The Use of Confocal Laser Endomicroscopy in Diagnosing Barrett’s Esophagus and Esophageal Adenocarcinoma
Abstract
1. Introduction
2. Studies Focusing on Diagnostics of BE and EAC Using CLE
3. Meta-Analysis and Systematic Reviews Focusing on Diagnostics of BE and EAC Using CLE
4. Surveillance of BE by CLE and Surveillance by CLE of BE Neoplastic Lesions after Endoscopic Treatment
5. Advantages and Disadvantages
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fugazza, A.; Gaiani, F.; Carra, M.C.; Brunetti, F.; Lévy, M.; Sobhani, I.; Azoulay, D.; Catena, F.; De’Angelis, G.L.; De’Angelis, N. Confocal Laser Endomicroscopy in Gastrointestinal and Pancreatobiliary Diseases: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2016, 2016, 4638683. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, D.I.; Cârţână, T.; Ciurea, T.; Popescu, C.; Bădărău, A.; Saftoiu, A. Confocal laser endomicroscopy and immunoendoscopy for real-time assessment of vascularization in gastrointestinal malignancies. World J. Gastroenterol. 2011, 17, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.D. Confocal Microscopy: Principles and Modern Practices. Curr. Protoc. Cytom. 2020, 92, e68. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Carr-Locke, D.L.; Singh, S.K.; Neumann, H.; Bertani, H.; Galmiche, J.-P.; Arsenescu, R.I.; Caillol, F.; Chang, K.J.; Chaussade, S.; et al. Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. United Eur. Gastroenterol. J. 2015, 3, 230–254. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, K.; Canto, M. Confocal endomicroscopy. Curr. Opin. Gastroenterol. 2008, 24, 631–637. [Google Scholar] [CrossRef]
- Peter, S.; Council, L.; Bang, J.Y.; Neumann, H.; Mönkemüller, K.; Varadarajulu, S.; Wilcox, C.M. Poor agreement between endoscopists and gastrointestinal pathologists for the interpretation of probe-based confocal laser endomicroscopy findings. World J. Gastroenterol. 2014, 20, 17993–18000. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Leong, R.W. Current application of confocal endomicroscopy in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2008, 23, 1483–1491. [Google Scholar] [CrossRef]
- Fuks, D.; Pierangelo, A.; Validire, P.; Lefevre, M.; Benali, A.; Trebuchet, G.; Criton, A.; Gayet, B. Intraoperative confocal laser endomicroscopy for real-time in vivo tissue characterization during surgical procedures. Surg. Endosc. 2019, 33, 1544–1552. [Google Scholar] [CrossRef]
- Ellebrecht, D.B.; Latus, S.; Schlaefer, A.; Keck, T.; Gessert, N. Towards an Optical Biopsy during Visceral Surgical Interventions. Visc. Med. 2020, 36, 70–79. [Google Scholar] [CrossRef]
- Wallace, M.; Lauwers, G.Y.; Chen, Y.; Dekker, E.; Fockens, P.; Sharma, P.; Meining, A. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 2011, 43, 882–891. [Google Scholar] [CrossRef]
- Visaggi, P.; Barberio, B.; Ghisa, M.; Ribolsi, M.; Savarino, V.; Fassan, M.; Valmasoni, M.; Marchi, S.; de Bortoli, N.; Savarino, E. Modern Diagnosis of Early Esophageal Cancer: From Blood Biomarkers to Advanced Endoscopy and Artificial Intelligence. Cancers 2021, 13, 3162. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Bertani, H.; O’Donovan, M.; Santos, P.; Alastal, H.; Phillips, R.; Ortiz-Fernández-Sordo, J.; Iacucci, M.; Modolell, I.; Bonetti, L.R.; et al. Development and Validation of Confocal Endomicroscopy Diagnostic Criteria for Low-Grade Dysplasia in Barrett’s Esophagus. Clin. Transl. Gastroenterol. 2019, 10, e00014. [Google Scholar] [CrossRef]
- Gaddam, S.; Mathur, S.C.; Singh, M.; Arora, J.; Wani, S.B.; Gupta, N.; Overhiser, A.; Rastogi, A.; Singh, V.; Desai, N.; et al. Novel Probe-Based Confocal Laser Endomicroscopy Criteria and Interobserver Agreement for the Detection of Dysplasia in Barrett’s Esophagus. Am. J. Gastroenterol. 2011, 106, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Runge, T.M.; Abrams, J.A.; Shaheen, N.J. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol. Clin. N. Am. 2015, 44, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Katzka, D.A.; Iyer, P.G. Endoscopic Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 27–41. [Google Scholar] [CrossRef]
- Bujanda, D.E.; Hachem, C. Barrett’s Esophagus. Mo. Med. 2018, 115, 211–213. [Google Scholar]
- Cameron, A.J.; Ott, B.J.; Payne, W.S. The Incidence of Adenocarcinoma in Columnar-Lined (Barrett’s) Esophagus. N. Engl. J. Med. 1985, 313, 857–859. [Google Scholar] [CrossRef]
- Condon, A.; Muthusamy, V.R. The evolution of endoscopic therapy for Barrett’s esophagus. Ther. Adv. Gastrointest. Endosc. 2021, 14, 26317745211051834. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Jovani, M.; De Vivo, I.; Jacobson, B.C. Gene-Environment Interactions and the Risk of Barrett’s Esophagus in Three US Cohorts. Am. J. Gastroenterol. 2019, 114, 893–899. [Google Scholar] [CrossRef]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef]
- Wheeler, J.B.; Reed, C.E. Epidemiology of Esophageal Cancer. Surg. Clin. N. Am. 2012, 92, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Risk factors and molecular mechanisms of esophageal cancer: Differences between the histologic subtype. J. Cancer Metastasis Treat. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Falk, G.W. Barrett’s oesophagus: Frequency and prediction of dysplasia and cancer. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 125–138. [Google Scholar] [CrossRef]
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Sanghi, V.; Thota, P.N. Barrett’s esophagus: Novel strategies for screening and surveillance. Ther. Adv. Chronic Dis. 2019, 10, 2040622319837851. [Google Scholar] [CrossRef]
- Elsheaita, A.; El-Bially, M.A.; Shamseya, M.M.; Ahmed, S.S.; Madkour, M.A.; Shamseya, A.M.; Nouh, H.H. Seattle protocol vs narrow band imaging guided biopsy in screening of Barrett’s esophagus in gastroesophageal reflux disease patients. Medicine 2020, 99, e19261. [Google Scholar] [CrossRef]
- Naini, B.V.; Souza, R.F.; Odze, R.D. Barrett’s Esophagus. Am. J. Surg. Pathol. 2016, 40, e45–e66. [Google Scholar] [CrossRef]
- Vranić, L.; Nadarević, T.; Štimac, D. Probe-Based Confocal Laser Endomicroscopy and Barrett’s Esophagus: Just a Scientific Toy or Significant Improvement in Diagnosis? Dig. Dis. 2022, 40, 97–105. [Google Scholar] [CrossRef]
- Kolb, J.M.; Wani, S. Barrett’s esophagus: Current standards in advanced imaging. Transl. Gastroenterol. Hepatol. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Gossner, L.; Goetz, M.; Dahlmann, A.; Vieth, M.; Stolte, M.; Hoffman, A.; Jung, M.; Nafe, B.; Galle, P.R. In Vivo Histology of Barrett’s Esophagus and Associated Neoplasia by Confocal Laser Endomicroscopy. Clin. Gastroenterol. Hepatol. 2006, 4, 979–987. [Google Scholar] [CrossRef]
- Bajbouj, M.; Vieth, M.; Rösch, T.; Miehlke, S.; Becker, V.; Anders, M.; Pohl, H.; Madisch, A.; Schuster, T.; Schmid, R.; et al. Probe-based confocal laser endomicroscopy compared with standard four-quadrant biopsy for evaluation of neoplasia in Barrett’s esophagus. Endoscopy 2010, 42, 435–440. [Google Scholar] [CrossRef]
- Wallace, M.B.; Sharma, P.; Lightdale, C.; Wolfsen, H.; Coron, E.; Buchner, A.; Bajbouj, M.; Bansal, A.; Rastogi, A.; Abrams, J.; et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest. Endosc. 2010, 72, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, P.; Meining, A.R.; Coron, E.; Lightdale, C.J.; Wolfsen, H.C.; Bansal, A.; Bajbouj, M.; Galmiche, J.-P.; Abrams, J.A.; Rastogi, A.; et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: Final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest. Endosc. 2011, 74, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Dolak, W.; Mesteri, I.; Asari, R.; Preusser, M.; Tribl, B.; Wrba, F.; Schoppmann, S.F.; Hejna, M.; Trauner, M.; Häfner, M.; et al. A pilot study of the endomicroscopic assessment of tumor extension in Barrett’s esophagus–associated neoplasia before endoscopic resection. Endosc. Int. Open 2015, 3, E19–E28. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, C.; Taylor, A.C.F.; Desmond, P.V.; Macrae, F.; Williams, R. Added value of narrow band imaging and confocal laser endomicroscopy in detecting Barrett’s esophagus neoplasia. Endoscopy 2012, 44, 1089–1095, Erratum in Endoscopy 2013, 45, 484. [Google Scholar] [CrossRef]
- Bertani, H.; Frazzoni, M.; Dabizzi, E.; Pigò, F.; Losi, L.; Manno, M.; Manta, R.; Bassotti, G.; Conigliaro, R. Improved Detection of Incident Dysplasia by Probe-Based Confocal Laser Endomicroscopy in a Barrett’s Esophagus Surveillance Program. Am. J. Dig. Dis. 2013, 58, 188–193. [Google Scholar] [CrossRef]
- Caillol, F. Probe confocal laser endomicroscopy in the therapeutic endoscopic management of Barrett’s dysplasia. Ann. Gastroenterol. 2017, 30, 295–301. [Google Scholar] [CrossRef]
- Canto, M.I.; Anandasabapathy, S.; Brugge, W.; Falk, G.; Dunbar, K.B.; Zhang, Z.; Woods, K.; Almario, J.A.; Schell, U.; Goldblum, J.; et al. In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: A multicenter international randomized controlled trial (with video). Gastrointest. Endosc. 2014, 79, 211–221. [Google Scholar] [CrossRef]
- Shah, T.; Lippman, R.; Kohli, D.; Mutha, P.; Solomon, S.; Zfass, A. Accuracy of probe-based confocal laser endomicroscopy (pCLE) compared to random biopsies during endoscopic surveillance of Barrett’s esophagus. Endosc. Int. Open 2018, 6, E414–E420. [Google Scholar] [CrossRef]
- Richardson, C.; Colavita, P.; Dunst, C.; Bagnato, J.; Billing, P.; Birkenhagen, K.; Buckley, F.; Buitrago, W.; Burnette, J.; Leggett, P.; et al. Real-Time diagnosis of Barrett’s esophagus: A prospective, multicenter study comparing confocal laser endomicroscopy with conventional histology for the identification of intestinal metaplasia in new users. Surg. Endosc. 2019, 33, 1585–1591. [Google Scholar] [CrossRef]
- Kunovský, L.; Kala, Z.; Kroupa, R.; Grolich, T.; Dolina, J.; Dastych, M.; Vaculová, J.; Vlažný, J.; Moravčík, P.; Hollá, L.I.; et al. Confocal laser endomicroscopy in the diagnostics of esophageal diseases: A pilot study. Vnitr Lek. 2020, 66, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kollar, M.; Krajciova, J.; Prefertusova, L.; Sticova, E.; Maluskova, J.; Vackova, Z.; Martinek, J. Probe-Based confocal laser endomicroscopy versus biopsies in the diagnostics of oesophageal and gastric lesions: A prospective, pathologist-blinded study. United Eur. Gastroenterol. J. 2020, 8, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Bird-Lieberman, E.L.; Liu, X.; Nuckcheddy-Grant, T.; Bertani, H.; O’Donovan, M.; Fitzgerald, R.C. Autofluorescence-Directed Confocal Endomicroscopy in Combination with a Three-Biomarker Panel Can Inform Management Decisions in Barrett’s Esophagus. Am. J. Gastroenterol. 2015, 110, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, M.; Modolell, I.; Ortiz-Fernandez-Sordo, J.; Oukrif, D.; Pappas, A.; Januszewicz, W.; O’Donovan, M.; Hadjinicolaou, A.; Bianchi, M.; Blasko, A.; et al. Image-Enhanced Endoscopy and Molecular Biomarkers Vs Seattle Protocol to Diagnose Dysplasia in Barrett’s Esophagus. Clin. Gastroenterol. Hepatol, 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-Q.; Ma, S.-J.; Zhou, J.-H.; Zhong, X.-S.; Chen, Q. A meta-analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett’s esophagus. J. Gastroenterol. Hepatol. 2016, 31, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.J.; Rosch, T.; Vieth, M.; Koch, M.; Becker, V.; Anders, M.; Khalifa, A.C.; Meining, A. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut 2008, 57, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Trovato, C.; Sonzogni, A.; Ravizza, D.; Fiori, G.; Tamayo, D.; De Roberto, G.; de Leone, A.; De Lisi, S.; Crosta, C. Confocal laser endomicroscopy for in vivo diagnosis of Barrett’s oesophagus and associated neoplasia: A pilot study conducted in a single Italian centre. Dig. Liver Dis. 2013, 45, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Leggett, C.L.; Gorospe, E.C.; Chan, D.K.; Muppa, P.; Owens, V.; Smyrk, T.C.; Anderson, M.; Lutzke, L.S.; Tearney, G.; Wang, K.K. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest. Endosc. 2016, 83, 880–888.e2. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Y.-M.; Wang, T.-T.; Hu, B. Confocal laser endomicroscopy for detection of neoplasia in Barrett’s esophagus: A meta-analysis. Dis. Esophagus 2014, 27, 248–254. [Google Scholar] [CrossRef]
- Gupta, A.; Attar, B.M.; Koduru, P.; Murali, A.R.; Go, B.T.; Agarwal, R. Utility of confocal laser endomicroscopy in identifying high-grade dysplasia and adenocarcinoma in Barrett’s esophagus: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 369–377. [Google Scholar] [CrossRef]
- Xiong, Y.-Q.; Ma, S.-J.; Hu, H.-Y.; Ge, J.; Zhou, L.-Z.; Huo, S.-T.; Qiu, M.; Chen, Q. Comparison of narrow-band imaging and confocal laser endomicroscopy for the detection of neoplasia in Barrett’s esophagus: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 31–39. [Google Scholar] [CrossRef] [PubMed]
- ASGE Technology Committee; Thosani, N.; Abu Dayyeh, B.K.; Sharma, P.; Aslanian, H.R.; Enestvedt, B.K.; Komanduri, S.; Manfredi, M.; Navaneethan, U.; Maple, J.T.; et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2016, 83, 684–698.e7. [Google Scholar] [CrossRef]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Esteban, J.-M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017, 49, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Crook, J.E.; Saunders, M.; Lovat, L.; Coron, E.; Waxman, I.; Sharma, P.; Hwang, J.H.; Banks, M.; DePreville, M.; et al. Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett’s esophagus. Gastrointest. Endosc. 2012, 76, 539–547.e1. [Google Scholar] [CrossRef] [PubMed]
- Krajciova, J.; Kollar, M.; Maluskova, J.; Janicko, M.; Vackova, Z.; Spicak, J.; Martinek, J. Confocal Laser Endomicroscopy vs Biopsies in the Assessment of Persistent or Recurrent Intestinal Metaplasia/Neoplasia after Endoscopic Treatment of Barrett’s Esophagus related Neoplasia. J. Gastrointest. Liver Dis. 2020, 29, 305–312. [Google Scholar] [CrossRef]
- Kollar, M.; Spicak, J.; Honsova, E.; Krajciova, J.; Vackova, Z.; Martinek, J. Role of confocal laser endomicroscopy in patients with early esophageal neoplasia. Minerva Chir. 2018, 73, 417–427. [Google Scholar] [CrossRef]
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: Technical aspects and clinical applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7. [Google Scholar] [CrossRef]
- Robles-Medranda, C. Confocal endomicroscopy: Is it time to move on? World J. Gastrointest. Endosc. 2016, 8, 1–3. [Google Scholar] [CrossRef]
- Choi, K.-S.; Jung, H.-Y. Confocal Laser Endomicroscopy and Molecular Imaging in Barrett Esophagus and Stomach. Clin. Endosc. 2014, 47, 23–30. [Google Scholar] [CrossRef]
- Leggett, C.L.; Gorospe, E.C. Application of confocal laser endomicroscopy in the diagnosis and management of Barrett’s esophagus. Ann. Gastroenterol. 2014, 27, 193–199. [Google Scholar]
- Moravčík, P.; Hlavsa, J.; Kunovský, L.; Kala, Z.; Penka, I.; Dastych, M. Confocal Laser Endomicroscopy in the Diagnostics of Malignancy of the Gastrointestinal Tract. Klin. Onkol. 2017, 30, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.; Langner, C.; Neurath, M.F.; Vieth, M. Confocal Laser Endomicroscopy for Diagnosis of Barrett’s Esophagus. Front. Oncol. 2012, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.B.; Meining, A.; Canto, M.I.; Fockens, P.; Miehlke, S.; Roesch, T.; Lightdale, C.J.; Pohl, H.; Carr-Locke, D.; Löhr, M.; et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol. Ther. 2010, 31, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.M.; Gomez, V.; Heckman, M.G.; Shahid, M.W.; Achem, S.; Gill, K.R.; Laith, J.; Kahaleh, M.; Lo, S.K.; Picco, M.; et al. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest. Endosc. 2011, 73, 556–560. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Li, Z.; Zuo, X.-L.; Li, C.-Q.; Dong, Y.-Y.; Zhou, C.-J.; Li, Y.-Q. Learning Curve and Interobserver Agreement of Confocal Laser Endomicroscopy for Detecting Precancerous or Early-Stage Esophageal Squamous Cancer. PLoS ONE 2014, 9, e99089. [Google Scholar] [CrossRef]
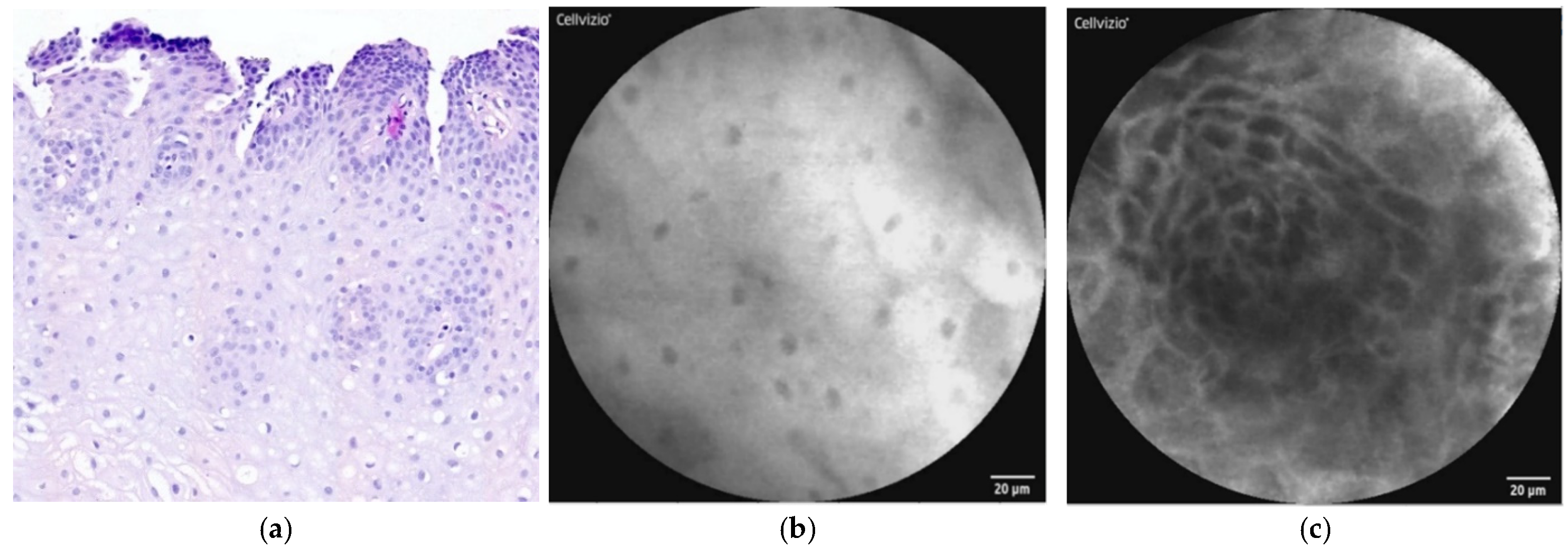
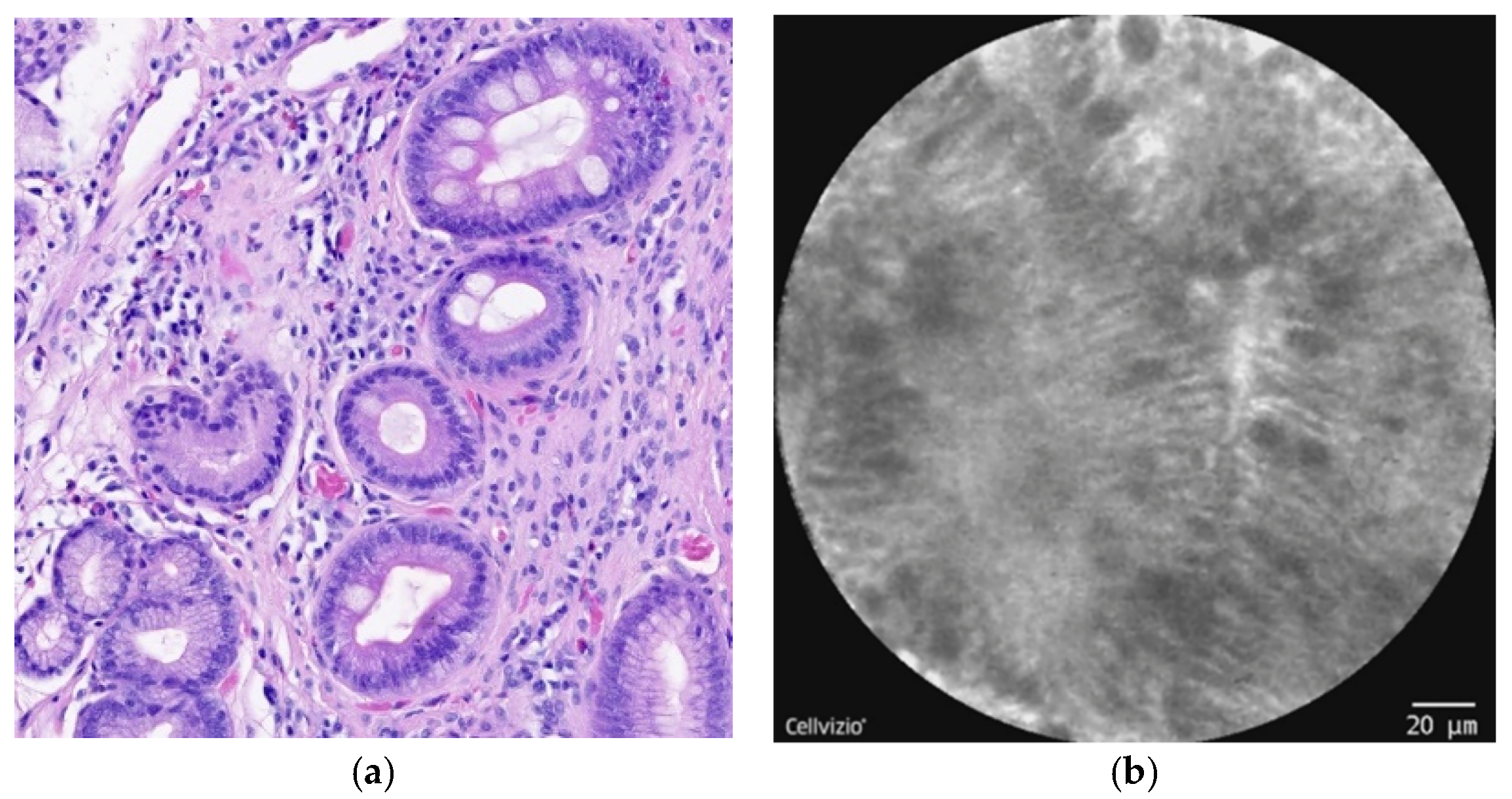

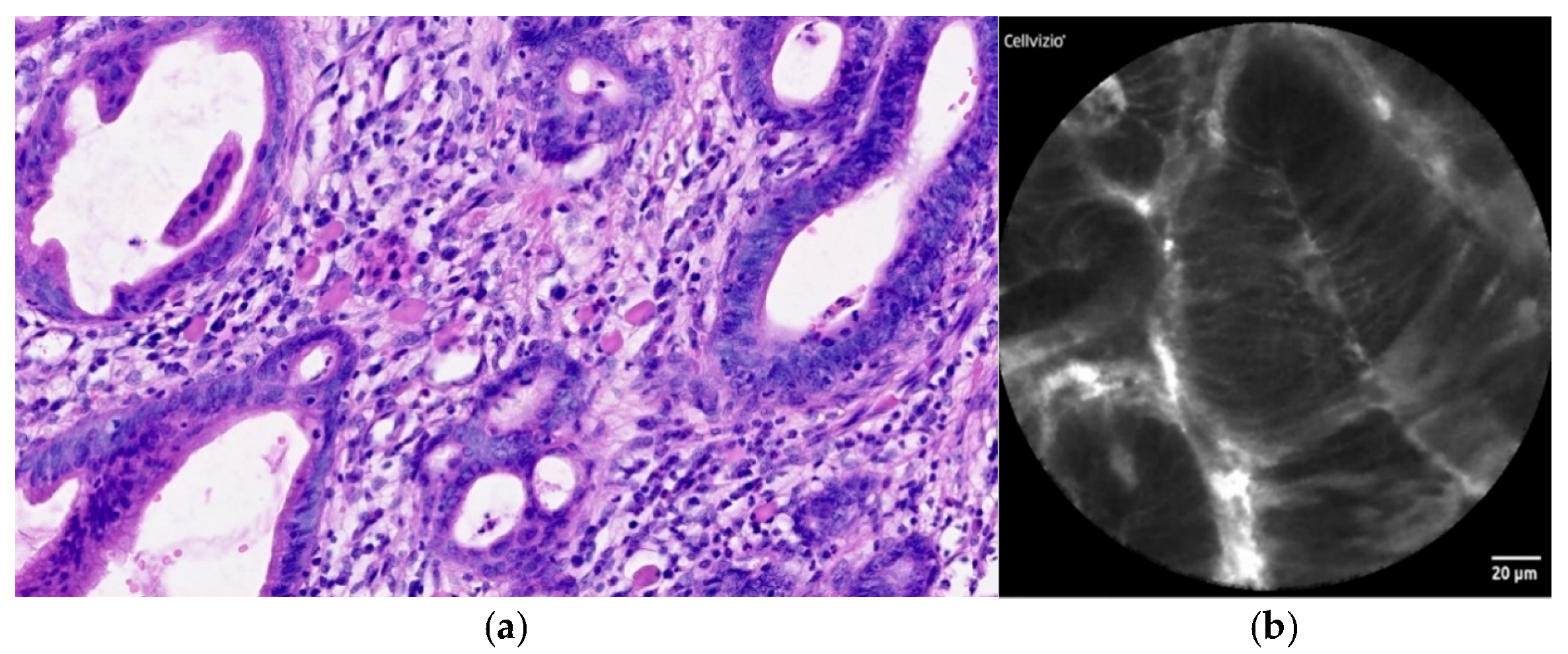
| Normal Squamous Epithelium | Nondysplastic BE | LGD * | HGD/EAC ** |
|---|---|---|---|
| Flat cells without crypts or villi | Uniform villiform architecture | Dark non-round glands | Saw-toothed epithelial surface |
| Bright vessels within papillae (intrapapillary capillary loops) | Columnar cells | Irregular gland shape | Unequal size and shape of glands |
| Dark goblet cells | Lack of goblet cells | Not-easily identifiable goblet cells | |
| Sharp cutoff of darkness | Non-equidistant glands | ||
| Variable cell size | Enlarged cells | ||
| Cellular stratification | Irregular and non-equidistant cells |
| Authors | Year | Type of CLE | No. of Patients | Sensitivity (%) * | Specificity (%) * |
|---|---|---|---|---|---|
| Kiesslich et al. [30] | 2006 | eCLE | 63 | 93 | 98 |
| Pohl et al. [46] | 2008 | pCLE | 75 | 75 | 58 |
| Bajbouj et al. [31] | 2009 | pCLE | 68 | 90 | 59 |
| Wallace et al. [32] | 2010 | pCLE | 5 | 88 | 96 |
| Sharma et al. [33] | 2011 | pCLE | 101 | 100 | 56 |
| Gaddam et al. [13] | 2011 | pCLE | 122 | 76 | 85 |
| Jayasekera et al. [35] | 2012 | eCLE | 50 | 76 | 80 |
| Trovaro et al. [47] | 2013 | eCLE | 48 | 83 | 95 |
| Bertani et al. [36] | 2013 | pCLE | 100 | 100 | 83 |
| Canto et al. [38] | 2014 | eCLE | 192 | 100 | 95 |
| Dolak et al. [34] | 2014 | eCLE | 38 | - | - |
| Legget et al. [48] | 2016 | pCLE | 27 | 76 | 79 |
| Caillol et al. [37] | 2017 | pCLE | 31 | 93 | 71 |
| Shah et al. [39] | 2017 | pCLE | 66 | 67 | 98 |
| Richardson et al. [40] | 2018 | pCLE | 172 | - | - |
| Kunovsky et al. [41] | 2020 | pCLE | 14 | - | - |
| Kollar et al. [42] | 2020 | pCLE | 67 | 88 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaculová, J.; Kroupa, R.; Kala, Z.; Dolina, J.; Grolich, T.; Vlažný, J.; Said, D.; Izakovičová Hollá, L.; Bořilová Linhartová, P.; Procházka, V.; et al. The Use of Confocal Laser Endomicroscopy in Diagnosing Barrett’s Esophagus and Esophageal Adenocarcinoma. Diagnostics 2022, 12, 1616. https://doi.org/10.3390/diagnostics12071616
Vaculová J, Kroupa R, Kala Z, Dolina J, Grolich T, Vlažný J, Said D, Izakovičová Hollá L, Bořilová Linhartová P, Procházka V, et al. The Use of Confocal Laser Endomicroscopy in Diagnosing Barrett’s Esophagus and Esophageal Adenocarcinoma. Diagnostics. 2022; 12(7):1616. https://doi.org/10.3390/diagnostics12071616
Chicago/Turabian StyleVaculová, Jitka, Radek Kroupa, Zdeněk Kala, Jiří Dolina, Tomáš Grolich, Jakub Vlažný, David Said, Lydie Izakovičová Hollá, Petra Bořilová Linhartová, Vladimír Procházka, and et al. 2022. "The Use of Confocal Laser Endomicroscopy in Diagnosing Barrett’s Esophagus and Esophageal Adenocarcinoma" Diagnostics 12, no. 7: 1616. https://doi.org/10.3390/diagnostics12071616
APA StyleVaculová, J., Kroupa, R., Kala, Z., Dolina, J., Grolich, T., Vlažný, J., Said, D., Izakovičová Hollá, L., Bořilová Linhartová, P., Procházka, V., Joukal, M., Jabandžiev, P., Slabý, O., & Kunovský, L. (2022). The Use of Confocal Laser Endomicroscopy in Diagnosing Barrett’s Esophagus and Esophageal Adenocarcinoma. Diagnostics, 12(7), 1616. https://doi.org/10.3390/diagnostics12071616








