Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded—Review
Abstract
1. Introduction
2. Materials and Methods
- Full text available in English
- Available patient data
- Human related studies
- Non-experimental studies, in-vivo
- Nephrological disease
- Transplanted kidney
- Prostate evaluation
- Articles not meeting the inclusion criteria
3. Results
3.1. Kidney
3.1.1. Normal Values for Renal Stiffness
Point Shear Wave Elastography (pSWE or ARFI)
2D SWE
3.1.2. Renal Masses
pSWE
2D SWE
RTE
3.1.3. Vesicoureteral Reflux
pSWE
2D SWE
RTE
3.1.4. Ureteropelvic Junction Obstruction (UPJO)
pSWE
2D SWE
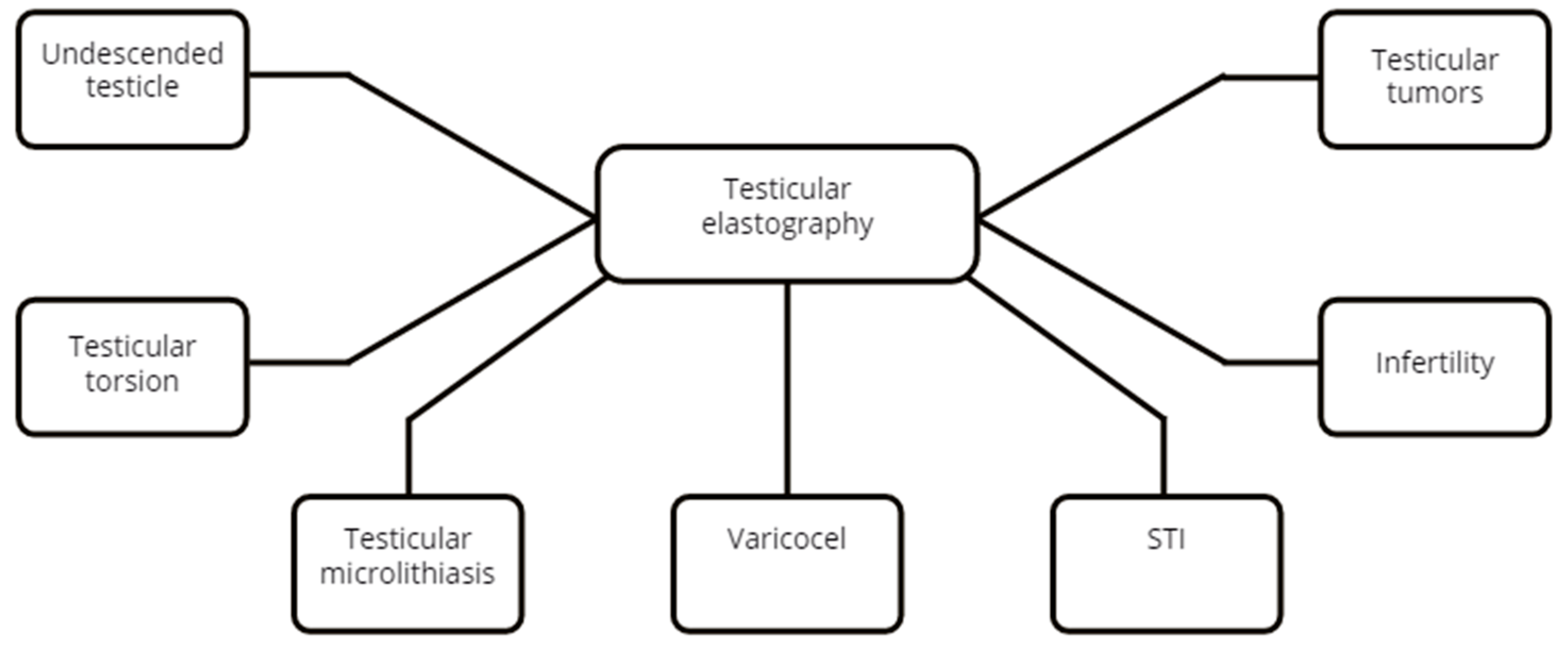
3.2. Testicle
3.2.1. Testicle Normal Values
pSWE
2D SWE
2D SWE
3.2.2. Undescended Testicle(UDT)
pSWE
2D SWE
RTE
3.2.3. Testicular Torsion
2D SWE
3.2.4. Testicular Microlithiasis
pSWE
3.2.5. Varicocele
pSWE
2D SWE
RTE
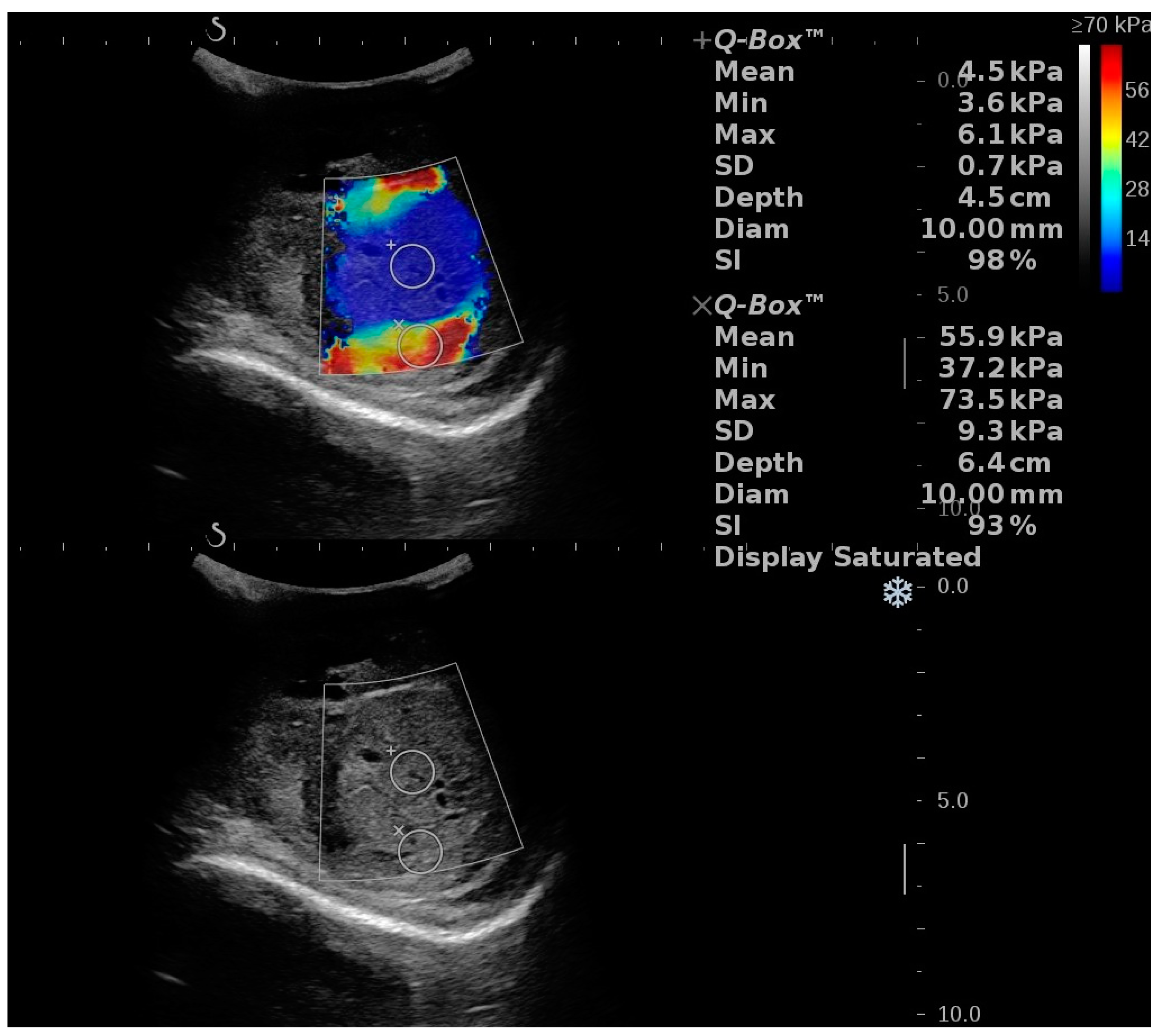
3.2.6. Segmental Testicular Infarction (STI)
3.2.7. Infertility
pSWE
2D SWE
RTE
3.2.8. Testicular Tumors
2D SWE and RTE
3.3. Penis
3.3.1. Normal Values
3.3.2. Erectile Dysfunction (ED)
2D SWE
RTE
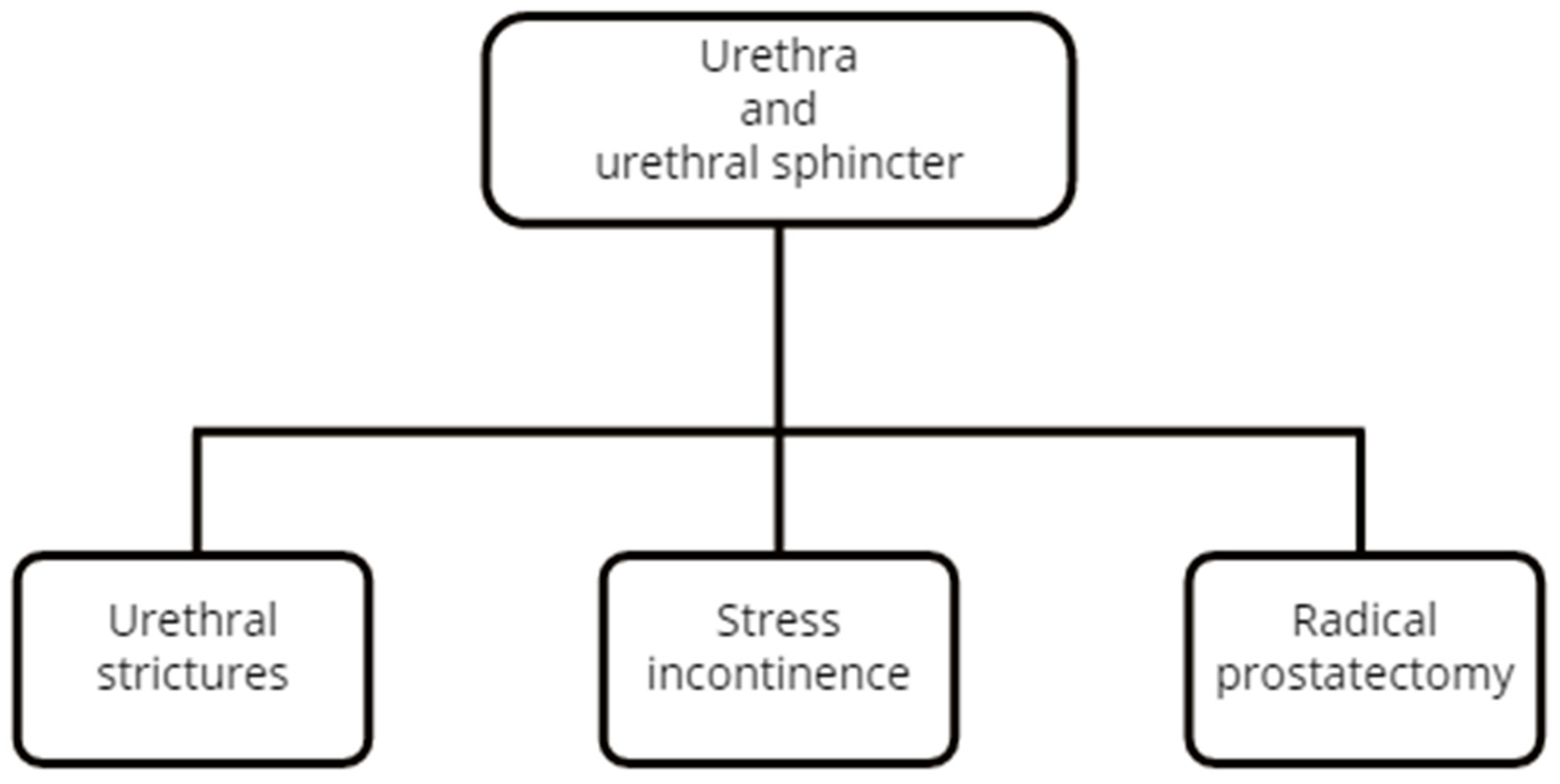
3.3.3. Peyronie’s Disease
3.4. Urethra
3.4.1. 2D SWE
3.4.2. RTE
3.5. Urethral Sphincter
3.5.1. 2D SWE Using the Transperineal Approach
3.5.2. 2D SWE Using the Transrectal Approach
3.6. Urinary Bladder
3.6.1. Bladder Neck Evaluation
pSWE
2D SWE
2D SWE vs. pSWE
RTE
3.6.2. Bladder Dysfunctions
2D SWE
pSWE
3.6.3. Cystitis
3.6.4. Bladder Cancer
pSWE
2D SWE
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 1: Basic Principles and Technology. Ultraschall Der Medizin 2013, 34, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, G.; Carlos, R.; Neal, C.; Dwamena, B. Ultrasonographic differentiation of malignant from benign breast lesions: A meta-analytic comparison of elasticity and BIRADS scoring. Breast Cancer Res. Treat. 2011, 133, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Talwalkar, J.; Kurtz, D.; Schoenleber, S.; West, C.; Montori, V. Ultrasound-Based Transient Elastography for the Detection of Hepatic Fibrosis: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2007, 5, 1214–1220. [Google Scholar] [CrossRef]
- Zhou, X.; Rao, J.; Wu, X.; Deng, R.; Ma, Y. Comparison of 2-D Shear Wave Elastography and Point Shear Wave Elastography for Assessing Liver Fibrosis. Ultrasound Med. Biol. 2021, 47, 408–427. [Google Scholar] [CrossRef]
- Dudea, S.; Jid, C.B. Ultrasound elastography in thyroid disease. Med. Ultrason. 2015, 17, 74. [Google Scholar] [CrossRef]
- Dudea-Simon, M.; Dudea, S.; Ciortea, R.; Malutan, A.; Mihu, D. Elastography of the uterine cervix in gynecology: Normal appearance, cervical intraepithelial neoplasia and cancer. A systematic review. Med. Ultrason. 2021, 23, 74. [Google Scholar] [CrossRef]
- Sang, L.; Wang, X.; Xu, D.; Cai, Y. Accuracy of shear wave elastography for the diagnosis of prostate cancer: A meta-analysis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, X.; Zhan, W.; Zhu, F.; Li, M.; Huang, J.; Li, Y.; Xue, L.; Liu, L. Real-Time Elastography in the Diagnosis of Patients Suspected of Having Prostate Cancer: A Meta-analysis. Ultrasound Med. Biol. 2014, 40, 1400–1407. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, H.; Kim, P.; Suh, C.; Yoon, H. Technical Performance of Acoustic Radiation Force Impulse Imaging for Measuring Renal Parenchymal Stiffness: A Systematic Review and Meta-Analysis. J. Ultrasound Med. 2021, 40, 2639–2653. [Google Scholar] [CrossRef]
- Palabiyik, F.; Inci, E.; Turkay, R.; Bas, D. Evaluation of Liver, Kidney, and Spleen Elasticity in Healthy Newborns and Infants Using Shear Wave Elastography. J. Ultrasound Med. 2017, 36, 2039–2045. [Google Scholar] [CrossRef]
- Grass, L.; Szekely, N.; Alrajab, A.; Bui-Ta, T.; Hoffmann, G.; Wühl, E.; Schenk, J. Point shear wave elastography (pSWE) using Acoustic Radiation Force Impulse (ARFI) imaging: A feasibility study and norm values for renal parenchymal stiffness in healthy children and adolescents. Med. Ultrason. 2017, 19, 366. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Panta, O.; Khanal, U.; Ghimire, R. Renal Cortical Elastography: Normal Values and Variations. J. Med. Ultrasound 2017, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Bob, F.; Sporea, I.; Sirli, I.; Petrica, L.; Schiller, A. Factors that influence kidney shear wave speed assessed by acoustic radiation force impulse elastography in patients without kidney pathology. Ultrasound Med. Biol. 2015, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Joo, D.; Han, W.; Jeong, H.; Oh, M.; Kim, Y.; Oh, Y.T. Renal tissue elasticity by acoustic radiation force impulse. Medicine 2021, 100, e23561. [Google Scholar] [CrossRef]
- Grenier, N.; Gennisson, J.L.; Cornelis, F.; Le Bras, Y.; Couzi, L. Renal ultrasound elastography. Diagn. Interv. Imaging 2013, 94, 545–550. [Google Scholar] [CrossRef]
- Sandhu, R.; Shin, J.; Wehrli, N.; Gao, J. Establishing normal values for shear-Wave elastography of the renal cortex in healthy adults. J. Med. Ultrasound 2018, 26, 81. [Google Scholar] [CrossRef]
- Arda, K.; Ciledag, N.; Aktas, E.; Arıbas, B.; Köse, K. Quantitative Assessment of Normal Soft-Tissue Elasticity Using Shear-Wave Ultrasound Elastography. Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef]
- Cai, Y.; Li, F.; Li, Z.; Du, L.; Wu, R. Diagnostic Performance of Ultrasound Shear Wave Elastography in Solid Small (≤4 cm) Renal Parenchymal Masses. Ultrasound Med. Biol. 2019, 45, 2328–2337. [Google Scholar] [CrossRef]
- Guo, L.H.; Liu, B.J.; Xu, H.X.; Liu, C.; Sun, L.P.; Zhang, Y.F.; Xu, J.M.; Wu, J.; Xu, X.H. Acoustic radiation force impulse elastography in differentiating renal solid masses: A preliminary experience. Int. J. Clin Exp. Pathol. 2014, 7, 7469–7476. [Google Scholar]
- Sagreiya, H.; Akhbardeh, A.; Li, D.; Sigrist, R.; Chung, B.; Sonn, G.; Tian, L.; Rubin, D.L.; Willmann, J.K. Point Shear Wave Elastography Using Machine Learning to Differentiate Renal Cell Carcinoma and Angiomyolipoma. Ultrasound Med. Biol. 2019, 45, 1944–1954. [Google Scholar] [CrossRef]
- Göya, C.; Daggulli, M.; Hamidi, C.; Yavuz, A.; Hattapoglu, S.; Cetincakmak, M.; Teke, M. The role of quantitative measurement by acoustic radiation force impulse imaging in differentiating benign renal lesions from malignant renal tumours. La Radiol. Med. 2014, 120, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, W.; Bedke, J.; Kruck, S.; Spira, D.; Stenzl, A.; Nikolaou, K.; Horger, M.; Kaufmann, S. Can contrast-enhanced ultrasound and acoustic radiation force impulse imaging characterize CT-indeterminate renal masses? A prospective evaluation with histological confirmation. World J. Urol. 2018, 37, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Yildiz, S.; Turkmen, I.; Sharifov, R.; Uysal, O.; Gucin, Z.; Armagan, A.; Kocakoc, E. Value of Shear Wave Elastography for differentiating benign and malignant renal lesions. Med. Ultrason. 2018, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.; Güven, S.; Keskin, Z.; Özbiner, H.; Kerimoğlu, Ü.; Yeşildağ, A. Strain elastography in the characterization of renal cell carcinoma and angiomyolipoma. Can. Urol. Assoc. J. 2015, 9, 67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onur, M.; Poyraz, A.; Bozgeyik, Z.; Onur, A.; Orhan, I. Utility of Semiquantitative Strain Elastography for Differentiation Between Benign and Malignant Solid Renal Masses. J. Ultrasound Med. 2015, 34, 639–647. [Google Scholar] [CrossRef]
- Tan, S.; Özcan, M.; Tezcan, F.; Balcı, S.; Karaoğlanoğlu, M.; Huddam, B.; Arslan, H. Real-Time Elastography for Distinguishing Angiomyolipoma from Renal Cell Carcinoma: Preliminary Observations. Am. J. Roentgenol. 2013, 200, W369–W375. [Google Scholar] [CrossRef]
- Inci, M.; Kalayci, T.; Tan, S.; Karasu, S.; Albayrak, E.; Cakir, V.; Ocal, F.; Ozkan, F. Diagnostic value of strain elastography for differentiation between renal cell carcinoma and transitional cell carcinoma of kidney. Abdom. Radiol. 2016, 41, 1152–1159. [Google Scholar] [CrossRef]
- Göya, C.; Hamidi, C.; Ece, A.; Okur, M.; Taşdemir, B.; Çetinçakmak, M.; Hattapoğlu, S.; Şahin, C. Acoustic radiation force impulse (ARFI) elastography for detection of renal damage in children. Pediatric Radiol. 2014, 45, 55–61. [Google Scholar] [CrossRef]
- Ucar, A.K.; Cicek, R.; Alis, D.; Akbas, S.; Habibi, H.A.; Arslan, M.; Eral, G.; Suleyman, A.; Caliskan, S.; Adaletli, I. Shear Wave Elastography in the Evaluation of the Kidneys in Pediatric Patients with Unilateral Vesicoureteral Reflux. J. Ultrasound Med. 2018, 38, 379–385. [Google Scholar] [CrossRef]
- Karabulut, B.; Bayram, G.; Oztorun, C.; Ozcift, B.; Tiryaki, T. Can Freehand Elastosonography Be an Alternative to Renal Scintigraphy in Pediatric Vesicoureteral Reflux Cases? Eur. J. Pediatric Surg. 2018, 29, 470–474. [Google Scholar] [CrossRef]
- Sohn, B.; Kim, M.; Han, S.; Im, Y.; Lee, M. Shear wave velocity measurements using acoustic radiation force impulse in young children with normal kidneys versus hydronephrotic kidneys. Ultrasonography 2014, 33, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Reddy, C.; Sai, V.; Shah, U. Utility of ultrasound elastography in postoperative follow-up of children with unilateral ureteropelvic junction obstruction. Indian J. Urol. 2020, 36, 101. [Google Scholar] [CrossRef] [PubMed]
- Trottmann, M.; Marcon, J.; D’Anastasi, M.; Bruce, M.; Stief, C.; Reiser, M.; Bucner, A.; Clevert, D.A. Shear-wave elastography of the testis in the healthy man—Determination of standard values. Clin. Hemorheol. Microcirc. 2016, 62, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Møller, H.; Osther, P.; Vedsted, P.; Holst, R.; Rafaelsen, S. Comparison of Tissue Stiffness Using Shear Wave Elastography in Men with Normal Testicular Tissue, Testicular Microlithiasis and Testicular Cancer. Ultrasound Int. Open 2017, 3, E150–E155. [Google Scholar] [CrossRef]
- Chen, F.; Mao, L.; Zhou, X.; Qiao, X.; Luo, Y.; Zhu, Z.; Chen, R.; Qiu, S.; Zeng, B. Application of Shear Wave Elastography to Evaluate the Stiffness of Normal Testes and Inflammatory Epididymal Tail Masses. Ultrasound Q. 2020, 37, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Marcon, J.; Trottmann, M.; Rübenthaler, J.; D’Anastasi, M.; Stief, C.; Reiser, M.; Clevert, D.A. Three-dimensional vs. two-dimensional shear-wave elastography of the testes—Preliminary study on a healthy collective. Clin. Hemorheol. Microcirc. 2017, 64, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Yoon, H.; Lee, Y.; Kim, M.; Han, S.; Roh, Y.; Lee, M. Normal Changes and Ranges of Pediatric Testicular Volume and Shear Wave Elasticity. Ultrasound Med. Biol. 2019, 45, 1638–1643. [Google Scholar] [CrossRef]
- Hattapoğlu, S.; Göya, C.; Arslan, S.; Alan, B.; Ekici, F.; Tekbaş, G.; Yildiz, I.; Hamidi, C. Evaluation of postoperative undescended testicles using point shear wave elastography in children. Ultrasonics 2016, 72, 191–194. [Google Scholar] [CrossRef]
- Ucar, A.; Alis, D.; Samanci, C.; Aslan, M.; Habibi, H.; Dikici, A.; Namdar, Y.; Gultekin, M.H.; Onal, B.; Adaletli, I. A preliminary study of shear wave elastography for the evaluation of unilateral palpable undescended testes. Eur. J. Radiol. 2017, 86, 248–251. [Google Scholar] [CrossRef]
- Shin, H.; Lee, Y.; Yoon, H.; Kim, M.; Han, S.; Kim, H.; Lee, J.; Lee, M. Testicular volume and elasticity changes in young children with undescended testes. Med. Ultrason. 2017, 19, 380. [Google Scholar] [CrossRef]
- Turna, O.; Alis, D. A comparative study of shear wave elastography in the evaluation of undescended and retractile testes in a pediatric population. J. Med. Ultrason. 2019, 46, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, G.; Wieczorek, A. Multiparametric ultrasonography of the pediatric scrotum and in boys with undescended testes. J. Ultrason. 2013, 13, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, M.; Xiang, F.; Song, Y.; Yu, C.; Zhang, Y.; Ramdhany, S.; Wang, J. Utility of Real-Time Shear Wave Elastography in the Assessment of Testicular Torsion. PLoS ONE 2015, 10, e0138523. [Google Scholar]
- Xue, E.; Yu, Y.; Lin, L.; Li, Z.; Su, H. Application value of real-time shear wave elastography in differential diagnosis of testicular torsion. Med. Ultrason. 2020, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Osther, P.; Rafaelsen, S. Testicular microlithiasis and preliminary experience of acoustic radiation force impulse imaging. Acta Radiol. Open 2016, 5, 205846011665868. [Google Scholar] [CrossRef]
- Aslan, S.; Bilgici, M.C.; Saglam, D.; Ozturk, M. The role of ARFI elastography to evaluate microstructural changes of patients with testicular microlithiasis. Acta Radiol. 2018, 59, 1517–1522. [Google Scholar] [CrossRef]
- Dede, O.; Teke, M.; Daggulli, M.; Utangaç, M.; Baş, O.; Penbegül, N. Elastography to assess the effect of varicoceles on testes: A prospective controlled study. Andrologia 2015, 48, 257–261. [Google Scholar] [CrossRef]
- Jedrzejewski, G.; Osemlak, P.; Wieczorek, A.; Nachulewicz, P. Prognostic values of shear wave elastography in adolescent boys with varicocele. J. Pediatric Urol. 2019, 15, 223.e1–223.e5. [Google Scholar] [CrossRef]
- Rocher, L.; Criton, A.; Gennisson, J.; Izard, V.; Ferlicot, S.; Tanter, M.; Benoit, G.; Belin, M.F.; Correas, J.M. Testicular Shear Wave Elastography in Normal and Infertile Men: A Prospective Study on 601 Patients. Ultrasound Med. Biol. 2017, 43, 782–789. [Google Scholar] [CrossRef]
- Ryu, Y.; Choi, Y.; Kim, J.; Cheon, J.; Kim, W.; Kim, I.; Park, J.E.; Im, Y.J.; Park, K. A preliminary study of shear-wave elastography for the evaluation of varicoceles in adolescents and young adults. Ultrasonography 2021, 41, 131. [Google Scholar] [CrossRef]
- Abdelwahab, K.; Eliwa, A.; Seleem, M.; El Galaly, H.; Ragab, A.; Desoky, E.; Naguib, M.; Ali, M.M.; Saber, S.; Kamel, H. Role of Preoperative Testicular Shear Wave Elastography in Predicting Improvement of Semen Parameters after Varicocelectomy for Male Patients with Primary Infertility. Urology 2017, 107, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Turna, O.; Aybar, M. Testicular stiffness in varicocele: Evaluation with shear wave elastography. Ultrasonography 2020, 39, 350–355. [Google Scholar] [CrossRef]
- Erdogan, H.; Durmaz, M.; Arslan, S.; Durmaz, F.G.; Cebeci, H.; Ergun, O.; Karaagac, S.S. Shear Wave Elastography Evaluation of Testes in Patients with Varicocele. Ultrasound Q. 2019, 36, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Camoglio, F.; Bruno, C.; Peretti, M.; Bianchi, F.; Bucci, A.; Scirè, G.; Patane, S.; Zampieri, N. The Role of Sonoelastography in the Evaluation of Testes with Varicocele. Urology 2017, 100, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.; Samir, M.; Blgozah, S. Evaluation of Normal and Varicocele-Bearing Testes Using Real-time Strain Elastography. J. Ultrasound Med. 2018, 38, 621–627. [Google Scholar] [CrossRef]
- Kucukdurmaz, F.; Sarica, M.; Emre, O.; Baykara, M.; Kizildag, B.; Resim, S. Evaluation of the diagnostic efficacy of strain elastography in infertile population with normal and abnormal semen parameters. Turk. J. Urol. 2017, 43, 261–267. [Google Scholar] [CrossRef]
- Ernst, S.; Saar, M.; Brenneis, H.; Kubale, R.; Ueberdiek, S.; Ohlmann, C.; Stoeckle, M.; Heinzelbecker, J. Segmental Testicular Infarction: Case Series and Literature Review of a Rare Diagnosis in Men with Acute Testicular Pain. Urol. Int. 2017, 101, 114–116. [Google Scholar] [CrossRef]
- Kantarci, F.; Olgun, D.C.; Mihmanli, I. Shear-Wave Elastography of Segmental Infarction of the Testis. Korean J. Radiol. 2012, 13, 820. [Google Scholar] [CrossRef]
- Patel, K.; Huang, D.; Sidhu, P. Metachronous bilateral segmental testicular infarction: Multi-parametric ultrasound imaging with grey-scale ultrasound, Doppler ultrasound, contrast-enhanced ultrasound (CEUS) and real-time tissue elastography (RTE). J. Ultrasound 2014, 17, 233–238. [Google Scholar] [CrossRef]
- Yavuz, A.; Yokus, A.; Taken, K.; Batur, A.; Ozgokce, M.; Arslan, H. Reliability of testicular stiffness quantification using shear wave elastography in predicting male fertility: A preliminary prospective study. Med. Ultrason. 2018, 20, 141. [Google Scholar] [CrossRef]
- Illiano, E.; Trama, F.; Ruffo, A.; Romeo, G.; Riccardo, F.; Crocetto, F.; Iacono, F.; Costantini, E. Testicular shear wave elastography in oligo-astheno-teratozoospermic individuals: A prospective case–control study. Int. Urol. Nephrol. 2021, 53, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, W.; Gao, Y.; Yang, Z.; Ding, L.; Xie, Y.; Xie, X.Y.; Hu, H.T.; Wang, Z. Preliminary investigation of the diagnostic value of shear wave elastography in evaluating the testicular spermatogenic function in patients with azoospermia. Andrologia 2021, 53, e14039. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, H.; Durmaz, M.; Özbakır, B.; Cebeci, H.; Özkan, D.; Gökmen, İ. Experience of using shear wave elastography in evaluation of testicular stiffness in cases of male infertility. J. Ultrasound 2020, 23, 529–534. [Google Scholar] [CrossRef]
- Abdelaal, A.; El-Azizi, H.; GamalEl Din, S.; Azzazi, O.A.M.; Mohamed, M.S. Evaluation of the potential role of shear wave elastography as a promising predictor of sperm retrieval in non-obstructive azoospermic patients: A prospective study. Andrology 2021, 9, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, J.; Wang, Z.; Li, F. The Value of Sonoelastography Scores and the Strain Ratio in Differential Diagnosis of Azoospermia. J. Urol. 2012, 188, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Huang, D.; Sidhu, P. Elastography of focal testicular lesions: Current concepts and utility. Ultrasonography 2019, 38, 302–310. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, R.; Wu, H.; Wu, L.; Zeng, J.; Xu, J.; Dong, F. Elastography for the differential diagnosis of malignant versus benign testicular lesions: A meta-analysis. Ultrasonography 2021, 40, 465–473. [Google Scholar] [CrossRef]
- Roy, C.; de Marini, P.; Labani, A.; Leyendecker, P.; Ohana, M. Shear-wave elastography of the testicle: Potential role of the stiffness value in various common testicular diseases. Clin. Radiol. 2020, 75, 560.e9–560.e17. [Google Scholar] [CrossRef]
- Marcon, J.; Trottmann, M.; Rûbenthaler, J.; Stief, C.G.; Reiser, M.F.; Clevert, D.A. Shear wave elastographyof the testes in a healthy study collective—Differences in standard values between ARFI and VTIQ techniques. Clin. Hemorheol. Microcirc. 2016, 64, 721–728. [Google Scholar] [CrossRef]
- Dikici, A.S.; Er, M.E.; Alis, D.; Samanci, C.; Ustabasioglu, F.E.; Demirag, C.; Durak, H.; Kantarci, F.; Mihmanli, I. Is there any difference between seminomas and nonseminomatous germ cell tumors on shear wave elastography? A preliminary study. J. Ultrasound Med. 2016, 35, 2575–2580. [Google Scholar] [CrossRef]
- Shin, H.; Kim, M.; Kim, H.; Roh, Y.; Lee, M. Comparison of shear wave velocities on ultrasound elastography between different machines, transducers, and acquisition depths: A phantom study. Eur. Radiol. 2016, 26, 3361–3367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, X.; Gao, F.; Li, F.; Bai, M.; Zhang, H.; Liu, Y.; Du, L.; Xing, J. A new method of measuring the stiffness of corpus cavernosum penis with ShearWave™ Elastography. Br. J. Radiol. 2015, 88, 20140671. [Google Scholar] [CrossRef] [PubMed]
- Inci, E.; Turkay, R.; Nalbant, M.; Yenice, M.; Tugcu, V. The value of shear wave elastography in the quantification of corpus cavernosum penis rigidity and its alteration with age. Eur. J. Radiol. 2017, 89, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Illiano, E.; Trama, F.; Ruffo, A.; Romeo, G.; Riccardo, F.; Iacono, F.; Costantini, E. Shear wave elastography as a new, non-invasive diagnostic modality for the diagnosis of penile elasticity: A prospective multicenter study. Ther. Adv. Urol. 2021, 13, 175628722110079. [Google Scholar] [CrossRef]
- Turkay, R.; Inci, E.; Yenice, M.; Tugcu, V. Shear wave elastography: Can it be a new radiologic approach for the diagnosis of erectile dysfunction? Ultrasound 2017, 25, 150–155. [Google Scholar] [CrossRef]
- Lee, J.; Jung, D.; Lee, S.; Kang, N.; Oh, Y.; Han, K. Stiffness of the Central Corpus Cavernosum on Shear-Wave Elastography Is Inversely Correlated with the Penile Rigidity Score in Patients with Erectile Dysfunction. World J. Mens Health 2021, 39, 123. [Google Scholar] [CrossRef]
- Cui, A.; Xu, L.; Mu, J.; Tong, M.; Peng, C.; Wu, T. The role of shear wave elastography on evaluation of the rigidity changes of corpus cavernosum penis in venogenic erectile dysfunction. Eur. J. Radiol. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, L.; Gao, J.; Zheng, H.; Huang, M.; Zhao, S.; Xie, X.; Zhang, C.; Zhang, X. Evaluation of Arterial Erectile Dysfunction Using Shear Wave Elastography. J. Ultrasound Med. 2020, 40, 1209–1216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Wu, X.; Zhao, S.; Zhang, X. Role of shear wave elastography measured in the flaccid state in predicting arteriogenic erectile dysfunction. Andrologia 2021, 53, e13996. [Google Scholar] [CrossRef]
- Cheng, H.; Niu, Z.; Xin, F.; Yang, L.; Ruan, L. A new method to quantify penile erection hardness: Real-time ultrasonic shear wave elastography. Transl. Androl. Urol. 2020, 9, 1735–1742. [Google Scholar] [CrossRef]
- Altinbas, N.; Hamidi, N. Penile Doppler ultrasonography and elastography evaluation in patients with erectile dysfunction. Pol. J. Radiol. 2018, 83, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, N.; Altinbas, N.; Gokce, M.; Suer, E.; Yagci, C.; Baltaci, S.; Turkolmez, K. Preliminary results of a new tool to evaluate cavernous body fibrosis following radical prostatectomy: Penile elastography. Andrology 2017, 5, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Goldenberg, E.; Pek, H.; Gilbert, B. Penile Sonoelastography for the Localization of a Non-Palpable, Non-Sonographically Visualized Lesion in a Patient with Penile Curvature from Peyronie’s Disease. J. Sex. Med. 2014, 11, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Riversi, V.; Tallis, V.; Trovatelli, S.; Belba, A.; Volterrani, L.; Iacoponi, F.; Ponchietti, R. Realtime-elastosonography of the penis in patients with Peyronie’s disease. Arch. Ital. Urol. Androl. 2012, 84, 174–177. [Google Scholar] [PubMed]
- Chung, P.; Leong, J.; Machado, P.; Wessner, C.; Trabulsi, E.; Halpern, E.; Eisenbrey, J.R.; Fosberg, F. Contrast-Enhanced Ultrasound and Shear Wave Elastography: Novel Methods for the Evaluation of Urethral Stricture Disease. J. Urol. 2022, 207, 152–160. [Google Scholar] [CrossRef]
- Talreja, S.; Tomar, V.; Yadav, S.; Jaipal, U.; Priyadarshi, S.; Agarwal, N.; Vyas, N. Comparison of sonoelastography with sonourethrography and retrograde urethrography in the evaluation of male anterior urethral strictures. Turk. J. Urol. 2016, 42, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Talreja, S.; Yadav, S.S.; Tomar, V.; Agarwal, N.; Jaipal, U.; Priyadarshi, S. ‘Real-time sonoelastography’ in anterior urethral strictures: A novel technique for assessment of spongiofibrosis. Cent. Eur. J. Urol. 2016, 69, 417. [Google Scholar]
- Aljuraifani, R.; Stafford, R.; Hug, F.; Hodges, P. Female striated urogenital sphincter contraction measured by shear wave elastography during pelvic floor muscle activation: Proof of concept and validation. Neurourol. Urodyn. 2017, 37, 206–212. [Google Scholar] [CrossRef]
- Stafford, R.; Aljuraifani, R.; Hug, F.; Hodges, P. Application of shear-wave elastography to estimate the stiffness of the male striated urethral sphincter during voluntary contractions. BJU Int. 2016, 119, 619–625. [Google Scholar] [CrossRef]
- Zhao, B.; Wen, L.; Chen, W.; Qing, Z.; Liu, D.; Liu, M. A Preliminary Study on Quantitative Quality Measurements of the Urethral Rhabdosphincter Muscle by Supersonic Shear Wave Imaging in Women with Stress Urinary Incontinence. J. Ultrasound Med. 2020, 39, 1615–1621. [Google Scholar] [CrossRef]
- Tyloch, D.; Tyloch, J.; Adamowicz, J.; Drewa, T. Shear Wave Elastography in the Evaluation of the Urethral Sphincter Complex after Radical Prostatectomy. Ultrasound Med. Biol. 2021, 47, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Su, C.; Jiang, D.; Yu, G. Application of Acoustic Radiation Force Impulse Imaging for Diagnosis of Female Bladder Neck Obstruction. J. Ultrasound Med. 2016, 35, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Ahmed, Y.; Azar, N.; El-Nashar, S.; Hijaz, A.; Mahajan, S. Trans-abdominal ultrasound shear wave elastography for quantitative assessment of female bladder neck elasticity. Int. Urogynecology J. 2016, 28, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Jiang, D.; Su, C.; Wang, X.; Zhao, X.; Yang, S. Value of Real-Time Shear Wave Elastography Versus Acoustic Radiation Force Impulse Imaging in the Diagnosis of Female Bladder Neck Obstruction. J. Ultrasound Med. 2019, 38, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Da, L.; Luo, J.; Li-Xia, L.; Yu, X.; Li-Mei, X.; Wei-Dong, R. Quantitative Assessment of Bladder Neck Compliance by Using Transvaginal Real-Time Elastography of Women. Ultrasound Med. Biol. 2013, 39, 1727–1734. [Google Scholar] [CrossRef]
- Sturm, R.; Yerkes, E.; Nicholas, J.; Snow-Lisy, D.; Saldano, D.D.; Gandor, P.; Halline, C.G.; Rosoklija, I.; Rychlik, K.; Johnson, E.K.; et al. Ultrasound Shear Wave Elastography: A Novel Method to Evaluate Bladder Pressure. J. Urol. 2017, 198, 422–429. [Google Scholar] [CrossRef]
- Do, M.; Kim, K.; Kim, L.; Im, Y.; Choi, Y.; Park, K. Ultrasonographic cystometry for neurogenic bladder using elastography. Neurourol. Urodyn. 2020, 40, 367–375. [Google Scholar] [CrossRef]
- Streur, C.; Smith, E.; Dillman, J.; Kraft, K. Acoustic radiation force imaging (ARFI) in the non-distended bladder does not predict abnormal urodynamic parameters in children. Can. Urol. Assoc. J. 2021, 16, E15. [Google Scholar] [CrossRef]
- Durmaz, M.; Yorulmaz, A.; Durmaz, F.G.; Arslan, S. Utility of 2-Dimensional Shear Wave Elastography for Assessment of the Bladder Wall in Children with Acute Cystitis. J. Ultrasound Med. 2021, 40, 1105–1111. [Google Scholar] [CrossRef]
- Onur, D.; Memik, T.; Mansur, D.; Necmettin, P. Use of Acoustic Radiation Force Impulse Elastography to Discrimination Benign and Malignant Masses for Bladder. Int. J. Radiol. Imaging Technol. 2018, 4, 39. [Google Scholar] [CrossRef]
- Martin, J.; Carballido, E.; Ahmed, A.; Farhan, B.; Dutta, R.; Smith, C.; Youssef, R. Squamous cell carcinoma of the urinary bladder: Systematic review of clinical characteristics and therapeutic approaches. Arab. J. Urol. 2016, 14, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Zhou, A.Y.; Liu, M.W.; Zhang, Y.; Xu, P. Shear Wave Elasticity Differentiation between Low- and High-Grade Bladder Urothelial Carcinoma and Correlation with Collagen Fiber Content. J. Ultrasound Med. 2020, 40, 113–122. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, V.; Dudea, S.M.; Crisan, N.; Stanca, V.D.; Dudea-Simon, M.; Andras, I.; Mihaly, Z.A.; Coman, I. Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded—Review. Diagnostics 2022, 12, 1727. https://doi.org/10.3390/diagnostics12071727
Simon V, Dudea SM, Crisan N, Stanca VD, Dudea-Simon M, Andras I, Mihaly ZA, Coman I. Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded—Review. Diagnostics. 2022; 12(7):1727. https://doi.org/10.3390/diagnostics12071727
Chicago/Turabian StyleSimon, Vasile, Sorin Marian Dudea, Nicolae Crisan, Vasile Dan Stanca, Marina Dudea-Simon, Iulia Andras, Zoltan Attila Mihaly, and Ioan Coman. 2022. "Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded—Review" Diagnostics 12, no. 7: 1727. https://doi.org/10.3390/diagnostics12071727
APA StyleSimon, V., Dudea, S. M., Crisan, N., Stanca, V. D., Dudea-Simon, M., Andras, I., Mihaly, Z. A., & Coman, I. (2022). Elastography in the Urological Practice: Urinary and Male Genital Tract, Prostate Excluded—Review. Diagnostics, 12(7), 1727. https://doi.org/10.3390/diagnostics12071727






