Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings
Abstract
1. Introduction
2. Materials and Methods
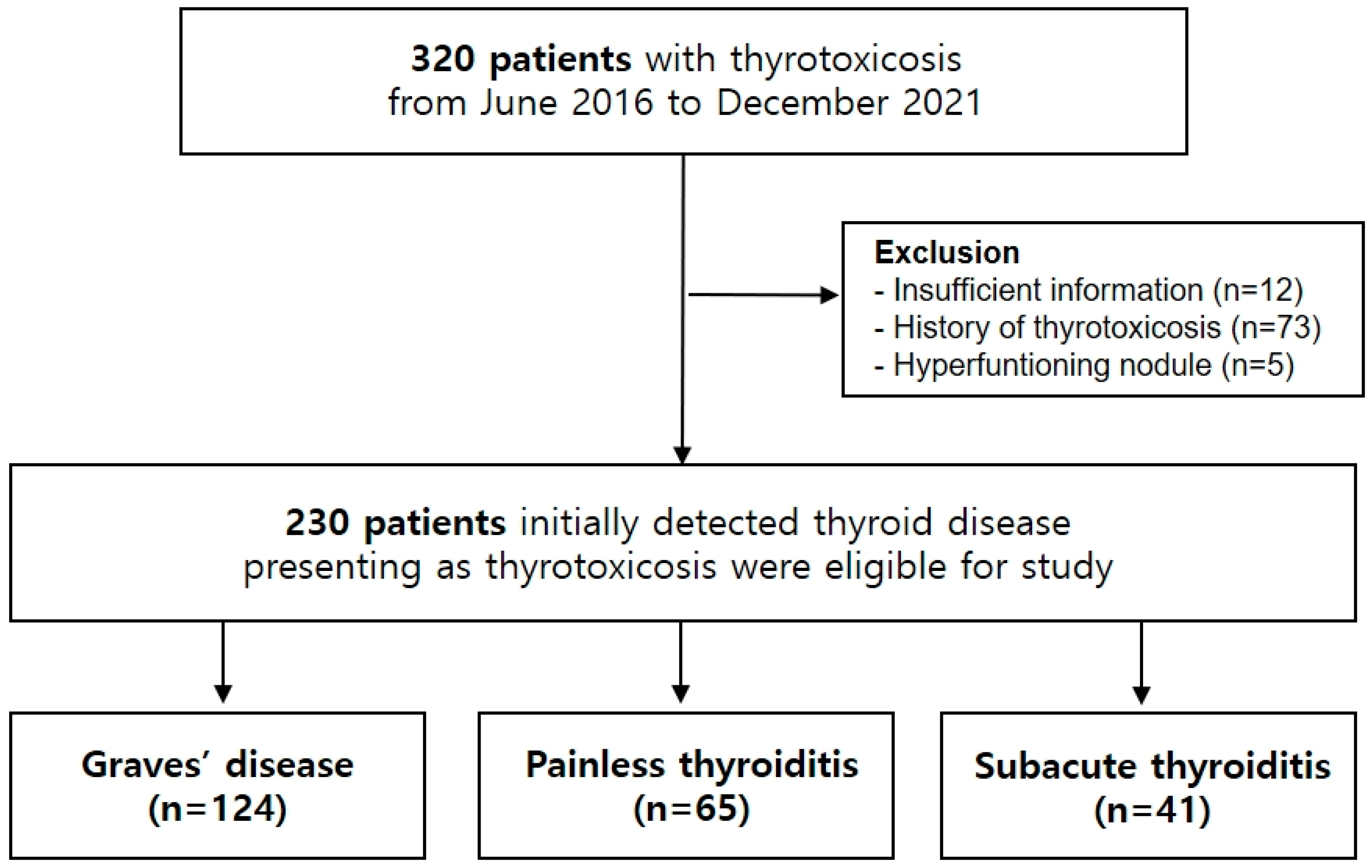
2.1. Patients
2.2. Measurements
2.3. Clinical Validation
2.4. Statistical Analyses
3. Results and Discussion
3.1. Baseline Characteristics of the Study Cohort
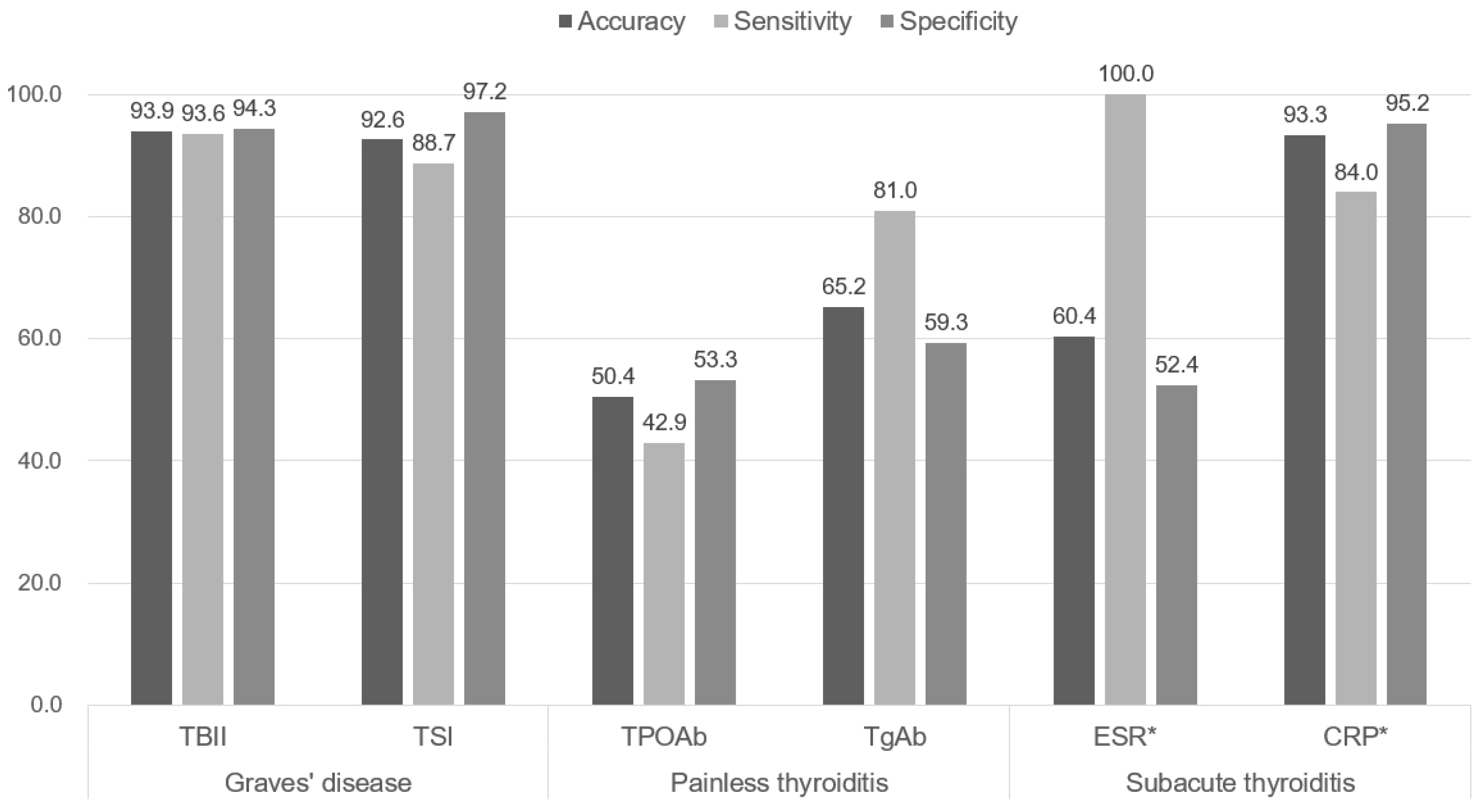
3.2. Predictive Values of Disease Specific Biomarkers
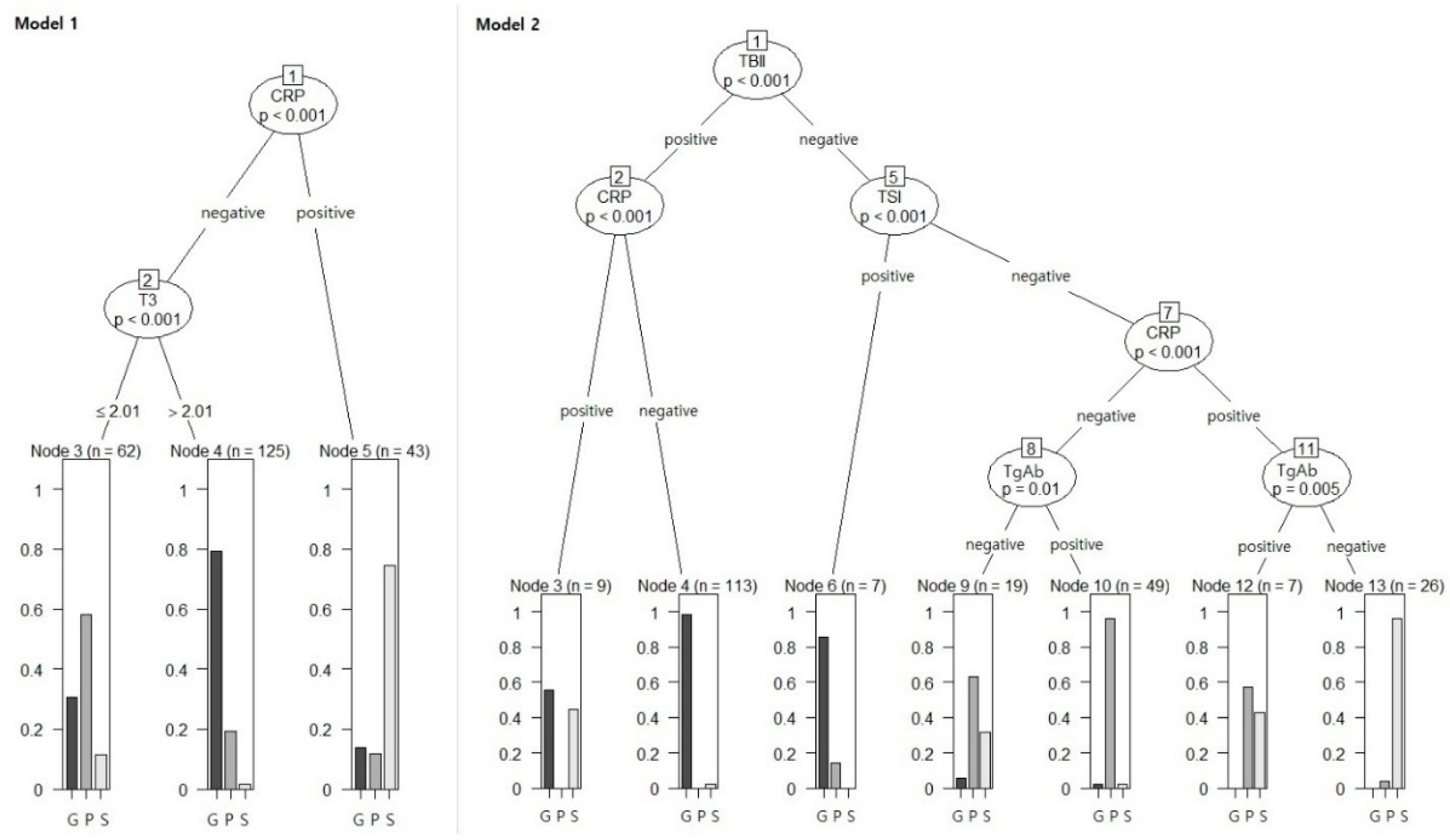
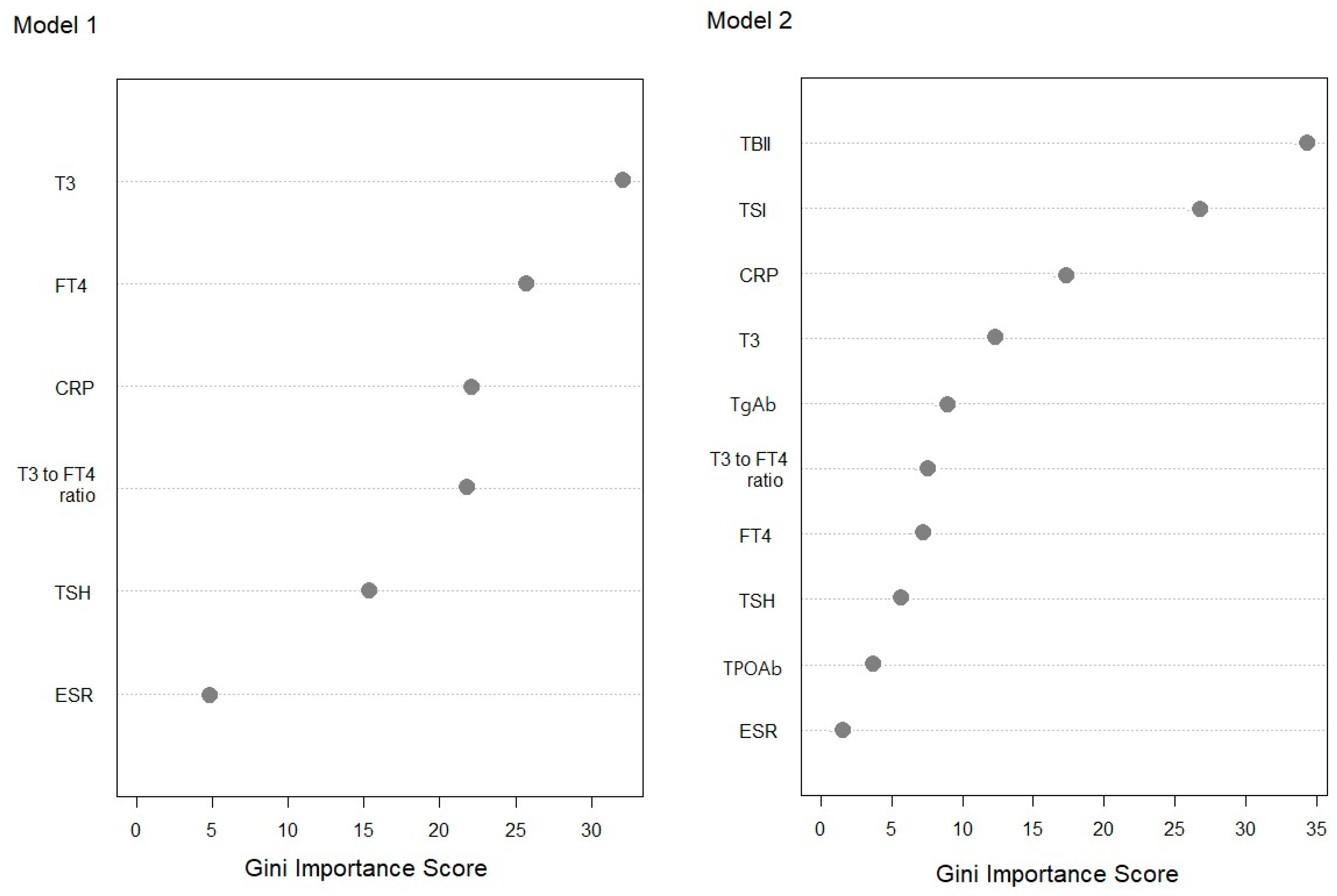
3.3. Comparisons of Machine Learning Algorithms
3.4. Clinical Validation Based on the Review of Medical Records
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Bartalena, L.; Hegedus, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European thyroid association guideline for the management of graves’ hyperthyroidism. Eur. Thyroid J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Nikolai, T.F.; Brosseau, J.; Kettrick, M.A.; Roberts, R.; Beltaos, E. Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (silent thyroiditis). Arch. Intern. Med. 1980, 140, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Fatourechi, V.; Aniszewski, J.P.; Fatourechi, G.Z.; Atkinson, E.J.; Jacobsen, S.J. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J. Clin. Endocrinol. Metab. 2003, 88, 2100–2105. [Google Scholar] [CrossRef]
- Barbesino, G.; Tomer, Y. Clinical review: Clinical utility of TSH receptor antibodies. J. Clin. Endocrinol. Metab. 2013, 98, 2247–2255. [Google Scholar] [CrossRef]
- Perdomo, C.M.; García-Goñi, M.; Sancho, L.; Paricio, J.; Lozano, M.D.; de la Higuera, M.; Currás, M.; Arbizu, J.; Galofré, J.C. Evaluation of the role of thyroid scintigraphy in the differential diagnosis of thyrotoxicosis. Clin. Endocrinol. 2021, 94, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Grayson, R.R. Factors which influence the radioactive iodine thyroidal uptake test. Am. J. Med. 1960, 28, 397–415. [Google Scholar] [CrossRef]
- Schulz, S.L.; Seeberger, U.; Hengstmann, J.H. Color Doppler sonography in hypothyroidism. Eur. J. Ultrasound 2003, 16, 183–189. [Google Scholar] [CrossRef]
- Reinwein, D.; Benker, G.; König, M.P.; Pinchera, A.; Schatz, H.; Schleusener, A. The different types of hyperthyroidism in Europe. Results of a prospective survey of 924 patients. J. Endocrinol. Investig. 1988, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Hakoshima, M.; Katsuyama, H. Differences in clinical and laboratory findings among graves’ disease, painless thyroiditis and subacute thyroiditis patients with hyperthyroidism. J. Endocrinol. Metab. 2019, 9, 37–42. [Google Scholar] [CrossRef]
- Amino, N.; Yabu, Y.; Miki, T.; Morimoto, S.; Kumahara, Y.; Mori, H.; Iwatani, Y.; Nishi, K.; Nakatani, K.; Miyai, K. Serum ratio of triiodothyronine to thyroxine, and thyroxine-binding globulin and calcitonin concentrations in Graves’ disease and destruction-induced thyrotoxicosis. J. Clin. Endocrinol. Metab. 1981, 53, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Knudsen, N.; Pedersen, I.B.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. Determinants of serum T4 and T3 at the time of diagnosis in nosological types of thyrotoxicosis: A population-based study. Eur. J. Endocrinol. 2013, 169, 537–545. [Google Scholar] [CrossRef][Green Version]
- Woeber, K.A. Triiodothyronine production in Graves’ hyperthyroidism. Thyroid 2006, 16, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hidaka, Y.; Tada, H.; Takano, T.; Kashiwai, T.; Tatsumi, K.I.; Ichihara, K.; Amino, N. Simple and practical parameters for differentiation between destruction-induced thyrotoxicosis and Graves’ thyrotoxicosis. Clin. Endocrinol. 2002, 57, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ladenson, P.W. Discordant hypothyroxinemia and hypertriiodothyroninemia in treated patients with hyperthyroid Graves’ disease. J. Clin. Endocrinol. Metab. 1986, 63, 102–106. [Google Scholar] [CrossRef]
- Evans, C.; Morgenthaler, N.G.; Lee, S.; Llewellyn, D.H.; Clifton-Bligh, R.; John, R.; Lazarus, J.H.; Chatterjee, V.K.; Ludgate, M. Development of a luminescent bioassay for thyroid stimulating antibodies. J. Clin. Endocrinol. Metab. 1999, 84, 374–377. [Google Scholar] [CrossRef]
- Lytton, S.D.; Ponto, K.A.; Kanitz, M.; Matheis, N.; Kohn, L.D.; Kahaly, G.J. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2010, 95, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Cerrone, D.; Harii, N.; Thornton, M.; Kohn, L.D.; Dagia, N.M.; Bucci, I.; Carpentieri, M.; Di Nenno, B.; Di Blasio, A.; et al. A TSHR-LH/CGR chimera that measures functional thyroid-stimulating autoantibodies (TSAb) can predict remission or recurrence in Graves’ patients undergoing antithyroid drug (ATD) treatment. J. Clin. Endocrinol. Metab. 2012, 97, E1080–E1087. [Google Scholar] [CrossRef]
- Cheng, X.; Chai, X.; Ma, C.; Jia, Q.; Zhao, H.; Dong, Z.; Zhang, Z.; Hu, Y.; Song, A.; Yang, G.; et al. Clinical diagnostic performance of a fully automated TSI immunoassay vs. that of an automated anti-TSHR immunoassay for Graves’ disease: A Chinese multicenter study. Endocrine 2021, 71, 139–148. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, S.M.; Rapoport, B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: Potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid 2013, 23, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lytton, S.D.; Kahaly, G.J. Bioassays for TSH-receptor autoantibodies: An update. Autoimmun. Rev. 2010, 10, 116–122. [Google Scholar] [CrossRef]
- Kiaei, D.; Molinaro, R. A negative thyretain TSI bioassay result does not exclude the possibility of the presence of TSI. Horm. Metab. Res. 2020, 52, 124–125. [Google Scholar] [CrossRef]
- Rahhal, S.N.; Eugster, E.A. Thyroid stimulating immunoglobulin is often negative in children with Graves’ disease. J. Pediatr. Endocrinol. Metab. 2008, 21, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Jang, H.W.; Kim, S.K.; Choi, J.Y.; Kim, J.Y.; Hur, K.Y.; Kim, J.H.; Min, Y.K.; Chung, J.H.; Kim, S.W. Diagnostic value of a chimeric TSH receptor (Mc4)-based bioassay for Graves’ disease. Korean J. Intern. Med. 2011, 26, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Takasu, N.; Oshiro, C.; Akamine, H.; Komiya, I.; Nagata, A.; Sato, Y.; Yoshimura, H.; Ito, K. Thyroid-stimulating antibody and TSH-binding inhibitor immunoglobulin in 277 Graves’ patients and in 686 normal subjects. J. Endocrinol. Investig. 1997, 20, 452–461. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Olchovsky, D.; Tsvetov, G.; Shimon, I. Subacute thyroiditis: Clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J. Endocrinol. Investig. 2007, 30, 631–635. [Google Scholar] [CrossRef]
- Pearce, E.N.; Bogazzi, F.; Martino, E.; Brogioni, S.; Pardini, E.; Pellegrini, G.; Parkes, A.B.; Lazarus, J.H.; Pinchera, A.; Braverman, L.E. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid 2003, 13, 643–648. [Google Scholar] [CrossRef]
- Osei-Bimpong, A.; Meek, J.H.; Lewis, S.M. ESR or CRP? A comparison of their clinical utility. Hematology 2007, 12, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.; Brownlee, Y.; Foster, P.; Geekie, C.; Kelly, P.; Robertson, S.; Wade, E.; Braun, H.B.; Staub, U.; Michel, G.; et al. The quantitative measurement of autoantibodies to thyroglobulin and thyroid peroxidase by automated microparticle based immunoassays in Hashimoto’s disease, Graves’ disease and a follow-up study on postpartum thyroid disease. Clin. Lab. 2000, 46, 57–61. [Google Scholar] [PubMed]
- Frohlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Ohye, H. Recurrent severe painless thyroiditis requiring multiple treatments with radioactive iodine. Thyroid 2008, 18, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, E.; Amino, N.; Kudo, T.; Ito, M.; Fukata, S.; Nishikawa, M.; Nakamura, H.; Miyauchi, A. Comparison of thyroglobulin and thyroid peroxidase antibodies measured by five different kits in autoimmune thyroid diseases. Endocr. J. 2017, 64, 955–961. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kwak, M.K.; Hong, S.M.; Hong, E.G. Changes in Thyroid Peroxidase and Thyroglobulin Antibodies Might Be Associated with Graves’ Disease Relapse after Antithyroid Drug Therapy. Endocrinol. Metab. 2019, 34, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Pei, S.; Chen, Y.; He, X.; Dong, Y.; Zhang, L.; Mo, X.; Huang, W.; Cong, S.; et al. Machine learning-assisted system for thyroid nodule diagnosis. Thyroid 2019, 29, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.K.; Ren, T.T.; Yin, Y.F.; Shi, H.; Wang, H.X.; Zhou, B.Y.; Wang, X.R.; Li, X.; Zhang, Y.F.; Liu, C.; et al. A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Thyroid 2021, 31, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cortes, C. Suppor-vector network. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Hand, D.J.; Yu, K. Idiot’s bayes: Not so stupid after all? Int. Stat. Rev. 2001, 69, 385–398. [Google Scholar]
- Yadav, S.S.; Jadhav, S.M. Deep convolutional neural network based medical image classification for disease diagnosis. J. Big Data 2019, 6, 113. [Google Scholar] [CrossRef]
- Laurberg, P.; Cerqueira, C.; Ovesen, L.; Rasmussen, L.B.; Perrild, H.; Andersen, S.; Pedersen, I.B.; Carlé, A. Iodine intake as a determinant of thyroid disorders in populations. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 13–27. [Google Scholar] [CrossRef]
| Graves’ Disease (n = 124) | Painless Thyroiditis (n = 65) | Subacute Thyroiditis (n = 41) | p | |
|---|---|---|---|---|
| Age years, mean (SD) | 46.89 (14.81) | 47.05 (11.30) | 51.71 (13.46) | 0.132 |
| Female, number (%) | 85 (67.7) | 53 (81.5) | 33 (80.5) | 0.070 |
| Thyroid function test | ||||
| TSH μIU/mL, mean (SD) | 0.011 (0.033) | 0.040 (0.159) | 0.038 (0.091) | 0.081 |
| T3 ng/mL, mean (SD) | 3.45 (1.75) | 1.96 (0.83) | 2.08 (0.70) | <0.001 |
| FT4 ng/dL, mean (SD) | 4.09 (2.15) | 2.72 (1.09) | 3.12 (1.58) | <0.001 |
| Thyroid auto-antibodies | ||||
| TBII positive, number (%) | 116 (93.5) | 0 (0.0) | 6 (14.6) | <0.001 |
| TSI positive, number (%) | 111 (89.5) | 1 (1.5) | 2 (4.7) | <0.001 |
| TPOAb positive, number (%) | 74 (59.7) | 27 (41.5) | 5 (11.6) | <0.001 |
| TgAb positive, number (%) | 62 (50.4) | 52 (80.0) | 5 (12.2) | <0.001 |
| Inflammatory markers | ||||
| ESR mm/h, mean (SD) | 13.39 (11.34) | 14.61 (12.26) | 66.08 (35.42) | <0.001 |
| CRP mg/L, mean (SD) | 1.36 (1.80) | 1.93 (3.67) | 35.46 (58.16) | <0.001 |
| Accuracy Classifier | Training Set (n = 161) | Test Set (n = 69) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | G | P | S | Overall | G | P | S | |
| Model 1 | ||||||||
| CART | 0.74 | 0.89 | 0.72 | 0.87 | 0.70 | 0.75 | 0.62 | 0.85 |
| RF | 0.80 | 0.82 | 0.77 | 0.87 | 0.70 | 0.69 | 0.58 | 0.85 |
| LDA | 0.74 | 0.79 | 0.72 | 0.87 | 0.70 | 0.74 | 0.60 | 0.85 |
| SVM | 0.76 | 0.80 | 0.73 | 0.87 | 0.65 | 0.69 | 0.51 | 0.85 |
| kNN | 0.75 | 0.73 | 0.65 | 0.81 | 0.67 | 0.78 | 0.63 | 0.70 |
| NB | 0.64 | 0.66 | 0.54 | 0.82 | 0.70 | 0.67 | 0.54 | 0.77 |
| NN | 0.74 | 0.79 | 0.71 | 0.87 | 0.68 | 0.69 | 0.58 | 0.80 |
| Model 2 | ||||||||
| CART | 0.90 | 0.95 | 0.93 | 0.83 | 0.86 | 0.91 | 0.88 | 0.82 |
| RF | 0.98 | 0.98 | 0.99 | 0.97 | 0.90 | 0.96 | 0.90 | 0.86 |
| LDA | 0.91 | 0.96 | 0.93 | 0.86 | 0.87 | 0.93 | 0.88 | 0.86 |
| SVM | 0.92 | 0.97 | 0.93 | 0.87 | 0.87 | 0.93 | 0.88 | 0.86 |
| kNN | 0.82 | 0.86 | 0.80 | 0.89 | 0.78 | 0.79 | 0.75 | 0.89 |
| NB | 0.88 | 0.94 | 0.89 | 0.84 | 0.84 | 0.90 | 0.88 | 0.81 |
| NN | 0.91 | 0.95 | 0.95 | 0.84 | 0.88 | 0.92 | 0.95 | 0.86 |
| Diagnosed as Graves’ Disease | Graves’ Disease (n = 124) | Painless Thyroiditis (n = 65) | Subacute Thyroiditis (n = 41) | Accuracy for Graves’ Disease |
|---|---|---|---|---|
| T3 † | 101 | 18 | 17 | 0.75 |
| TBII | 116 | 0 | 6 | 0.94 |
| Thyroid scan * | 48/73 | 1/48 | 2/32 | 0.82 |
| Initial ATD Prescription | 79 | 11 | 3 | 0.74 |
| RF Model 2 | 122 | 0 | 1 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Baek, H.-S.; Ha, J.; Kim, M.K.; Kwon, H.-S.; Song, K.-H.; Lim, D.-J.; Baek, K.-H. Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings. Diagnostics 2022, 12, 1468. https://doi.org/10.3390/diagnostics12061468
Kim J, Baek H-S, Ha J, Kim MK, Kwon H-S, Song K-H, Lim D-J, Baek K-H. Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings. Diagnostics. 2022; 12(6):1468. https://doi.org/10.3390/diagnostics12061468
Chicago/Turabian StyleKim, Jinyoung, Han-Sang Baek, Jeonghoon Ha, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Dong-Jun Lim, and Ki-Hyun Baek. 2022. "Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings" Diagnostics 12, no. 6: 1468. https://doi.org/10.3390/diagnostics12061468
APA StyleKim, J., Baek, H.-S., Ha, J., Kim, M. K., Kwon, H.-S., Song, K.-H., Lim, D.-J., & Baek, K.-H. (2022). Differential Diagnosis of Thyrotoxicosis by Machine Learning Models with Laboratory Findings. Diagnostics, 12(6), 1468. https://doi.org/10.3390/diagnostics12061468






