Clinical Application of Diffusion Tensor Imaging for a Brachial Plexus Injury
Abstract
:- FIESTA: This steady-state sequence is not very susceptible to flow artifact, so it provides an excellent signal-to-background contrast for nerve roots and allows the detection of pseudomyelomeningoceles. Its fast acquisition time, <2 min, allows it to be incorporated in a standard acquisition protocol for the spine, or even implemented in emergency departments with an available MRI [1]. It has substituted the previous gold standard, the CT-myelography, which has the disadvantages of contrast media administration in the spinal canal and the use of ionizing radiations (particularly important in the obstetric lesions) [2].
- STIR: Nerves are isointense compared to muscle in T2-weighted sequences, and an hyperintensity can be a consequence of various pathological processes. The presence of a nodular formation on the course of the nerve is indicative of a neuroma, the detection of which MRI has shown a great accuracy of up to 99% [1,3]. The STIR sequence is often preferred over the T2 with fat suppression due to the homogenous fat saturation obtained. Moreover, the depiction of nerve fibers can be further enhanced with the administration of gadolinium, which suppresses the signal generated by the vessels improving the signal-to-background of the nerves [3]. This sequence also allows examining for muscular signal alteration, to properly stage the nerve injury [4].
- DWI/T2-weighted trace: DWI significatively improves the conspicuity of the nerves compared to T1- and T2-weighted sequences, and is the best alternative to DTI, over which it has the advantages of lower acquisition time and limited need of post-processing [5,6]. The most used b values are 600–700 (so that b × ADC = 1) [7]. A lower b-value with a reduced number of excitations can be added to the sequence, to calculate the ADC map, particularly useful in oncological patients. T2-weighted trace is the isotropic DWI resulting from the DTI post-processing, today a task often performed automatically by the machine’s console, so every study comprising a DTI sequence has isotropic DWI images available [8].
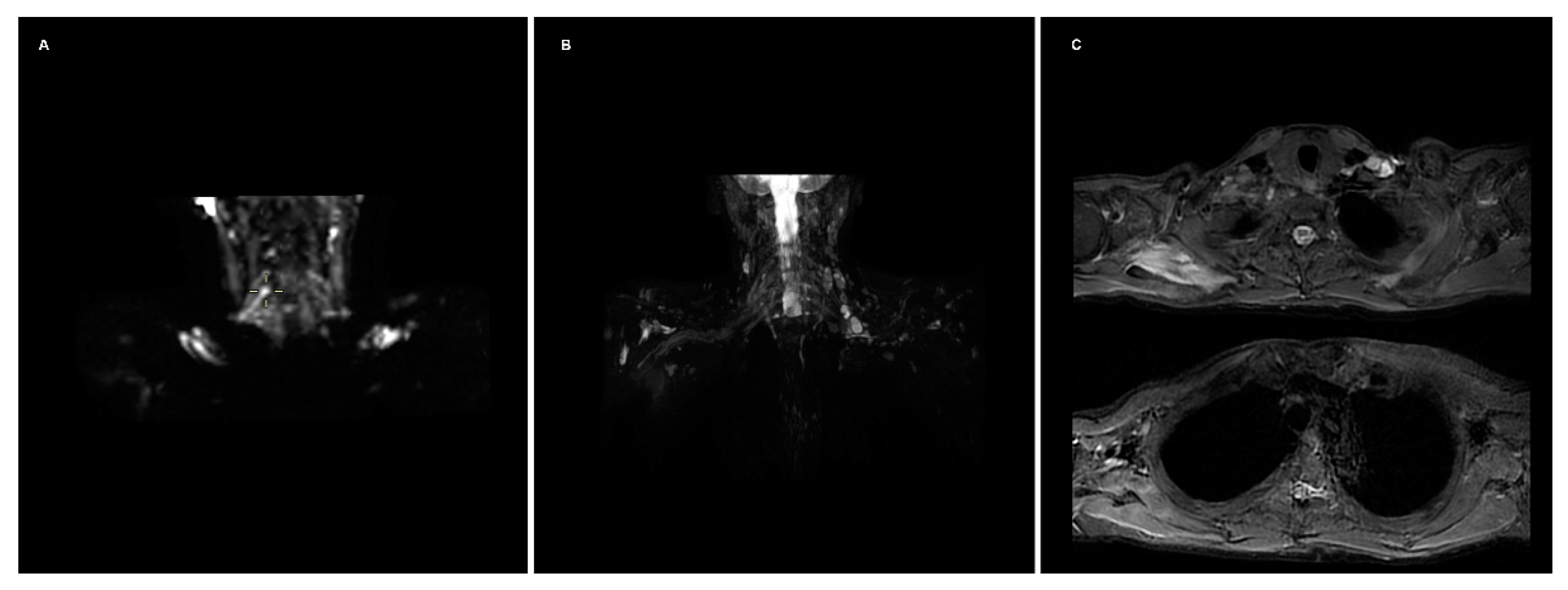
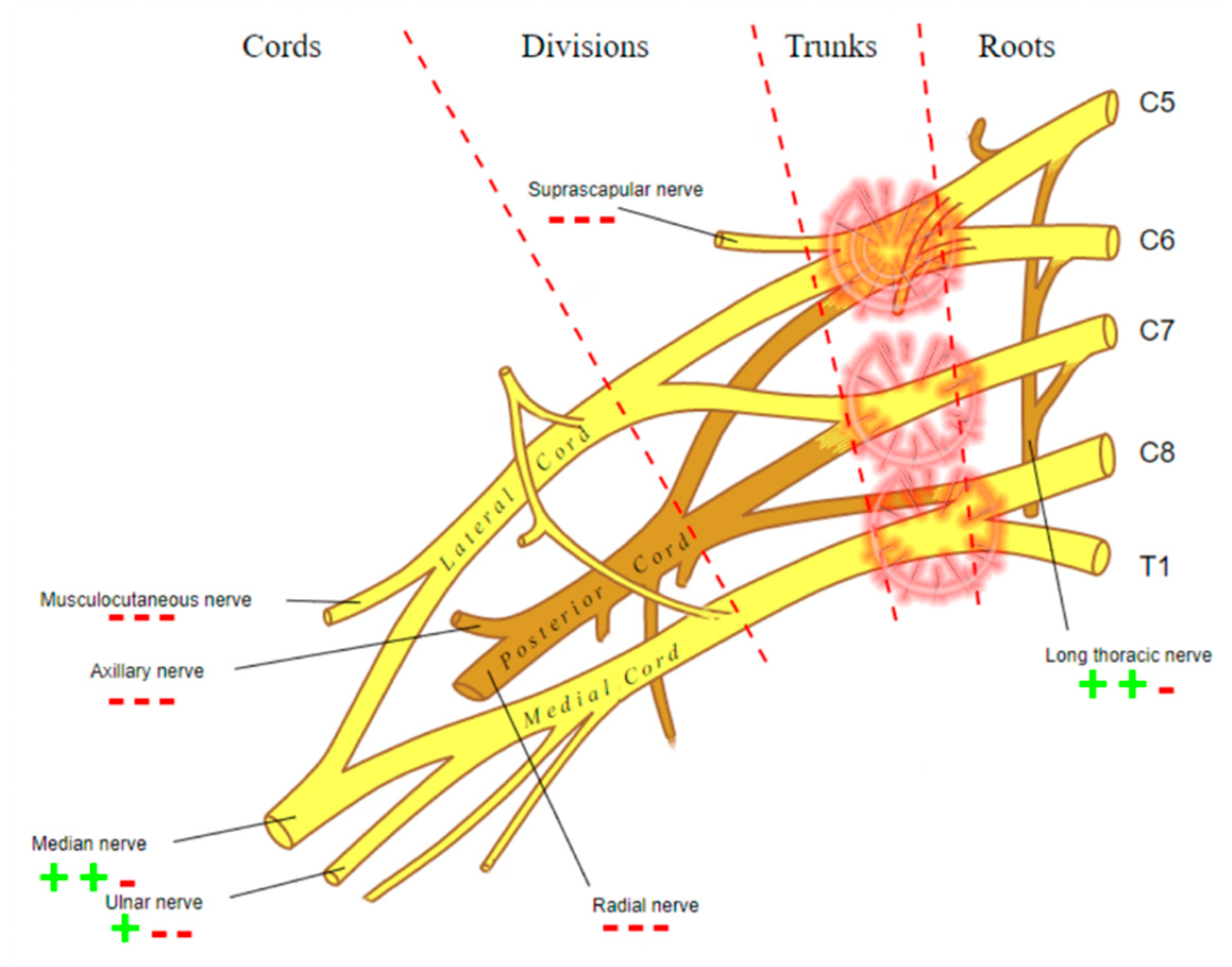
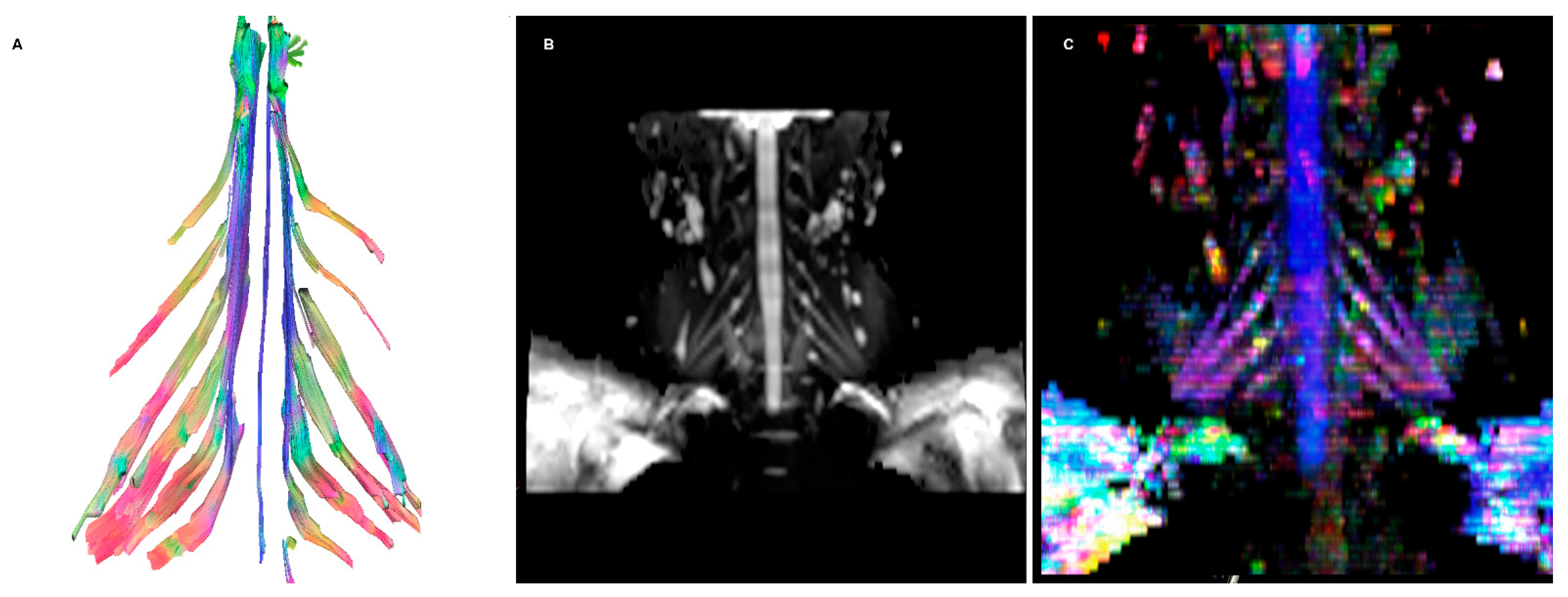
- DTI: Pioneered on the peripheral nervous system in the last decade, the DTI sequences provide the data to perform the nerve fiber tractography, clearly depicting the anatomy of the nerves and allowing to calculate novel in vivo biomarkers, such as fractional and more recently quantitative anisotropy [9,10]. Quantitative anisotropy is less susceptible to edema surrounding the fibers, so a decrease in fractional anisotropy alone should be regarded as edema, while a reduction in quantitative anisotropy is necessary to confirm a structural change [11]. In recent years, vendors have provided radiologists with proprietary applications to perform in-line post-processing, even if advanced studies are possible only on third-party software. Distortions caused by field inhomogeneity can be addressed with post-processing correction available in most software, with the optional addition of an inversed-encoding b0 sequence (“blip-in-blip-out” method) [12]. Manufacturers are addressing this topic by developing coils with a dedicated geometry [3]. A detailed visualization of the nerve fibers is extremely helpful in surgical planning, giving more information when choosing between nerve graft or a nerve transfer [13]. In the last few years, the multishell technique has emerged, and it consists of acquiring at least two DTI sequences with different b-values, increasing the accuracy of the tractography and reducing the in-scanner motion artifacts [14]. The multishell technique is being tested on the central nervous system, and very sparse applications on peripheral nerves have been published [15,16]. In this paper, the authors present a first attempt of multishell acquisition of the brachial plexus performed on a healthy volunteer (Figure 4). Diffusion signal in vivo originates from water represented in different structures, not only neurons but free water and glial cells as well. Conventional DTI-based fiber tracking results in a hampered representation of the tracks when they run in proximity to other water molecules with such different orientation modules, thus not matching the prediction of the Stejskal–Tanner equation:S = signal; b = b-value; D = Diffusion constant.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gad, D.M.; Hussein, M.T.; Omar, N.N.M.; Kotb, M.M.; Abdel-Tawab, M.; Yousef, H.A.Z. Role of MRI in the Diagnosis of Adult Traumatic and Obstetric Brachial Plexus Injury Compared to Intraoperative Findings. Egypt. J. Radiol. Nucl. Med. 2020, 51, 195. [Google Scholar] [CrossRef]
- Nagano, A.; Ochiai, N.; Sugioka, H.; Hara, T.; Tsuyama, N. Usefulness of Myelography in Brachial Plexus Injuries. J. Hand Surg. Edinb. Scotl. 1989, 14, 59–64. [Google Scholar] [CrossRef]
- Sneag, D.B.; Queler, S. Technological Advancements in Magnetic Resonance Neurography. Curr. Neurol. Neurosci. Rep. 2019, 19, 75. [Google Scholar] [CrossRef]
- Mitchell, C.H.; Brushart, T.M.; Ahlawat, S.; Belzberg, A.J.; Carrino, J.A.; Fayad, L.M. MRI of Sports-Related Peripheral Nerve Injuries. Am. J. Roentgenol. 2014, 203, 1075–1084. [Google Scholar] [CrossRef]
- Andreou, A.; Sohaib, A.; Collins, D.J.; Takahara, T.; Kwee, T.C.; Leach, M.O.; MacVicar, D.A.; Koh, D.-M. Diffusion-Weighted MR Neurography for the Assessment of Brachial Plexopathy in Oncological Practice. Cancer Imaging 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahara, T.; Hendrikse, J.; Yamashita, T.; Mali, W.P.T.M.; Kwee, T.C.; Imai, Y.; Luijten, P.R. Diffusion-Weighted MR Neurography of the Brachial Plexus: Feasibility Study. Radiology 2008, 249, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, P.B.; Monahan, W.G. Selection of the Optimum b Factor for Diffusion-Weighted Magnetic Resonance Imaging Assessment of Ischemic Stroke. Magn. Reson. Med. 2004, 51, 996–1001. [Google Scholar] [CrossRef]
- Chou, M.-C.; Tzeng, W.-S.; Chung, H.-W.; Wang, C.-Y.; Liu, H.-S.; Juan, C.-J.; Lo, C.-P.; Hsueh, C.-J.; Chen, C.-Y. T2-Enhanced Tensor Diffusion Trace-Weighted Image in the Detection of Hyper-Acute Cerebral Infarction: Comparison with Isotropic Diffusion-Weighted Image. Eur. J. Radiol. 2010, 74, e89–e94. [Google Scholar] [CrossRef]
- Gasparotti, R.; Lodoli, G.; Meoded, A.; Carletti, F.; Garozzo, D.; Ferraresi, S. Feasibility of Diffusion Tensor Tractography of Brachial Plexus Injuries at 1.5 T. Investig. Radiol. 2013, 48, 104–112. [Google Scholar] [CrossRef]
- Gasparotti, R. Gasparotti New Techniques in Spinal Imaging. Neuroradiology 2011, 53, 195–197. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Lu, T.; Qiu, B.; Tang, Y.; Ou, S.; Tie, X.; Sun, C.; Xu, K.; Wang, Y. Differences Between Generalized Q-Sampling Imaging and Diffusion Tensor Imaging in the Preoperative Visualization of the Nerve Fiber Tracts Within Peritumoral Edema in Brain. Neurosurgery 2013, 73, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Gallichan, D.; Andersson, J.L.R.; Jenkinson, M.; Robson, M.D.; Miller, K.L. Reducing Distortions in Diffusion-Weighted Echo Planar Imaging with a Dual-Echo Blip-Reversed Sequence. Magn. Reson. Med. 2010, 64, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Silbermann-Hoffman, O.; Teboul, F. Post-Traumatic Brachial Plexus MRI in Practice. Diagn. Interv. Imaging 2013, 94, 925–943. [Google Scholar] [CrossRef] [Green Version]
- Castellaro, M.; Moretto, M.; Baro, V.; Brigadoi, S.; Zanoletti, E.; Anglani, M.; Denaro, L.; Dell’Acqua, R.; Landi, A.; Causin, F.; et al. Multishell Diffusion MRI-Based Tractography of the Facial Nerve in Vestibular Schwannoma. Am. J. Neuroradiol. 2020, 41, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Sneag, D.B.; Zochowski, K.C.; Tan, E.T.; Queler, S.C.; Burge, A.; Endo, Y.; Lin, B.; Fung, M.; Shin, J. Denoising of Diffusion MRI Improves Peripheral Nerve Conspicuity and Reproducibility. J. Magn. Reson. Imaging 2020, 51, 1128–1137. [Google Scholar] [CrossRef]
- Nemmi, F.; Levardon, M.; Péran, P. Brain-age Estimation Accuracy Is Significantly Increased Using Multishell Free-water Reconstruction. Hum. Brain Mapp. 2022, 43, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Hrabe, J.; Kaur, G.; Guilfoyle, D.N. Principles and Limitations of NMR Diffusion Measurements. J. Med. Phys. Assoc. Med. Phys. India 2007, 32, 34–42. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [Green Version]
- Yeh, F.-C.; Wedeen, V.J.; Tseng, W.-Y.I. Generalized Q-Sampling Imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef]
- Henssen, D.J.H.A.; Weber, R.C.; de Boef, J.; Mollink, J.; Kozicz, T.; Kurt, E.; van Cappellen van Walsum, A.-M. Post-Mortem 11.7 Tesla Magnetic Resonance Imaging vs. Polarized Light Imaging Microscopy to Measure the Angle and Orientation of Dorsal Root Afferents in the Human Cervical Dorsal Root Entry Zone. Front. Neuroanat. 2019, 13, 66. [Google Scholar] [CrossRef]
- Szaro, P.; McGrath, A.; Ciszek, B.; Geijer, M. Magnetic Resonance Imaging of the Brachial Plexus. Part 1: Anatomical Considerations, Magnetic Resonance Techniques, and Non-Traumatic Lesions. Eur. J. Radiol. Open 2021, 9, 100392. [Google Scholar] [CrossRef] [PubMed]
- Koide, K.; Sugiyama, A.; Yokota, H.; Mukai, H.; Wang, J.; Nakamura, K.; Misawa, S.; Ito, S.; Kuwabara, S. Nerve Hypertrophy and Altered Diffusion in Anti-Myelin-Associated Glycoprotein Neuropathy Detected by Brachial Plexus Magnetic Resonance Neurography. Eur. Neurol. 2022, 85, 95–103. [Google Scholar] [CrossRef] [PubMed]

| Sequence | FOV | Voxel Size (mm) | TE/TR (ms) | b Value (s/mm2) | Acquisition Time (mm:ss) |
|---|---|---|---|---|---|
| Fiesta | 18 | 0.6 × 0.7 × 2 | 1.5/5.6 | 2:58 | |
| 3D STIR | 42 | 1.5 × 1.5 × 1.5 | 120/2800 | 4:19 | |
| DWI | 40 | 4.0 × 4.2 × 3.6 | Min/5099 | 625 | 3:41 |
| DTI | 36 | 3.6 × 3.6 × 3.6 | Min/7792 | 800 (30 dir.) | 4:19 |
| Multishell DTI | 36 | 3.6 × 3.6 × 3.6 | Min/7792 | 300–600–1000 | 14:51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vara, G.; Tuzzato, G.; Bianchi, G.; Miceli, M.; Spinardi, L.; Golfieri, R.; Rinaldi, R.; Facchini, G. Clinical Application of Diffusion Tensor Imaging for a Brachial Plexus Injury. Diagnostics 2022, 12, 1687. https://doi.org/10.3390/diagnostics12071687
Vara G, Tuzzato G, Bianchi G, Miceli M, Spinardi L, Golfieri R, Rinaldi R, Facchini G. Clinical Application of Diffusion Tensor Imaging for a Brachial Plexus Injury. Diagnostics. 2022; 12(7):1687. https://doi.org/10.3390/diagnostics12071687
Chicago/Turabian StyleVara, Giulio, Gianmarco Tuzzato, Giuseppe Bianchi, Marco Miceli, Luca Spinardi, Rita Golfieri, Raffaella Rinaldi, and Giancarlo Facchini. 2022. "Clinical Application of Diffusion Tensor Imaging for a Brachial Plexus Injury" Diagnostics 12, no. 7: 1687. https://doi.org/10.3390/diagnostics12071687
APA StyleVara, G., Tuzzato, G., Bianchi, G., Miceli, M., Spinardi, L., Golfieri, R., Rinaldi, R., & Facchini, G. (2022). Clinical Application of Diffusion Tensor Imaging for a Brachial Plexus Injury. Diagnostics, 12(7), 1687. https://doi.org/10.3390/diagnostics12071687








