Calculation of Aortic VAlve and LVOT Areas by a Modified Continuity Equation Using Different Echocardiography Methods: The CAVALIER Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Echocardiography
2.3. CT
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sattin, M.; Burhani, Z.; Jaidka, A.; Millington, S.; Arntfield, R. Stroke Volume Determination by Echocardiography. Chest 2022, 161, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Mercado, P.; Maizel, J.; Beyls, C.; Titeca-Beauport, D.; Joris, M.; Kontar, L.; Riviere, A.; Bonef, O.; Soupison, T.; Tribouilloy, C.; et al. Transthoracic echocardiography: An accurate and precise method for estimating cardiac output in the critically ill patient. Crit. Care 2017, 21, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinh, V.A.; Ko, H.S.; Rao, R.; Bansal, R.C.; Smith, D.D.; Kim, T.E.; Nguyen, H.B. Measuring cardiac index with a focused cardiac ultrasound examination in the ED. Am. J. Emerg. Med. 2012, 30, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.F.; Kuo, L.C.; Nelson, J.G.; Limacher, M.C.; Quinones, M.A. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: Clinical validation of two new methods using the apical window. Circulation 1984, 70, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Farmer, K.L.; Soto, J.G.; Nelson, J.G.; Quinones, M.A. Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation 1986, 73, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Saitoh, T.; Shiota, M.; Izumo, M.; Gurudevan, S.V.; Tolstrup, K.; Siegel, R.J.; Shiota, T. Comparison of left ventricular outflow geometry and aortic valve area in patients with aortic stenosis by 2-dimensional versus 3-dimensional echocardiography. Am. J. Cardiol. 2012, 109, 1626–1631. [Google Scholar] [CrossRef] [Green Version]
- Doddamani, S.; Bello, R.; Friedman, M.A.; Banerjee, A.; Bowers, J.H., Jr.; Kim, B.; Vennalaganti, P.R.; Ostfeld, R.J.; Gordon, G.M.; Malhotra, D.; et al. Demonstration of left ventricular outflow tract eccentricity by real time 3D echocardiography: Implications for the determination of aortic valve area. Echocardiography 2007, 24, 860–866. [Google Scholar] [CrossRef]
- Silva, C.D.; Pedro, F.; Deister, L.; Sahlen, A.; Manouras, A.; Shahgaldi, K. Two-dimensional color Doppler echocardiography for left ventricular stroke volume assessment: A comparison study with three-dimensional echocardiography. Echocardiography 2012, 29, 766–772. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Yamamoto, H.; Horiguchi, J.; Kunita, E.; Okada, T.; Yamazato, R.; Hidaka, T.; Kihara, Y. Underestimation of aortic valve area in calcified aortic valve disease: Effects of left ventricular outflow tract ellipticity. Int. J. Cardiol. 2012, 157, 347–353. [Google Scholar] [CrossRef]
- Bunting, K.V.; Steeds, R.P.; Slater, L.T.; Rogers, J.K.; Gkoutos, G.V.; Kotecha, D. A Practical Guide to Assess the Reproducibility of Echocardiographic Measurements. J. Am. Soc. Echocardiogr. 2019, 32, 1505–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaspar, T.; Adawi, S.; Sachner, R.; Asmer, I.; Ganaeem, M.; Rubinshtein, R.; Shiran, A. Three-dimensional imaging of the left ventricular outflow tract: Impact on aortic valve area estimation by the continuity equation. J. Am. Soc. Echocardiogr. 2012, 25, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.; Guetta, V.; Konen, E.; Goitein, O.; Segev, A.; Raanani, E.; Spiegelstein, D.; Hay, I.; Di Segni, E.; Eldar, M.; et al. Deformation dynamics and mechanical properties of the aortic annulus by 4-dimensional computed tomography: Insights into the functional anatomy of the aortic valve complex and implications for transcatheter aortic valve therapy. J. Am. Coll. Cardiol. 2012, 59, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, A.V.; von Bardeleben, R.S.; Strasser, C.; Mohr-Kahaly, S.; Blankenberg, S.; Espinola-Klein, C.; Munzel, T.F.; Schnabel, R. Direct measurement of left ventricular outflow tract by transthoracic real-time 3D-echocardiography increases accuracy in assessment of aortic valve stenosis. Int. J. Cardiol. 2009, 136, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Buellesfeld, L.; Stortecky, S.; Kalesan, B.; Gloekler, S.; Khattab, A.A.; Nietlispach, F.; Delfine, V.; Huber, C.; Eberle, B.; Meier, B.; et al. Aortic root dimensions among patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2013, 6, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Nieznańska, M.; Zatorska, K.; Stokłosa, P.; Ryś, M.; Duchnowski, P.; Szymański, P.; Hryniewiecki, T.; Michałowska, I. Comparison of the geometry of the left ventricle outflow tract, the aortic root and the ascending aorta in patients with severe tricuspid aortic stenosis versus healthy controls. Int. J. Cardiovasc. Imaging 2020, 36, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Tops, L.F.; Wood, D.A.; Delgado, V.; Schuijf, J.; Mayo, J.R.; Pasupati, S.; Lamers, F.P.; van der Wall, E.E.; Schalij, M.J.; Webb, J.G.; et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 2008, 1, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Norum, I.B.; Edvardsen, T.; Ruddox, V.; Gunther, A.; Dahle, G.; Otterstad, J.E. Three-dimensional versus two-dimensional transthoracic echocardiography for left ventricular outflow tract measurements in severe aortic stenosis. A cross-sectional study using computer tomography and Haegar sizers as reference. Scand. Cardiovasc. J. 2020, 54, 220–226. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Hagendorff, A.; Knebel, F.; Helfen, A.; Knierim, J.; Sinning, C.; Stöbe, S.; Fehske, W.; Ewen, S. Expert consensus document on the assessment of the severity of aortic valve stenosis by echocardiography to provide diagnostic conclusiveness by standardized verifiable documentation. Clin. Res. Cardiol. 2020, 109, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Kratzer, H.; Helmreich, G.; Kuehn, P. Determination of aortic valve area by Doppler echocardiography using the continuity equation: A critical evaluation. Cardiology 1990, 77, 101–111. [Google Scholar] [CrossRef]
- Yuan, T.; Lu, Y.; Bian, C.; Cai, Z. Early Aortic Valve Replacement vs. Conservative Management in Asymptomatic Severe Aortic Stenosis Patients With Preserved Ejection Fraction: A Meta-Analysis. Front. Cardiovasc. Med. 2020, 7, 621149. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Messika-Zeitoun, D.; Ben-Yehuda, O.; Hahn, R.T.; Burwash, I.G.; Van Mieghem, N.M.; Spitzer, E.; Leon, M.B.; Bax, J.; Otto, C.M. Moderate Aortic Stenosis and Heart Failure With Reduced Ejection Fraction: Can Imaging Guide Us to Therapy? JACC Cardiovasc. Imaging 2019, 12, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, E.; Ren, B.; Kroon, H.; van Gils, L.; Manintveld, O.; Daemen, J.; Zijlstra, F.; de Jaegere, P.P.; Geleijnse, M.L.; Van Mieghem, N.M. Moderate Aortic Stenosis and Reduced Left Ventricular Ejection Fraction: Current Evidence and Challenges Ahead. Front. Cardiovasc. Med. 2018, 5, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzer, E.; Van Mieghem, N.M.; Pibarot, P.; Hahn, R.T.; Kodali, S.; Maurer, M.S.; Nazif, T.M.; Rodes-Cabau, J.; Paradis, J.M.; Kappetein, A.P.; et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am. Heart J. 2016, 182, 80–88. [Google Scholar] [CrossRef]
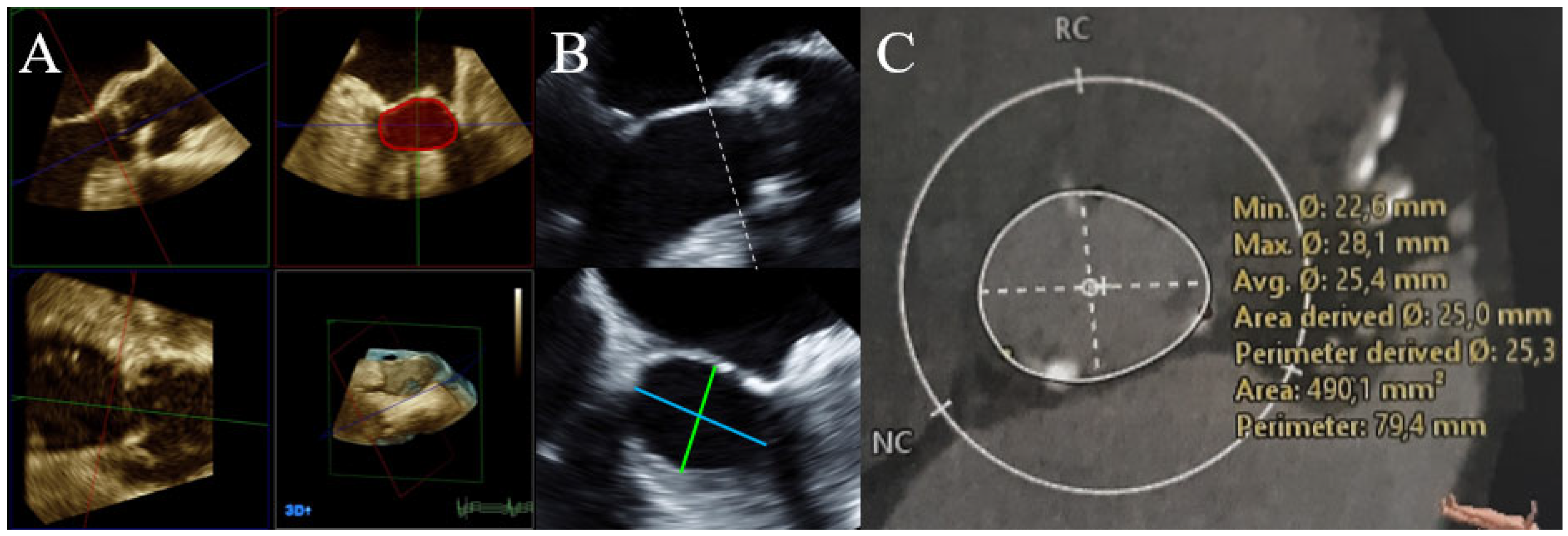

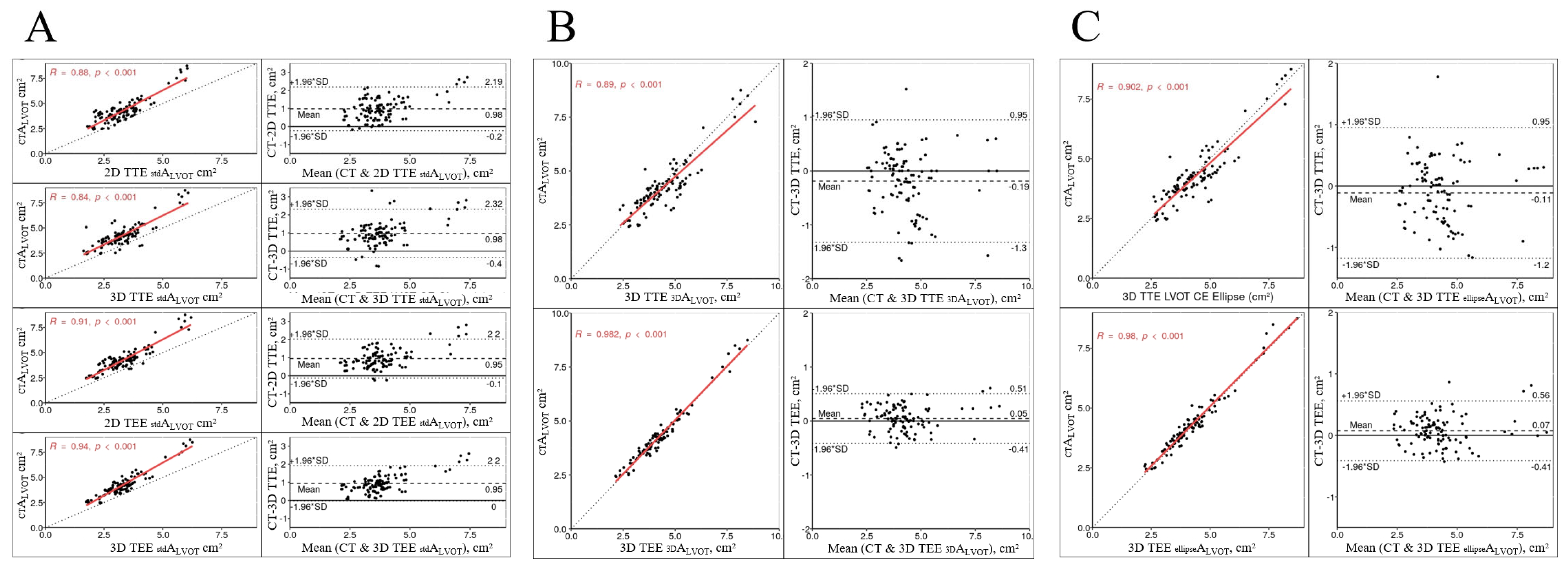
| Characteristic | n = 107 |
|---|---|
| Age, years | 74 [64, 80] |
| Male sex | 59 (55) |
| NYHA class | |
| 0 | 19 (18) |
| I | 17 (16) |
| II | 28 (26) |
| III | 32 (30) |
| IV | 11 (10) |
| CAD | 55 (51) |
| aHT | 93 (87) |
| D.m. II | 42 (39) |
| HLP | 59 (55) |
| Obesity | 48 (45) |
| Characteristic | n = 107 |
|---|---|
| LV EDD, mm | 47 [42, 54] |
| LV ESD, mm | 32 [25, 40] |
| IVSD, mm | 13.0 [10.9, 14.1] |
| LV EF, % | 57 [41, 65] |
| Vmax AV, cm/s | 321 [148, 415] |
| Maximum PG AV, mm Hg | 40 [9; 69] |
| Mean PG AV, mm Hg | 23 [5, 40] |
| VTI AV, cm | 51 [27, 86] |
| Vmax LVOT, cm/s | 89 [72, 100] |
| VTI LVOT, cm | 19 [15, 25] |
| AS grade | |
| 0 | 52 (49) |
| I | 1 (1) |
| II | 11 (10) |
| III | 43 (40) |
| LVOT | 2D-TTE | 3D-TTE | 2D-TEE | 3D-TEE | CT MPR | p-Value |
|---|---|---|---|---|---|---|
| a/p dimension (cm) | 2.01 [1.86, 2.17] | 2.02 [1.90, 2.16] | 2.00 [1.89, 2.17] | 2.02 [1.92, 2.13] | 2.04 [1.90, 2.13] | 0.98 |
| m/l dimension (cm) | 2.67 [2.38, 2.95] | 2.53 [2.30, 2.77] | 2.59 [2.42, 2.90] | 0.002 | ||
| eccentricity (=a/p: m/L) | 0.75 [0.70, 0.82] | 0.79 [0.75, 0.84] | 0.76 [0.71, 0.81] | <0.001 | ||
| (cm2) | 3.18 [2.74, 3.70] | 3.17 [2.84, 3.66] | 3.17 [2.82, 3.68] | 3.23 [2.87, 3.58] | 4.15 [3.63, 4.53] | <0.001 |
| (cm2) | 4.22 [3.64, 5.06] * | 4.11 [3.59, 4.61] * | 0.14 | |||
| (cm2) | 4.24 [3.57, 4.92] | 4.00 [3.49, 4.52] | 0.20 |
| Echocardiographic Method | Bias | LOA (Lower; Upper) |
|---|---|---|
| TTE | ||
| ) | −0.4 † | −1; 0.21 |
| ) | −0.41 † | −1.13; 0.31 |
| ) | −0.07 *¶ | −0.71; 0.56 |
| ) | 0.06 *¶ | −0.45; 0.56 |
| TEE | ||
| ) | −0.4 † | −0.96; 0.16 |
| ) | −0.41 † | 1.05; 0.24 |
| ) | −0.03 *¶ | −0.38; 0.32 |
| ) | −0.04 *¶ | −0.4; 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruf, T.F.; Cannard, B.E.; Strasser, R.H.; Linke, A.; Sveric, K.M. Calculation of Aortic VAlve and LVOT Areas by a Modified Continuity Equation Using Different Echocardiography Methods: The CAVALIER Study. Diagnostics 2022, 12, 1656. https://doi.org/10.3390/diagnostics12071656
Ruf TF, Cannard BE, Strasser RH, Linke A, Sveric KM. Calculation of Aortic VAlve and LVOT Areas by a Modified Continuity Equation Using Different Echocardiography Methods: The CAVALIER Study. Diagnostics. 2022; 12(7):1656. https://doi.org/10.3390/diagnostics12071656
Chicago/Turabian StyleRuf, Tobias Friedrich, Béatrice Elise Cannard, Ruth H. Strasser, Axel Linke, and Krunoslav M. Sveric. 2022. "Calculation of Aortic VAlve and LVOT Areas by a Modified Continuity Equation Using Different Echocardiography Methods: The CAVALIER Study" Diagnostics 12, no. 7: 1656. https://doi.org/10.3390/diagnostics12071656
APA StyleRuf, T. F., Cannard, B. E., Strasser, R. H., Linke, A., & Sveric, K. M. (2022). Calculation of Aortic VAlve and LVOT Areas by a Modified Continuity Equation Using Different Echocardiography Methods: The CAVALIER Study. Diagnostics, 12(7), 1656. https://doi.org/10.3390/diagnostics12071656






