Reduced Cerebellar Volume in Term Infants with Complex Congenital Heart Disease: Correlation with Postnatal Growth Measurements
Abstract: Objectives
1. Introduction
2. Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. Structural Segmentation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, J.H.; Rotermann, I.; Logoteta, J.; Jung, O.; Peter, D.; Dütschke, P.; Scheewe, J.; Kramer, H.-H.; Peter, D. Neurodevelopmental outcome in hypoplastic left heart syndrome: Impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J. Thorac. Cardiovasc. Surg. 2016, 151, 1358–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabbutt, S.; Nord, A.S.; Jarvik, G.P.; Bernbaum, J.; Wernovsky, G.; Gerdes, M.; Zackai, E.; Clancy, R.R.; Nicolson, S.C.; Spray, T.L.; et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics 2008, 121, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sarajuuri, A.; Jokinen, E.; Mildh, L.; Tujulin, A.-M.; Mattila, I.; Valanne, L.; Lönnqvist, T. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics 2012, 130, e1636–e1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J. Int. Neuropsychol. Soc. 2014, 21, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Calderon, J.; Bellinger, D.C. Executive function deficits in congenital heart disease: Why is intervention important? Cardiol. Young 2015, 25, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuillen, P.S.; Barkovich, A.J.; Hamrick, S.E.G.; Perez, M.; Ward, P.; Glidden, D.V.; Azakie, A.; Karl, T.; Miller, S.P. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 2007, 38, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Newburger, J.W.; Sleeper, L.A.; Bellinger, D.C.; Goldberg, C.S.; Tabbutt, S.; Lu, M.; Mussatto, K.A.; Williams, I.A.; Gustafson, K.E.; Mital, S.; et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: The single ventricle reconstruction trial. Circulation 2012, 125, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Mahle, W.T.; Tavani, F.; Zimmerman, R.A.; Nicolson, S.C.; Galli, K.K.; Gaynor, J.W.; Clancy, R.R.; Montenegro, L.M.; Spray, T.L.; Chiavacci, R.M.; et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002, 106, I109–I114. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar] [CrossRef]
- Gerstle, M.; Beebe, D.W.; Drotar, D.; Cassedy, A.; Marino, B.S. Executive Functioning and School Performance among Pediatric Survivors of Complex Congenital Heart Disease. J. Pediatr. 2016, 173, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, K.A.; Hoffmann, R.; Hoffman, G.; Tweddell, J.S.; Bear, L.; Cao, Y.; Tanem, J.; Brosig, C. Risk Factors for Abnormal Developmental Trajectories in Young Children With Congenital Heart Disease. Circulation 2015, 132, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellinger, D.C.; Newburger, J.W. Neuropsychological, psychosocial, and quality-of-life outcomes in children and adolescents with congenital heart disease. Prog. Pediatr. Cardiol. 2010, 29, 87–92. [Google Scholar] [CrossRef]
- Ramoǧlu, M.G.; Kavuncuoǧlu, S.; Özbek, S.; Aldemir, E. Perinatal and somatic growth properties of preterm babies born from spontaneous and in vitro fertilization multiple pregnancies. Turk Pediatr. Ars. 2014, 49, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bocca-tjeertes, I.; Bos, A.; Kerstjens, J.; De Winter, A.; de Winter, A.; Reijneveld, S. Symmetrical and asymmetrical growth restriction in preterm-born children. Pediatrics 2014, 133, e650–e656. [Google Scholar] [CrossRef] [Green Version]
- Cheong, J.L.Y.; Hunt, R.W.; Anderson, P.J.; Howard, K.; Thompson, D.K.; Wang, H.X.; Bear, M.J.; Nursing, B.A.; Inder, T.E.; Doyle, L.W. Head Growth in Preterm Infants: Correlation With Magnetic Resonance Imaging and Neurodevelopmental Outcome. Pediatrics 2008, 121, e1534–e1540. [Google Scholar] [CrossRef]
- Klarić, A.Š.; Galić, S.; Kolundžić, Z.; Bošnjak, V.M. Neuropsychological development in preschool children born with asymmetrical intrauterine growth restriction and impact of postnatal head growth. J. Child Neurol. 2013, 28, 867–873. [Google Scholar] [CrossRef]
- Poryo, M.; Paes, L.A.; Pickardt, T.; Bauer, U.M.M.; Meyer, S.; Wagenpfeil, S.; Abdul-Khaliq, H.; Kerst, G.; Vazquez-Jimenez, J.F.; Gkalpakiotis, D.; et al. Somatic Development in Children with Congenital Heart Defects. J. Pediatr. 2018, 192, 136–143.e4. [Google Scholar] [CrossRef]
- Steurer, M.A.; Peyvandi, S.; Costello, J.M.; Moon-Grady, A.J.; Habib, R.H.; Hill, K.D.; Jacobs, M.L.; Jelliffe-Pawlowski, L.L.; Keller, R.L.; Pasquali, S.K.; et al. Association between Z-score for birth weight and postoperative outcomes in neonates and infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2021, 162, 1838–1847.e4. [Google Scholar] [CrossRef]
- Volpe, J.J. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef] [Green Version]
- Gertsvolf, N.; Votava-Smith, J.K.; Ceschin, R.; del Castillo, S.; Lee, V.; Lai, H.A.; Bluml, S.; Paquette, L.; Panigrahy, A. Association between Subcortical Morphology and Cerebral White Matter Energy Metabolism in Neonates with Congenital Heart Disease. Sci. Rep. 2018, 8, 14057. [Google Scholar] [CrossRef] [Green Version]
- Ortinau, C.; Inder, T.; Lambeth, J.; Wallendorf, M.; Finucane, K.; Beca, J. Congenital heart disease affects cerebral size but not brain growth. Pediatr. Cardiol. 2012, 33, 1138–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Rhein, M.; Buchmann, A.; Hagmann, C.; Dave, H.; Bernet, V.; Scheer, I.; Knirsch, W.; Latal, B. Severe Congenital Heart Defects Are Associated with Global Reduction of Neonatal Brain Volumes. J. Pediatr. 2015, 167, 1259–1263.e1. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, A.; Lee, V.; Ceschin, R.; Zuccoli, G.; Beluk, N.; Khalifa, O.; Votava-Smith, J.K.; DeBrunner, M.; Munoz, R.; Domnina, Y.; et al. Brain Dysplasia Associated with Ciliary Dysfunction in Infants with Congenital Heart Disease. J. Pediatr. 2016, 178, 141–148.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquette, L.B.; Wisnowski, J.L.; Ceschin, R.; Pruetz, J.D.; Detterich, J.A.; Del Castillo, S.; Nagasunder, A.C.; Kim, R.; Painter, M.J.; Gilles, F.H.; et al. Abnormal cerebral microstructure in premature neonates with congenital heart disease. Am. J. Neuroradiol. 2013, 34, 2026–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wang, Y.; Lao, Y.; Ceschin, R.; Mi, L.; Nelson, M.D.; Panigrahy, A.; Leporé, N. Abnormal ventricular development in preterm neonates with visually normal MRIs. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9681, 96810H. [Google Scholar]
- Multicentre, W.H.O.; Reference, G.; Group, S. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- Ceschin, R.; Zahner, A.; Reynolds, W.; Gaesser, J.; Zuccoli, G.; Lo, C.W.; Gopalakrishnan, V.; Panigrahy, A. A computational framework for the detection of subcortical brain dysmaturation in neonatal MRI using 3D Convolutional Neural Networks. Neuroimage 2018, 178, 183–197. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Serag, A.; Aljabar, P.; Ball, G.; Counsell, S.J.; Boardman, J.P.; Rutherford, M.A.; Edwards, A.D.; Hajnal, J.V.; Rueckert, D. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage 2012, 59, 2255–2265. [Google Scholar] [CrossRef]
- Avants, B.; Tustison, N.; Song, G. Advanced Normalization Tools (ANTS). Insight J. 2009, 2, 1–35. [Google Scholar] [CrossRef]
- Gousias, I.S.; Edwards, A.D.; Rutherford, M.A.; Counsell, S.J.; Hajnal, J.V.; Rueckert, D.; Hammers, A. Magnetic resonance imaging of the newborn brain: Manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 2012, 62, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2008. Available online: https://cran.r-project.org/ (accessed on 25 June 2022).
- Cockerill, J.; Uthaya, S.; Doré, C.J.; Modi, N. Accelerated postnatal head growth follows preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F184. [Google Scholar] [CrossRef] [PubMed]
- Guellec, I.; Marret, S.; Baud, O.; Cambonie, G.; Lapillonne, A.; Roze, J.; Fresson, J.; Flamant, C.; Charkaluk, M.-L.; Arnaud, C.; et al. Intrauterine Growth Restriction, Head Size at Birth, and Outcome in Very Preterm Infants. J. Pediatr. 2015, 167, 975–981.e2. [Google Scholar] [CrossRef]
- Lee, K.A.; Hayes, B.C. Head size and growth in the very preterm infant: A literature review. Res. Rep. Neonatol. 2015, 2015, 1–7. [Google Scholar]
- Nosarti, C.; Woo, K.; Walshe, M.; Murray, R.M.; Cuddy, M.; Rifkin, L.; Allin, M.P.G.; Nam, K.W.; Walshe, M.; Murray, R.M.; et al. Preterm birth and structural brain alterations in early adulthood. NeuroImage Clin. 2014, 6, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Hapuoja, L.; Kretschmar, O.; Rousson, V.; Dave, H.; Naef, N.; Latal, B. Somatic growth in children with congenital heart disease at 10 years of age: Risk factors and longitudinal growth. Early Hum. Dev. 2021, 156, 105349. [Google Scholar] [CrossRef]
- Miller, T.A.; Zak, V.; Shrader, P.; Ravishankar, C.; Pemberton, V.L.; Newburger, J.W.; Shillingford, A.J.; Dagincourt, N.; Cnota, J.F.; Lambert, L.M.; et al. Growth Asymmetry, Head Circumference, and Neurodevelopmental Outcomes in Infants with Single Ventricles. J. Pediatr. 2015, 168, 220–225.e1. [Google Scholar] [CrossRef] [Green Version]
- Limperopoulos, C.; Tworetzky, W.; McElhinney, D.B.; Newburger, J.W.; Brown, D.W.; Robertson, R.L.; Guizard, N.; McGrath, E.; Geva, J.; Annese, D.; et al. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010, 121, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Owen, M.; Shevell, M.; Donofrio, M.; Majnemer, A.; McCarter, R.; Vezina, G.; Bouyssi-Kobar, M.; Evangelou, I.; Freeman, D.; Weisenfeld, N.; et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J. Pediatr. 2014, 164, 1121–1127.e1. [Google Scholar] [CrossRef] [Green Version]
- Peyvandi, S.; De Santiago, V.; Chakkarapani, E.; Chau, V.; Campbell, A.; Poskitt, K.J.; Xu, D.; Barkovich, A.J.; Miller, S.; McQuillen, P. Association of Prenatal Diagnosis of Critical Congenital Heart Disease With Postnatal Brain Development and the Risk of Brain Injury. JAMA Pediatr. 2016, 94158. [Google Scholar] [CrossRef] [Green Version]
- Ortinau, C.; Beca, J.; Lambeth, J.; Ferdman, B.; Alexopoulos, D.; Shimony, J.S.; Wallendorf, M.; Neil, J.; Inder, T. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2012, 143, 1264–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ní Bhroin, M.; Abo Seada, S.; Bonthrone, A.F.; Kelly, C.J.; Christiaens, D.; Schuh, A.; Pietsch, M.; Hutter, J.; Tournier, J.D.; Cordero-Grande, L.; et al. Reduced structural connectivity in cortico-striatal-thalamic network in neonates with congenital heart disease. NeuroImage Clin. 2020, 28, 102423. [Google Scholar] [CrossRef] [PubMed]
- Xydis, V.; Drougia, A.; Giapros, V.; Argyropoulou, M.; Andronikou, S. Brain growth in preterm infants is affected by the degree of growth restriction at birth. J. Matern. Neonatal Med. 2012, 7058, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Votava-Smith, J.K.; Tran, N.; Kim, R.; Lee, V.; Ceschin, R.; Lai, H.; Johnson, J.A.; De Toledo, J.S.; Blüml, S.; et al. Structural network topology correlates of microstructural brain dysmaturation in term infants with congenital heart disease. Hum. Brain Mapp. 2018, 39, 4593–4610. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, V.; Votava-Smith, J.K.; Zhuang, X.; Brian, J.; Marshall, L.; Panigrahy, A.; Paquette, L. Fetuses with single ventricle congenital heart disease manifest impairment of regional brain growth. Prenat Diagn. 2018, 38, 1042–1048. [Google Scholar] [CrossRef]
- Paquette, L.B.; Votava-Smith, J.K.; Ceschin, R.; Nagasunder, A.C.; Jackson, H.A.; Blüml, S.; Wisnowski, J.L.; Panigrahy, A. Abnormal Development of Thalamic Microstructure in Premature Neonates with Congenital Heart Disease. Pediatr. Cardiol. 2015, 36, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Limperopoulos, C.; Chilingaryan, G.; Guizard, N.; Robertson, R.L.; Du Plessis, A.J. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr. Res. 2010, 68, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Tam, E.W.Y. Potential mechanisms of cerebellar hypoplasia in prematurity. Neuroradiology 2013, 55, 41–46. [Google Scholar] [CrossRef]
- Aguilar, A.; Meunier, A.; Strehl, L.; Martinovic, J.; Bonniere, M.; Attie-Bitach, T.; Encha-Razavi, F.; Spassky, N. Analysis of human samples reveals impaired SHH-dependent cerebellar development in Joubert syndrome/Meckel syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16951–16956. [Google Scholar] [CrossRef] [Green Version]
- Badaly, D.; Beers, S.R.; Ceschin, R.; Lee, V.K.; Sulaiman, S.; Zahner, A.; Wallace, J.; Berdaa-Sahel, A.; Burns, C.; Lo, C.W.; et al. Cerebellar and Prefrontal Structures Associated with Executive Functioning in Pediatric Patients with Congenital Heart Defects. Front. Neurol. Appl. Neuroimaging 2022, 13, 827780. [Google Scholar] [CrossRef]
- Cheng, H.H.; Ferradal, S.L.; Vyas, R.; Wigmore, D.; McDavitt, E.; Soul, J.S.; Franceschini, M.A.; Newburger, J.W.; Grant, P.E. Abnormalities in cerebral hemodynamics and changes with surgical intervention in neonates with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2020, 159, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Habas, C. Functional Connectivity of the Cognitive Cerebellum. Front. Syst. Neurosci. 2021, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, H.; Faber, J.; Timmann, D.; Klockgether, T. Update cerebellum and cognition. J. Neurol. 2021, 268, 3921–3925. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Fumagalli, M.; Alomar, Y.I.; Passera, S.; Cavallaro, G.; Mosca, F.; Villamor, E. Cerebellar hemorrhage in preterm infants: A meta-analysis on risk factors and neurodevelopmental outcome. Front. Physiol. 2019, 10, 800. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The Cerebro-Cerebellum as a Locus of Forward Model: A Review. Front. Syst. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
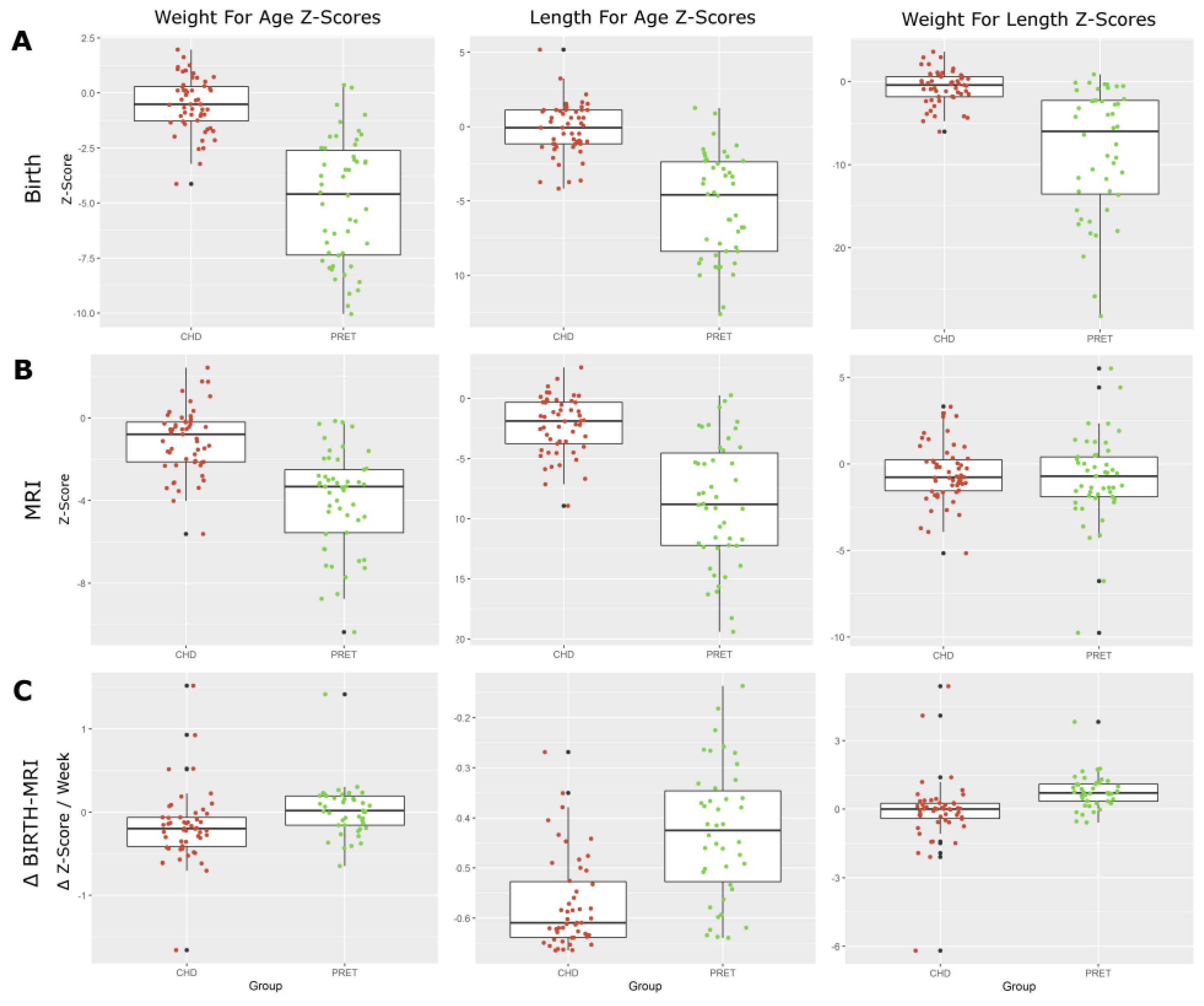
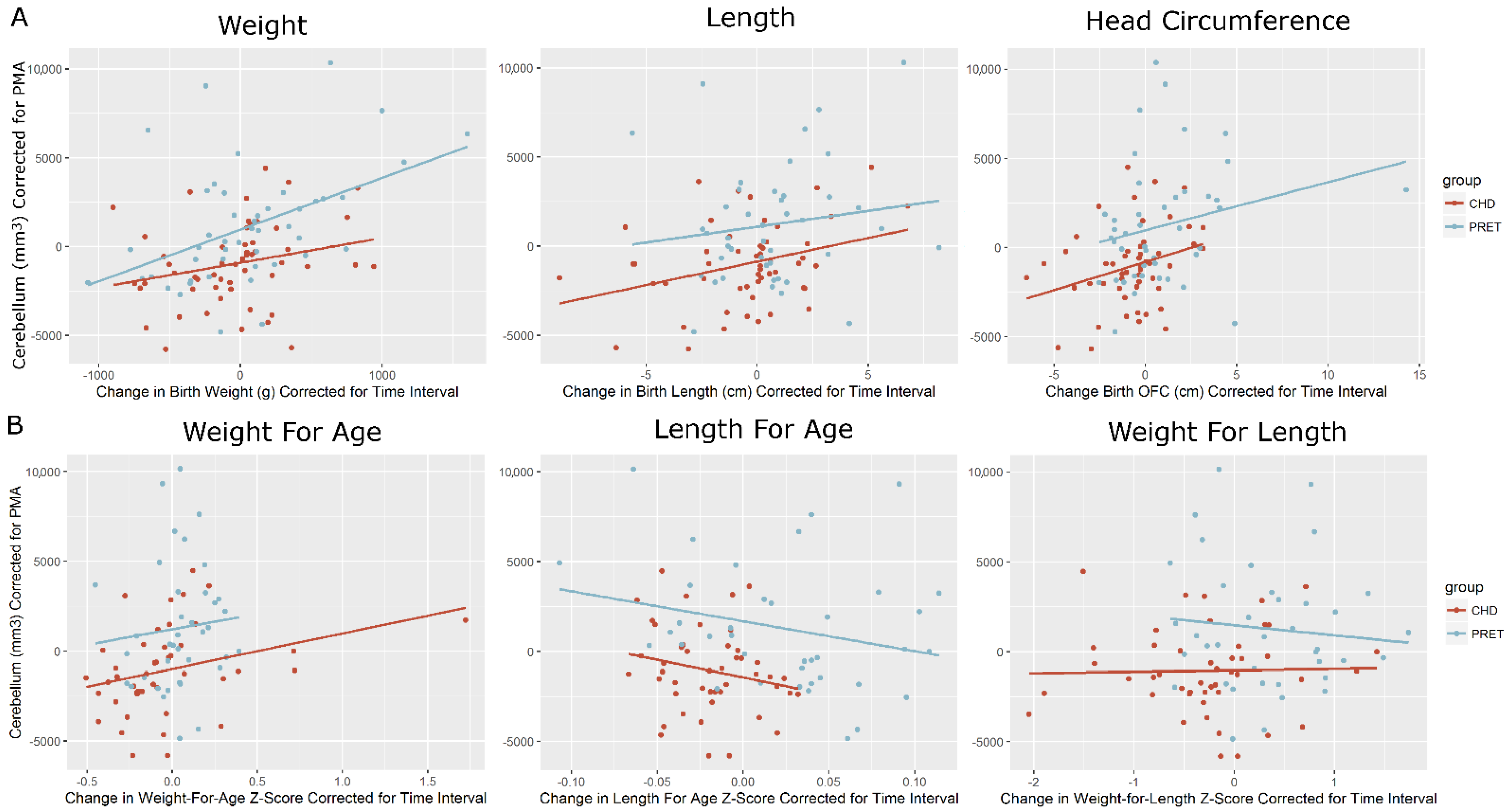
| CHD | Preterm | ||
|---|---|---|---|
| Change in Metric (Birth—MRI) | Mean (SD) | Mean (SD) | (p<) |
| Weight for Age Z-Score (WAZ) | −0.18 (0.44) | 0.02 (0.31) | 0.012 |
| Length for Age Z-Score (LAZ) | −0.57 (0.09) | −0.43 (0.13) | 0.000 |
| Weight for Length Z-Score (WLZ) | −0.08 (1.48) | 0.76 (0.75) | 0.001 |
| Head Circumference for Age Z-Score (HCAZ) | −0.14 (0.87) | 0.03 (0.52) | 0.263 |
| Asymmetry (WAZ—HCAZ) | −0.03 (0.80) | −0.01 (0.45) | 0.898 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceschin, R.; Zahner, A.; Reynolds, W.; Beluk, N.; Panigrahy, A. Reduced Cerebellar Volume in Term Infants with Complex Congenital Heart Disease: Correlation with Postnatal Growth Measurements. Diagnostics 2022, 12, 1644. https://doi.org/10.3390/diagnostics12071644
Ceschin R, Zahner A, Reynolds W, Beluk N, Panigrahy A. Reduced Cerebellar Volume in Term Infants with Complex Congenital Heart Disease: Correlation with Postnatal Growth Measurements. Diagnostics. 2022; 12(7):1644. https://doi.org/10.3390/diagnostics12071644
Chicago/Turabian StyleCeschin, Rafael, Alexandria Zahner, William Reynolds, Nancy Beluk, and Ashok Panigrahy. 2022. "Reduced Cerebellar Volume in Term Infants with Complex Congenital Heart Disease: Correlation with Postnatal Growth Measurements" Diagnostics 12, no. 7: 1644. https://doi.org/10.3390/diagnostics12071644
APA StyleCeschin, R., Zahner, A., Reynolds, W., Beluk, N., & Panigrahy, A. (2022). Reduced Cerebellar Volume in Term Infants with Complex Congenital Heart Disease: Correlation with Postnatal Growth Measurements. Diagnostics, 12(7), 1644. https://doi.org/10.3390/diagnostics12071644






