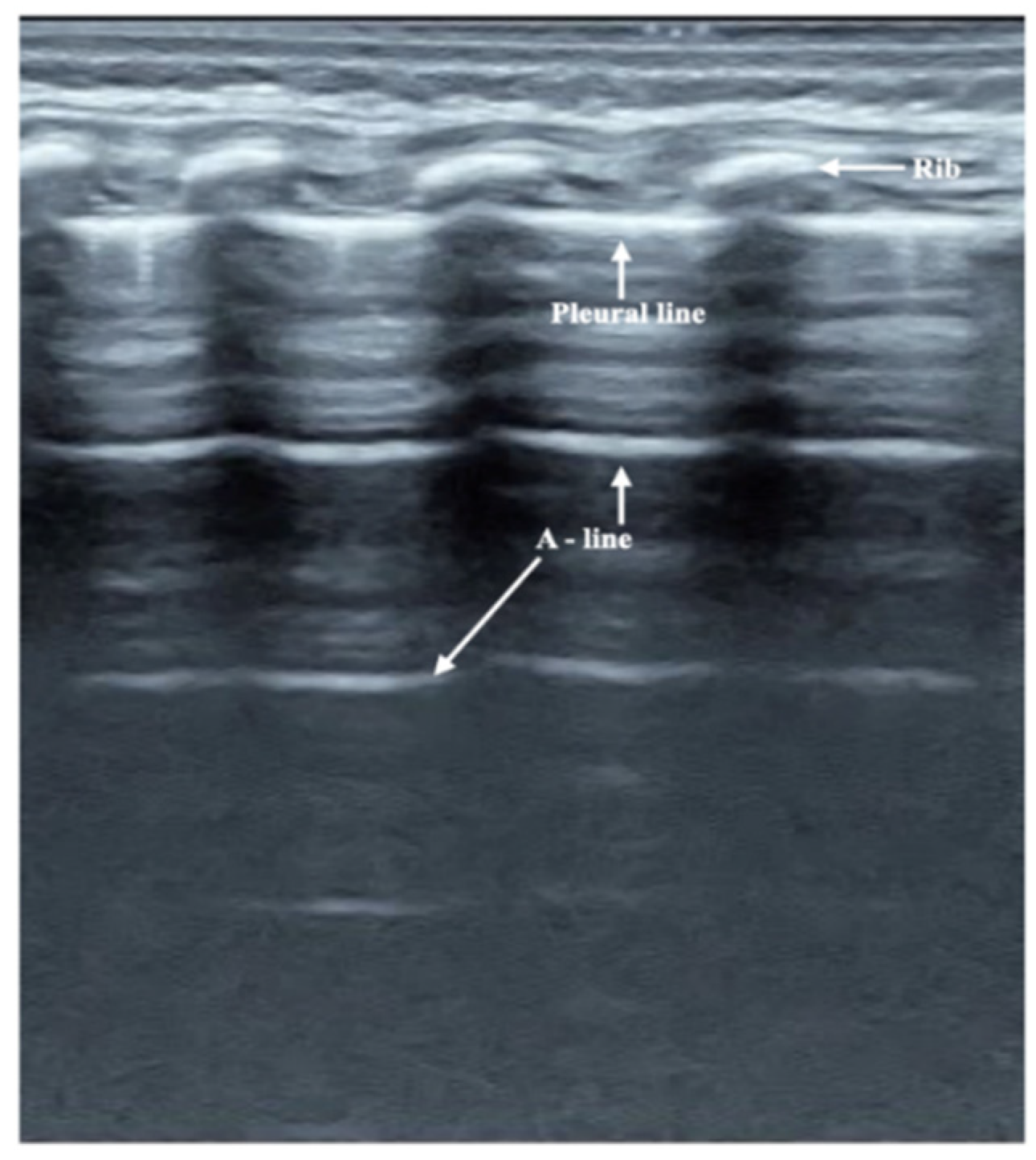
3.2. Ultrasound Manifestation of Neonatal Normal Lung
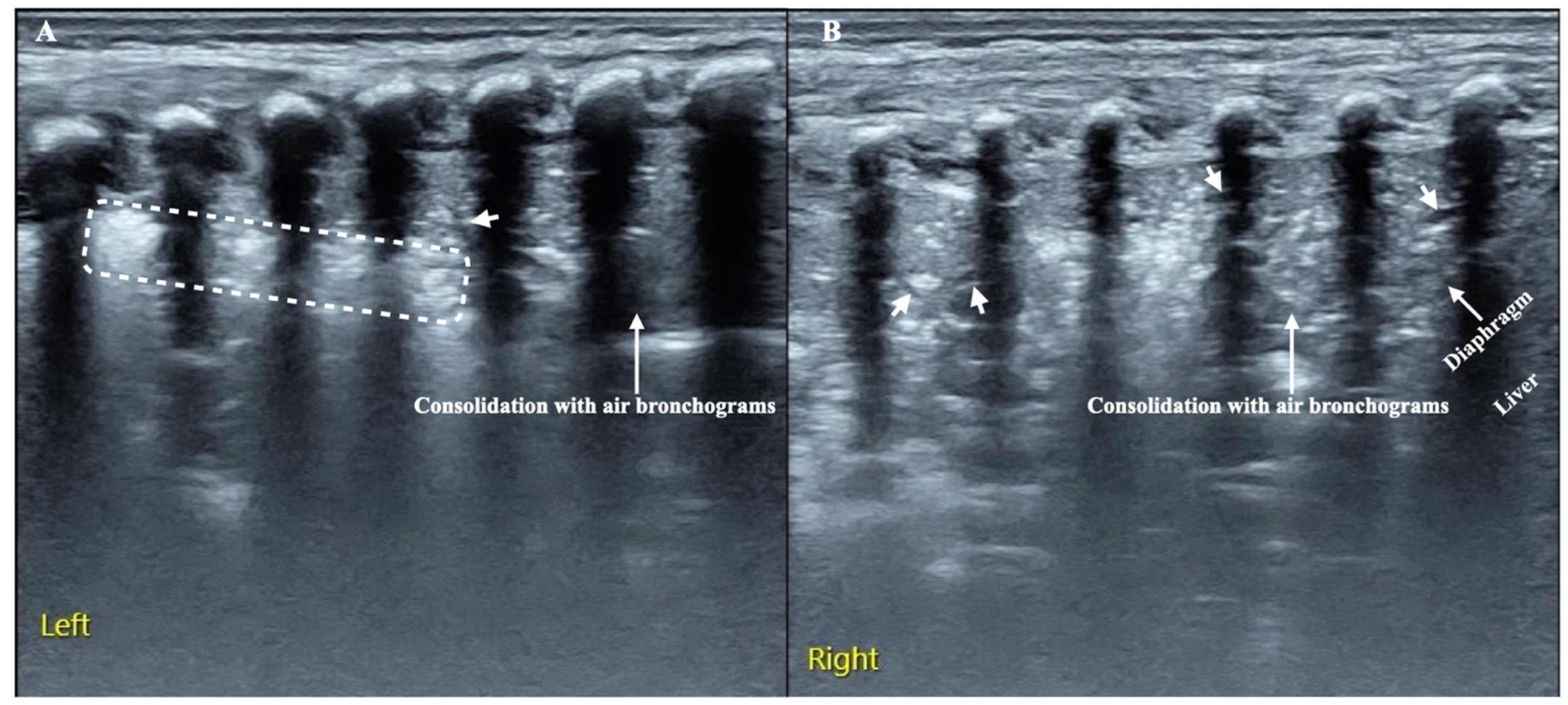
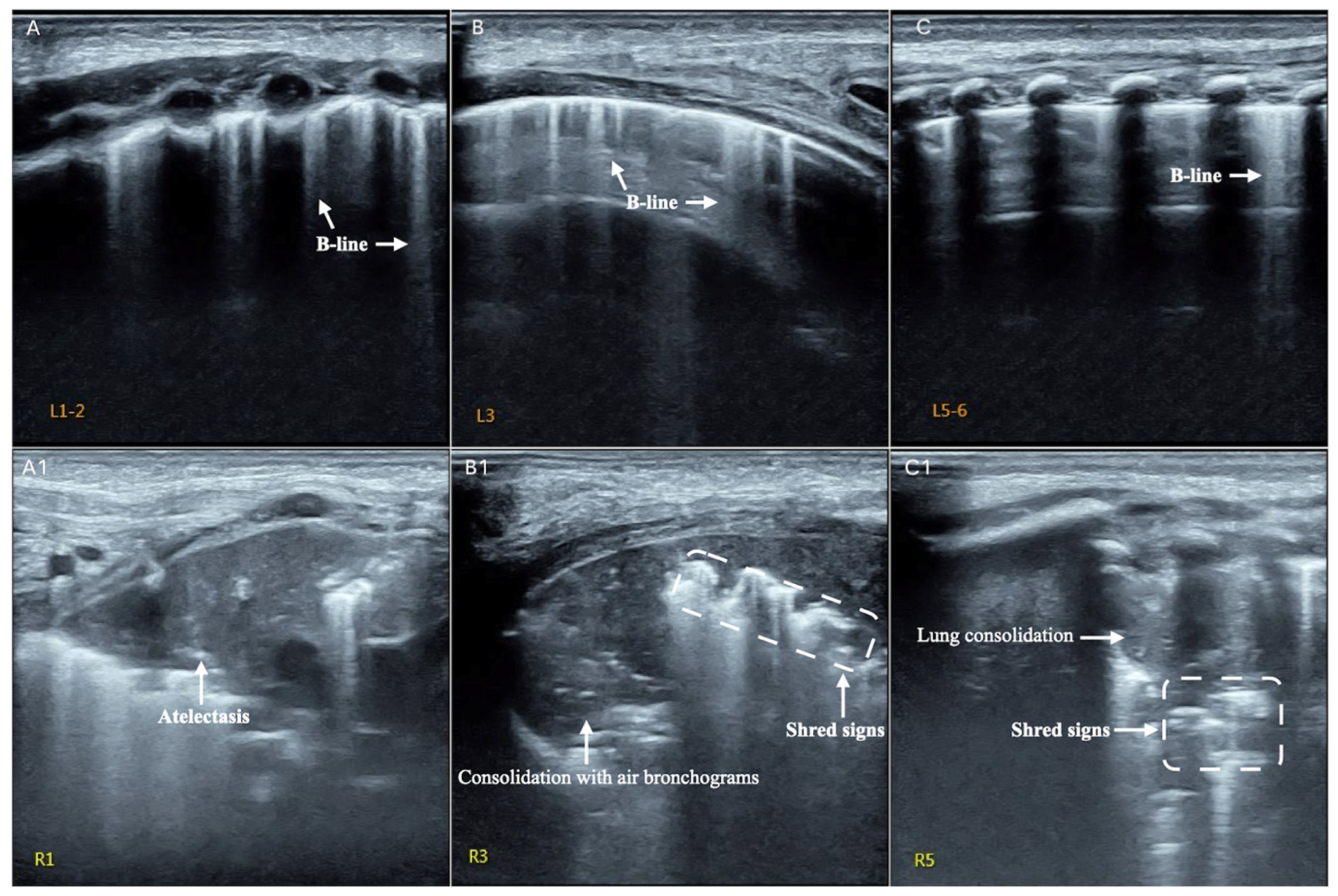
3.3. LUS Findings of FP
3.4. Typical Case Presentations
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LUS | lung ultrasound |
| NICU | neonatal intensive care unit |
| CXR | chest X-ray |
| NFI | neonatal fungal infection |
| FP | fungal pneumonia |
| MAS | meconium aspiration syndrome |
| RDS | respiratory distress syndrome |
| TTN | transient tachypnea of newborn |
| BPD | bronchopulmonary dysplasia |
| CRP | c-reactive protein |
| G test | 1, 3-β-d-glucan test |
References
- Hooven, T.A.; Polin, R.A. Pneumonia. Semin. Fetal Neonatal Med. 2017, 22, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Oeser, C.; Vergnano, S.; Naidoo, R.; Anthony, M.; Chang, J.; Chow, P.; Clarke, P.; Embleton, N.; Kennea, N.; Pattnayak, S.; et al. Neonatal invasive fungal infection in England 2004–2010. Clin. Microbiol. Infect. 2014, 20, 936–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.J. Risk factors for fungal infection in extremely low birthweight infants registered in the Korean neonatal network from 2013 to 2015: Male sex and hypotension. Pediatr. Int. 2020, 62, 477–483. [Google Scholar] [CrossRef]
- Ishiwada, N.; Kitajima, H.; Morioka, I.; Takeuchi, N.; Endo, M.; Watanabe, A.; Kamei, K. Nationwide survey of neonatal invasive fungal infection in Japan. Med. Mycol. 2018, 56, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Wu, H.; Xia, S.; Zhu, X.; Chen, C.; Qiu, G.; Zhou, W.; Shi, Y.; Mia, L.; Sun, J.; et al. Invasive candidiasis in preterm neonates in China: A retrospective study from 11 NICUs during 2009–2011. Pediatr. Infect. Dis. J. 2014, 33, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Piastra, M.; Yousef, N.; Brat, R.; Manzoni, P.; Mokhtari, M.; De Luca, D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 2014, 90 (Suppl. S2), S41–S43. [Google Scholar] [CrossRef]
- Liu, J.; Cao, H.-Y.; Fu, W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J. Int. Med. Res. 2016, 44, 1534–1542. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cao, H.Y.; Wang, H.-W.; Kong, X.Y. The Role of Lung Ultrasound in Diagnosis of Respiratory Distress Syndrome in Newborn Infants. Iran. J. Pediatr. 2015, 25, e323. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Yan, W.; Liu, J. Diagnostic value of lung ultrasound for neonatal respiratory distress syndrome: A meta-analysis and systematic review. Med. Ultrason. 2020, 22, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.-X.; Li, X.-W.; Wang, Y.; Chen, S.-W.; Fu, W. Lung Ultrasonography to Diagnose Transient Tachypnea of the Newborn. Chest 2016, 149, 1269–1275. [Google Scholar] [CrossRef]
- He, L.; Sun, Y.; Sheng, W.; Yao, Q. Diagnostic performance of lung ultrasound for transient tachypnea of the newborn: A meta-analysis. PLoS ONE 2021, 16, e0248827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chi, J.-H.; Ren, X.-L.; Li, J.; Chen, Y.-J.; Lu, Z.-L.; Liu, Y.; Fu, W.; Xia, R.-M. Lung ultrasonography to diagnose pneumothorax of the newborn. Am. J. Emerg. Med. 2017, 35, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Szymońska, I.; Wentrys, L.; Jagła, M.; Olszewska, M.; Wasilewska, W.; Smykla, B.; Kwinta, P. Lung ultrasound reduces the number of chest X-rays in newborns with pneumothorax. Dev. Period. Med. 2019, 23, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-L.; Fu, W.; Liu, J.; Liu, Y.; Xia, R.-M. Lung ultrasonography to diagnose pulmonary hemorrhage of the newborn. J. Matern. Fetal Neonatal Med. 2016, 30, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chi, J.-H.; Fu, W.; Zhang, L.; Qiu, R.-X. Lung Ultrasonography to Diagnose Bronchopulmonary Dysplasia of Premature Infants. Iran. J. Pediatr. 2021, 31, e109598. [Google Scholar] [CrossRef]
- Di Mauro, A.; Ammirabile, A.; Quercia, M.; Panza, R.; Capozza, M.; Manzionna, M.M.; Laforgia, N. Acute Bronchiolitis: Is There a Role for Lung Ultrasound? Diagnostics 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Quercia, M.; Panza, R.; Calderoni, G.; Di Mauro, A.; Laforgia, N. Lung Ultrasound: A New Tool in the Management of Congenital Lung Malformation. Am. J. Perinatol. 2019, 36 (Suppl. S2), S99–S105. [Google Scholar] [CrossRef] [Green Version]
- Merli, L.; Nanni, L.; Curatola, A.; Pellegrino, M.; De Santis, M.; Silvaroli, S.; Paradiso, F.V.; Buonsenso, D. Congenital lung malformations: A novel application for lung ultrasound? J. Ultrasound 2021, 24, 349–353. [Google Scholar] [CrossRef]
- Liu, J.; Liu, F.; Liu, Y.; Wang, H.-W.; Feng, Z.-C. Lung Ultrasonography for the Diagnosis of Severe Neonatal Pneumonia. Chest 2014, 146, 383–388. [Google Scholar] [CrossRef]
- Öktem, A.; Zenciroğlu, A.; Üner, Ç.; Aydoğan, S.; Dilli, D.; Okumuş, N. Efficiency of Lung Ultrasonography in the Diagnosis and Follow-up of Viral Pneumonia in Newborn. Am. J. Perinatol. 2021; in press. [Google Scholar] [CrossRef]
- Gregorio-Hernández, R.; Escobar-Izquierdo, A.B.; Cobas-Pazos, J.; Martínez-Gimeno, A. Point-of-care lung ultrasound in three neonates with COVID-19. Eur. J. Pediatr. 2020, 179, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, E.R.; Ciuca, I.M.; Iacob, R.; Iacob, E.R.; Marc, M.S.; Birsasteanu, F.; Manolescu, D.L.; Iacob, D. Is Lung Ultrasound Helpful in COVID-19 Neonates?—A Systematic Review. Diagnostics 2021, 11, 2296. [Google Scholar] [CrossRef]
- Tusor, N.; De Cunto, A.; Basma, Y.; Klein, J.L.; Meau-Petit, V. Ventilator-associated pneumonia in neonates: The role of point of care lung ultrasound. Eur. J. Pediatr. 2021, 180, 137–146. [Google Scholar] [CrossRef]
- Gao, Y.-Q.; Qiu, R.-X.; Liu, J.; Zhang, L.; Ren, X.-L.; Qin, S.-J. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: A 3-year clinical practice report from a neonatal intensive care unit in China. J. Matern. Fetal Neonatal Med. 2020; 1–8, in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattaross, L.; Zhang, H.; Yeh, T.F.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. 2019, 145, e58990. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, G.; Kurepa, D.; Volpicelli, G.; Sorantin, E.; Lovrenski, J.; Alonso-Ojembarrena, A.; Hsieh, K.-S.; Lodha, A.; Yeh, T.F.; et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J. Matern. Fetal Neonatal Med. 2021, 35, 1003–1016. [Google Scholar] [CrossRef]
- Liao, D.; Li, J.; Lv, J.; Sun, T.; Deng, S. Evaluation of the Diagnostic Value of Peripheral Blood Parameters for Neonatal Pneumonia. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Kumar, C.S.; Subramanian, S.; Murki, S.; Rao, J.V.; Bai, M.; Penagaram, S.; Singh, H.; Bondili, P.B.; Madireddy, A.; Lingaldinna, S.; et al. Predictors of Mortality in Neonatal Pneumonia: An INCLEN Childhood Pneumonia Study. Indian Pediatr. 2021, 58, 1040–1045. [Google Scholar] [CrossRef]
- Liu, J.; Lovrenski, J.; Hlaing, A.Y.; Kurepa, D. Neonatal lung diseases: Lung ultrasound or chest x-ray. J. Matern. Fetal Neonatal Med. 2019, 34, 1177–1182. [Google Scholar] [CrossRef]
- Zong, H.F.; Guo, G.; Liu, J. Meta-analysis of lung ultrasound for the diagnosis of neonatal pneumonia. Chin. J. Pract. Pediatr. 2019, 34, 911–916. [Google Scholar] [CrossRef]
- Leonart, L.; Tonin, F.; Ferreira, V.L.; Penteado, S.T.D.S.; Motta, F.D.A.; Pontarolo, R. Fluconazole Doses Used for Prophylaxis of Invasive Fungal Infection in Neonatal Intensive Care Units: A Network Meta-Analysis. J. Pediatr. 2017, 185, 129–135.e6. [Google Scholar] [CrossRef] [PubMed]
- Antolín, L.F.; Sharland, M.; Warris, A. Management of Invasive Fungal Disease in Neonates and Children. Pediatr. Infect. Dis. J. 2019, 38, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.-W.; Liu, F.; Li, Q.-P.; Kong, X.-Y.; Feng, Z.-C. The Diagnosis of Neonatal Pulmonary Atelectasis Using Lung Ultrasonography. Chest 2015, 147, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, X.-L.; Fu, W.; Liu, Y.; Xia, R.-M. Bronchoalveolar lavage for the treatment of neonatal pulmonary atelectasis under lung ultrasound monitoring. J. Matern. Fetal Neonatal Med. 2016, 30, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, H.-R.; Wei, H.-L.; Chen, C.; Qiu, R.-X.; Ren, X.-L.; Zhang, L.; Gao, Y.-Q. Efficacy of Bronchoalveolar Lavage as Adjunct Therapy in the Treatment of Neonatal Severe Pneumonia: A Prospective Case–Control Study. J. Trop. Pediatr. 2020, 66, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, D.; Boyar, V.; Zaghloul, N.; Beachy, J.; Zaytseva, A.; Teng, D.; Cooper, R.; Klewer, S.; Amodio, J. Structured Neonatal Point-of-Care Ultrasound Training Program. Am. J. Perinatol. 2021, 38, e284–e291. [Google Scholar] [CrossRef]
| Ultrasound Manifestation | Pneumonia (n = 7) | Controls (n = 20) | p-Value |
|---|---|---|---|
| Lung consolidation | 7 (100) | 0 (0) | <0.001 |
| Pleural line abnormalities | 7 (100) | 0 (0) | <0.001 |
| Pleural effusion | 2 (28.6) | 0 (0) | <0.001 |
| Lung pulse | 2 (28.6) | 0 (0) | <0.001 |
| B-lines | 7 (100) | 3 (15) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ma, H.-R.; Fu, W. Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection. Diagnostics 2022, 12, 1776. https://doi.org/10.3390/diagnostics12081776
Liu J, Ma H-R, Fu W. Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection. Diagnostics. 2022; 12(8):1776. https://doi.org/10.3390/diagnostics12081776
Chicago/Turabian StyleLiu, Jing, Hai-Ran Ma, and Wei Fu. 2022. "Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection" Diagnostics 12, no. 8: 1776. https://doi.org/10.3390/diagnostics12081776
APA StyleLiu, J., Ma, H.-R., & Fu, W. (2022). Lung Ultrasound to Diagnose Pneumonia in Neonates with Fungal Infection. Diagnostics, 12(8), 1776. https://doi.org/10.3390/diagnostics12081776







