Sonographic Evaluation of Muscle Echogenicity for the Detection of Intensive Care Unit-Acquired Weakness: A Pilot Single-Center Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Inclusion and Exclusion Criteria
2.2. Assessment of Intensive Care Unit-Acquired Weakness
2.3. Muscle Ultrasound Protocol
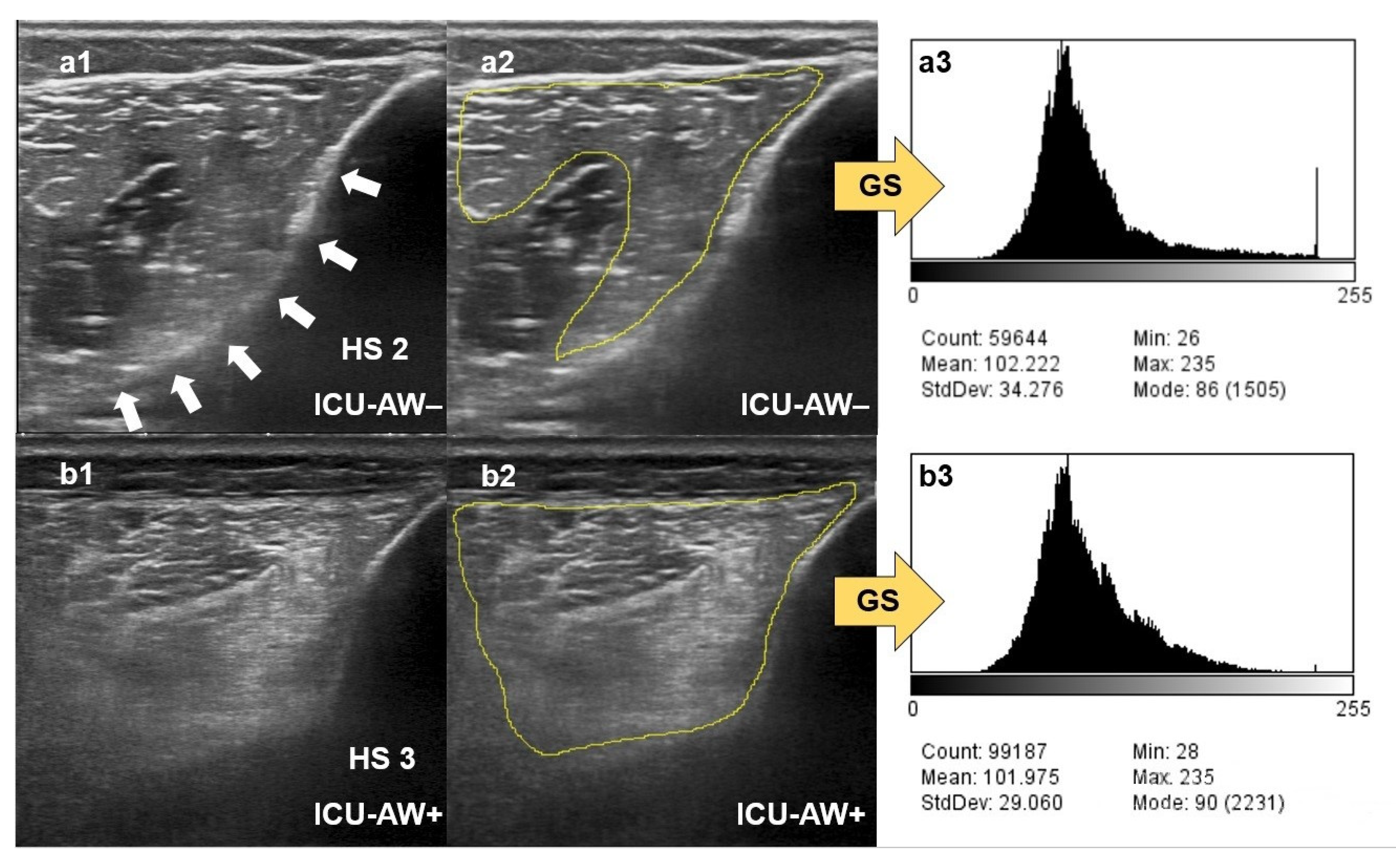
2.4. Qualitative Assessment of Muscle Echogenicity Using the Heckmatt Scale
2.5. Quantitative Greyscale Analysis of Muscle Echogenicity with ImageJ
2.6. Statistical Analysis
3. Results
3.1. Study Population Characteristics
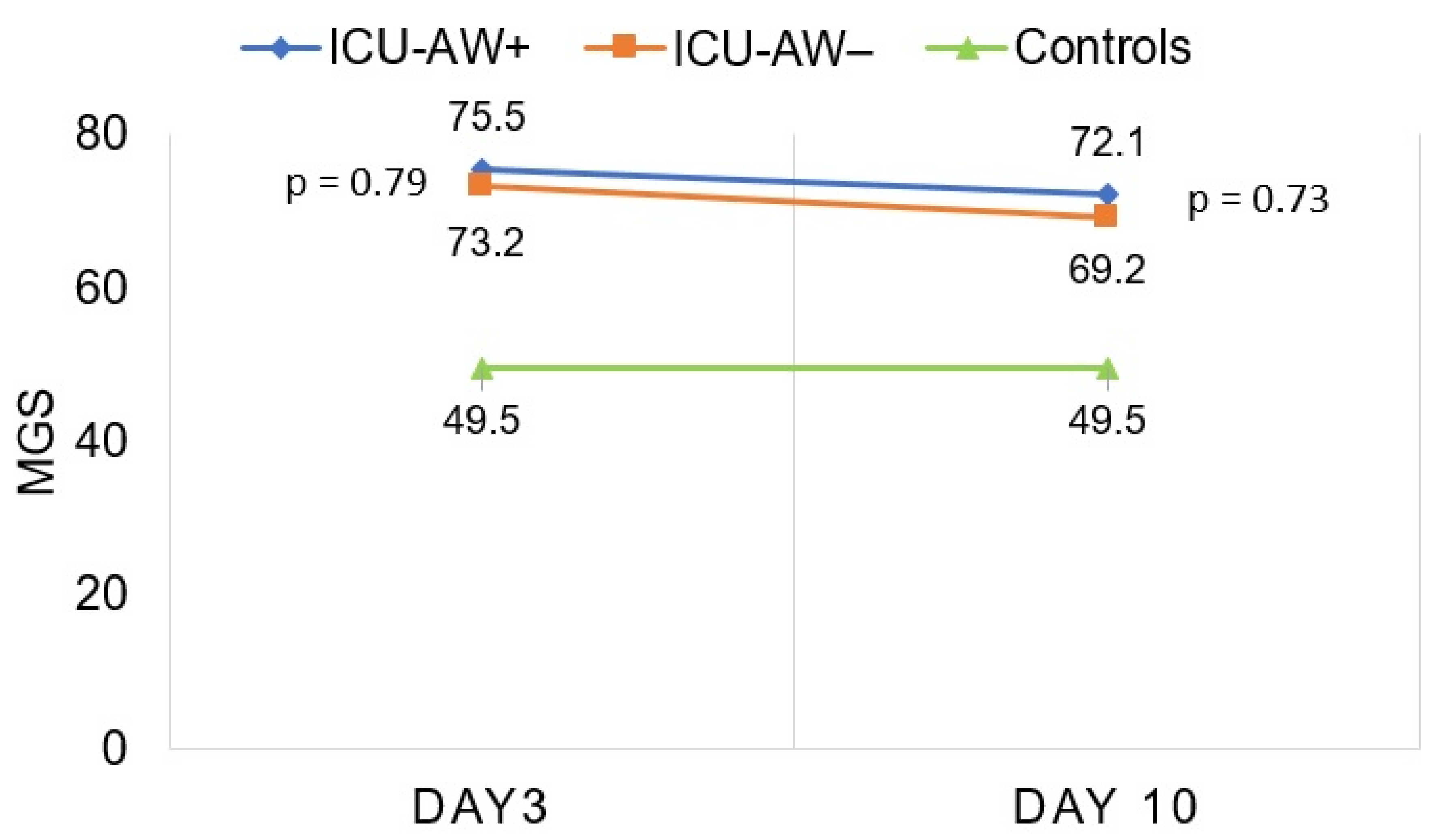
3.2. Quantitative Greyscale Analysis of Muscle Echogenicity in Patients with and without ICU-AW Compared to Controls
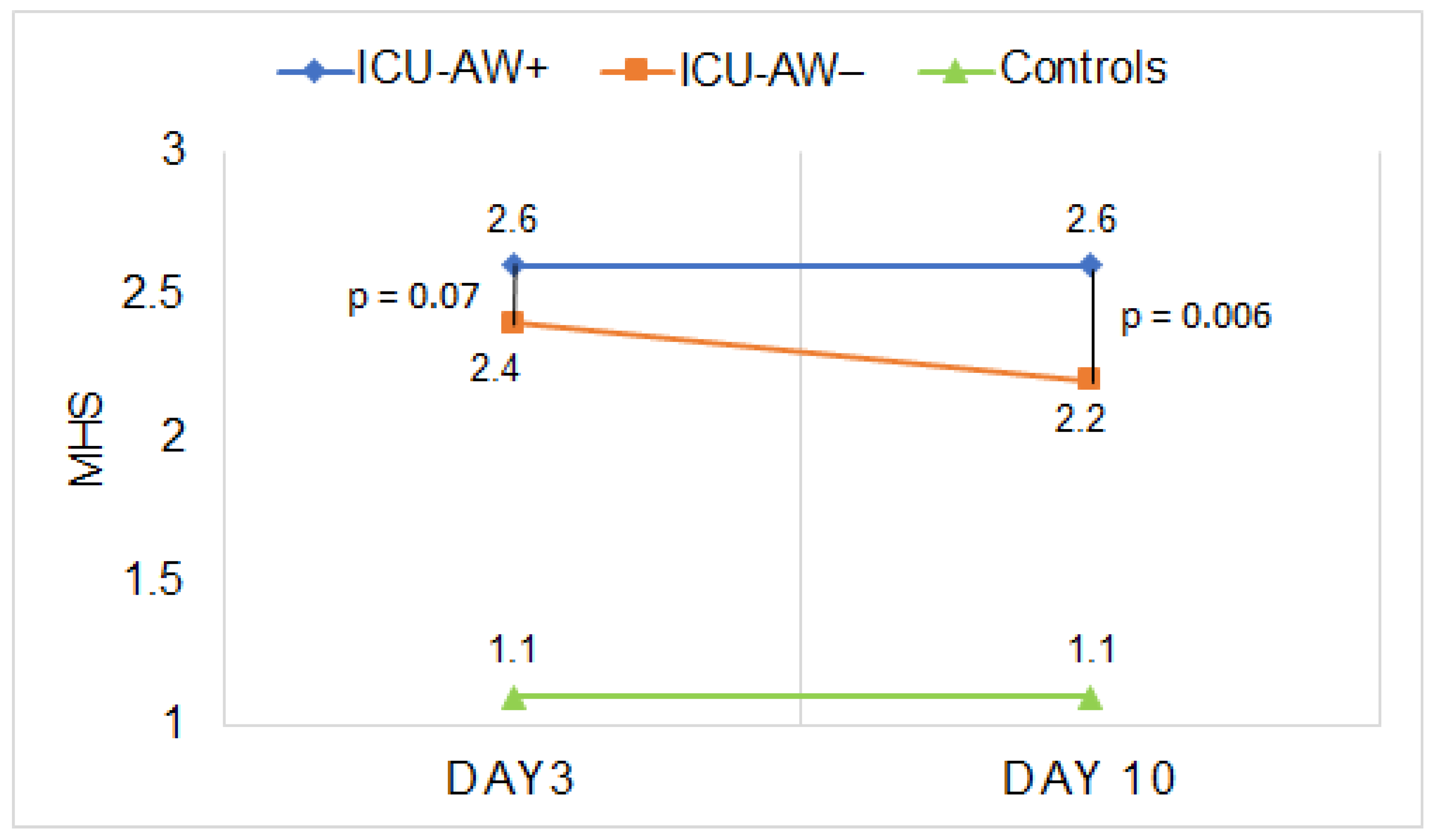
3.3. Qualitative Ultrasound Assessment of Muscle Echogenicity in Patients with and without ICU-AW Compared to Controls
3.4. Quantitative Greyscale Analysis of Muscle Echogenicity Inpatients with and without ICU-AW
3.5. Qualitative Ultrasound Assessment of Muscle Echogenicity in Patients with and without ICU-AW
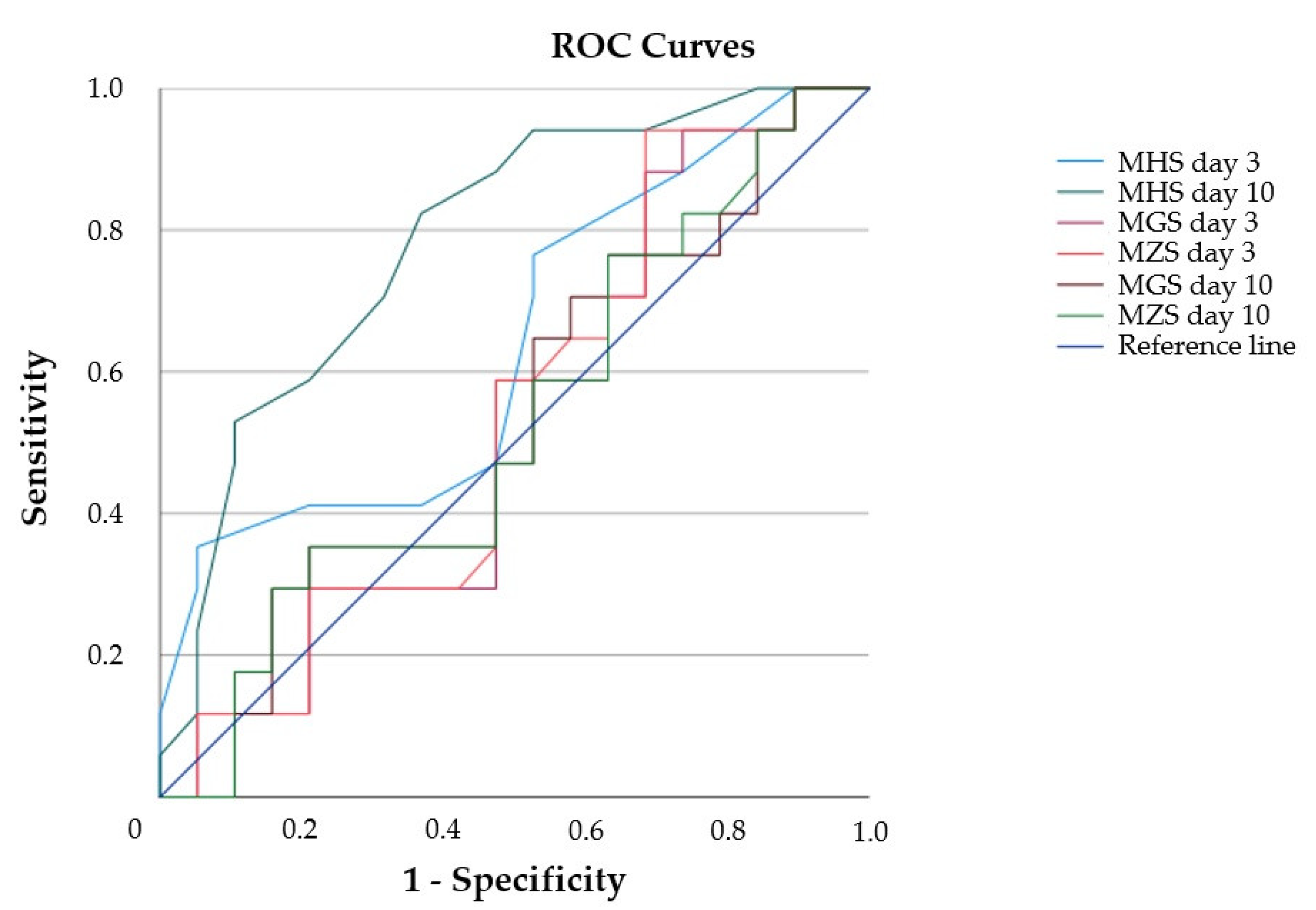
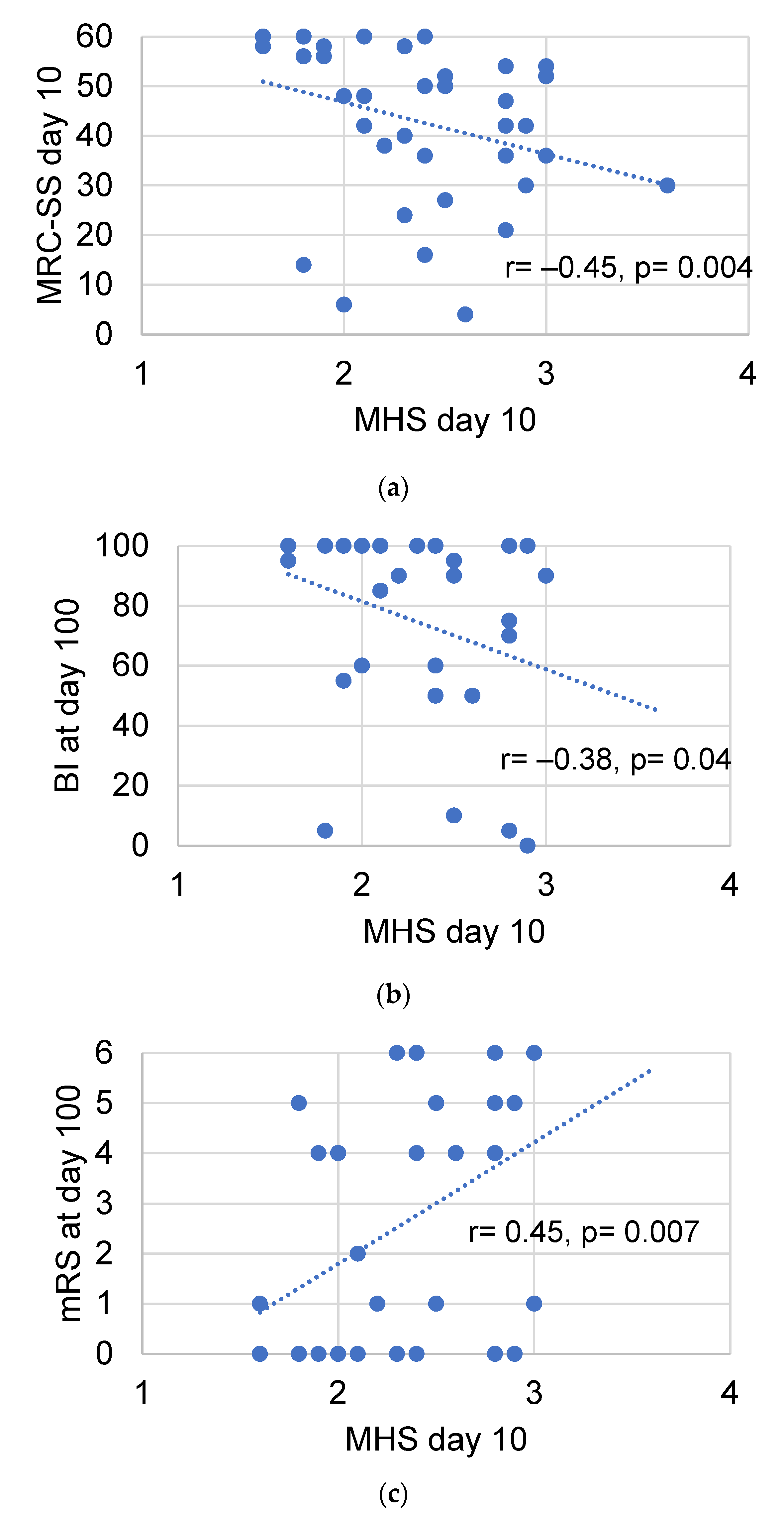
3.6. Diagnostic Value of Muscle Echogenicity and Relationship to Functional Patient Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connolly, B.; MacBean, V.; Crowley, C.; Lunt, A.; Moxham, J.; Rafferty, G.F.; Hart, N. Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: A systematic review. Crit. Care Med. 2015, 43, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunnell, A.; Ney, J.; Gellhorn, A.; Hough, C.L. Quantitative neuromuscular ultrasound in intensive care unit-acquired weakness: A systematic review. Muscle Nerve 2015, 52, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formenti, P.; Umbrello, M.; Coppola, S.; Froio, S.; Chiumello, D. Clinical review: Peripheral muscular ultrasound in the ICU. Ann. Intensive Care 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnacho-Montero, J.; Madrazo-Osuna, J.; Garcia-Garmendia, J.L.; Ortiz-Leyba, C.; Jiménez-Jiménez, F.; Barrero-Almodóvar, A.; Garnacho-Montero, M.; Moyano-Del-Estad, M. Critical illness polyneuropathy: Risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001, 27, 1288–1296. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Amaya-Villar, R.; García-Garmendía, J.L.; Madrazo-Osuna, J.; Ortiz-Leyba, C. Effect of critical illness polyneuropathy on the withdrawal from mechanical ventilation and the length of stay in septic patients. Crit. Care Med. 2005, 33, 349–354. [Google Scholar] [CrossRef]
- Gruther, W.; Benesch, T.; Zorn, C.; Paternostro-Sluga, T.; Quittan, M.; Fialka-Moser, V.; Spiss, C.; Kainberger, F.; Crevenna, R. Muscle wasting in intensive care patients: Ultrasound observation of the M. quadriceps femoris muscle layer. J. Rehabil. Med. 2008, 40, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Hadda, V.; Kumar, R.; Khilnani, G.C.; Kalaivani, M.; Madan, K.; Tiwari, P. Trends of loss of peripheral muscle thickness on ultrasonography and its relationship with outcomes among patients with sepsis. J. Intensive Care 2018, 6, 81. [Google Scholar] [CrossRef]
- Grimm, A.; Teschner, U.; Porzelius, C.; Ludewig, K.; Zielske, J.; Witte, O.W.; Brunkhorst, F.M.; Axer, H. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit. Care 2013, 17, R227. [Google Scholar] [CrossRef] [Green Version]
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Van Alfen, N.; Mah, J.K. Neuromuscular Ultrasound: A New Tool in Your Toolbox. Can. J. Neurol. Sci. 2018, 45, 504–515. [Google Scholar] [CrossRef]
- Heckmatt, J.Z.; Leeman, S.; Dubowitz, V. Ultrasound imaging in the diagnosis of muscle disease. J. Pediatr. 1982, 101, 656–660. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Matta, N.; Nawaz, S.; Stephenson, J.B.; McWilliam, R.C.; Hollman, A. Muscle ultrasound in the assessment of suspected neuromuscular disease in childhood. Neuromuscul. Disord. 1999, 9, 203–207. [Google Scholar] [CrossRef]
- Kenis-Coskun, O.; Gunes, T.; Isak, B.; Yagci, I. Intrarater and Interrater Reliability of Heckmatt Scoring System in Amyotrophic Lateral Sclerosis. J. Clin. Neurophysiol. 2021, 38, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kelmenson, D.A.; Quan, D.; Moss, M. What is the diagnostic accuracy of single nerve conduction studies and muscle ultrasound to identify critical illness polyneuromyopathy: A prospective cohort study. Crit. Care 2018, 22, 342. [Google Scholar] [CrossRef] [Green Version]
- Sarwal, A.; Parry, S.M.; Berry, M.J.; Hsu, F.C.; Lewis, M.T.; Justus, N.W. Interobserver Reliability of Quantitative Muscle Sonographic Analysis in the Critically Ill Population. J. Ultrasound Med. 2015, 34, 1191–1200. [Google Scholar] [CrossRef]
- Witteveen, E.; Sommers, J.; Wieske, L.; Doorduin, J.; van Alfen, N.; Schultz, M.J. Diagnostic accuracy of quantitative neuromuscular ultrasound for the diagnosis of intensive care unit-acquired weakness: A cross-sectional observational study. Ann. Intensive Care 2017, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Memmott, B.; Poulson, J.; Harmon, B.; Hammond, C. Quantitative Ultrasound Imaging to Assess Skeletal Muscles in Adults with Multiple Sclerosis: A Feasibility Study. J. Ultrasound Med. 2019, 38, 2915–2923. [Google Scholar] [CrossRef]
- Umbrello, M.; Guglielmetti, L.; Formenti, P.; Antonucci, E.; Cereghini, S.; Filardo, C. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19_related ARDS. Nutrition 2021, 91, 111449. [Google Scholar] [CrossRef]
- Formenti, P.; Umbrello, M.; Castagna, V.; Cenci, S.; Bichi, F.; Pozzi, T. Respiratory and peripheral muscular ultrasound characteristics in ICU COVID 19 ARDS patients. J. Crit. Care 2022, 67, 14–20. [Google Scholar] [CrossRef]
- Patejdl, R.; Klawitter, F.; Walter, U.; Zanaty, K.; Schwander, F.; Sellmann, T. A novel ex vivo model for critical illness neuromyopathy using freshly resected human colon smooth muscle. Sci. Rep. 2021, 11, 24249. [Google Scholar] [CrossRef] [PubMed]
- Weber-Carstens, S.; Deja, M.; Koch, S.; Spranger, J.; Bubser, F.; Wernecke, K.D. Risk factors in critical illness myopathy during the early course of critical illness: A prospective observational study. Crit. Care 2010, 14, R119. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.D.; Marshall, S.A.; Cornblath, D.R.; Hoke, A.; Needham, D.M.; De Jonghe, B.; Sharshar, T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit. Care Med. 2009, 37, S299–S308. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J.A.; Reilly, J.P.; Schweickert, W.D.; Anderson, B.J.; Khoury, V.; Shashaty, M.G. Quantitative peripheral muscle ultrasound in sepsis: Muscle area superior to thickness. J. Crit. Care 2018, 47, 324–330. [Google Scholar] [CrossRef]
- Pillen, S.; van Keimpema, M.; Nievelstein, R.A.J.; Verrips, A.; van Kruijsbergen-Raijmann, W.; Zwarts, M.J. Skeletal muscle ultrasonography: Visual versus quantitative evaluation. Ultrasound Med. Biol. 2006, 32, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.S.; Kwayisi, G.; Griffin, L.P.; Sarwal, A.; Walker, F.O.; Harris, J.M. Quantitative neuromuscular ultrasound in the intensive care unit. Muscle Nerve 2013, 47, 255–259. [Google Scholar] [CrossRef]
- Annetta, M.G.; Pittiruti, M.; Silvestri, D.; Grieco, D.L.; Maccaglia, A.; La Torre, M.F. Ultrasound assessment of rectus femoris and anterior tibialis muscles in young trauma patients. Ann. Intensive Care 2017, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Patejdl, R.; Walter, U.; Rosener, S.; Sauer, M.; Reuter, D.A.; Ehler, J. Muscular Ultrasound, Syndecan-1 and Procalcitonin Serum Levels to Assess Intensive Care Unit-Acquired Weakness. Can. J. Neurol. Sci. 2019, 46, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Yamada, Y.; Fukumoto, Y.; Ishihara, T.; Yokoyama, K.; Yoshida, T. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin. Interv. Aging 2013, 8, 993. [Google Scholar] [CrossRef] [Green Version]
- Rech, A.; Radaelli, R.; Goltz, F.R.; da Rosa, L.H.T.; Schneider, C.D.; Pinto, R.S. Echo intensity is negatively associated with functional capacity in older women. Age 2014, 36, 9708. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; Arts, I.M.P.; Zwarts, M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008, 37, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.M.P.; Pillen, S.; Schelhaas, H.J.; Overeem, S.; Zwarts, M.J. Normal values for quantitative muscle ultrasonography in adults. Muscle Nerve 2010, 41, 32–41. [Google Scholar] [CrossRef]
- Caresio, C.; Molinari, F.; Emanuel, G.; Minetto, M.A. Muscle echo intensity: Reliability and conditioning factors. Clin. Physiol. Funct. Imaging 2015, 35, 393–403. [Google Scholar] [CrossRef]
- Reimers, K.; Reimers, C.D.; Wagner, S.; Paetzke, I.; Pongratz, D.E. Skeletal muscle sonography: A correlative study of echogenicity and morphology. J. Ultrasound Med. 1993, 12, 73–77. [Google Scholar] [CrossRef]

| Healthy Controls | ICU-AW− | ICU-AW+ | p Value (ICU-AW − vs. Healthy Controls) | p Value (ICU-AW+ vs. Healthy Controls) | p Value (ICU-AW+ vs. ICU-AW−) | |
|---|---|---|---|---|---|---|
| Total (%) | 10 (26.3) | 20 (41.7) | 18 (37.5) | N/A | N/A | N/A |
| Female (%) | 4 (40) | 5 (25) | 8 (44.4) | 0.43 | 1 | 0.11 |
| Age, years (SD) | 53.1 (8.0) | 68.1 (14.2) | 70.8 (11.5) | 0.002 | <0.0001 | 0.52 |
| BMI, kg/cm2 (SD) | 27.3 (4.9) | 28.4 (5.1) | 28.6 (5.9) | 0.56 | 0.56 | 0.94 |
| APACHE II (SD) | N/A | 23.7 (6.2) | 26.2 (3.7) | N/A | N/A | 0.14 |
| SOFA day 3 (SD) | N/A | 10.1 (2.3) | 12.9 (2.8) | N/A | N/A | 0.001 |
| SOFA day 10 (SD) | N/A | 3.6 (3.0) | 6.6 (2.8) | N/A | N/A | 0.001 |
| MRC-SS day 3 (SD) | 60 (0) | 51.2 (12.4) | 30.5 (8.7) | <0.0001 | <0.0001 | 0.01 |
| MRC-SS day 10 (SD) | 60 (0) | 55.4 (4.2) | 29.5 (12.9) | <0.0001 | <0.0001 | <0.0001 |
| mRS 100 days after study inclusion (SD) | 0 (0) | 2.1 (2.6) | 3.1 (2.2) | 0.005 | <0.0001 | 0.32 |
| Barthel index before ICU admission (SD) | N/A | 97.5 (4.4) | 93.9 (16.4) | N/A | N/A | 0.68 |
| Barthel index 100 days after study inclusion (SD) | N/A | 87.9 (25.5) | 63.7 (36.0) | N/A | N/A | 0.03 |
| 28-day survival (%) | 10 (100) | 18 (90) | 18 (100) | 0.54 | 1 | 0.49 |
| 100-day survival (%) | 10 (100) | 16 (80) | 16 (88.9) | 0.27 | 0.52 | 0.66 |
| Healthy Controls | ICU-AW+ Day 3 | ICU-AW− Day 3 | ICU-AW+ Day 10 | ICU-AW− Day 10 | |
|---|---|---|---|---|---|
| Biceps brachii left | |||||
| GS | 49.4 (19.7) | 71.8 (25.3) | 66.4 (24.3) | 73.9 (21.5) | 63.4 (26.8) |
| z-Score | N/A | 0.9 (1.0) | 0.7 (0.9) | 0.9 (0.8) | 0.5 (1.0) |
| HS | 1.0 (0.0) | 2.6 (0.6) | 2.2 (0.5) | 2.4 (0.6) | 2.0 (0.7) |
| GSSD | 26.0 (4.4) | 27.4 | 25.9 | 27.8 | 28.5 |
| SFT, cm | 0.5 (0.1) | 0.6 (0.2) | 0.6 (0.2) | 0.7 (0.3) | 0.6 (0.2) |
| Biceps brachii right | |||||
| GS | 44.8 (17.2) | 74.7 (31.2) | 76.5 (38.0) | 70.5 (31.4) | 65.8 (32.2) |
| z-Score | N/A | 1.1 (1.1) | 1.1 (1.4) | 0.9 (1.1) | 0.8 (1.2) |
| HS | 1.0 (0.0) | 2.5 (0.5) | 2.3 (0.7) | 2.3 (0.8) | 2.0 (0.7) |
| GSSD | 27.9 (3.5) | 24.6 | 24.3 | 25.3 | 27.1 |
| SFT, cm | 0.5 (0.1) | 0.7 (0.2) | 0.6 (0.1) | 0.7 (0.3) | 0.6 (0.2) |
| Brachioradialis left | |||||
| GS | 41.6 (17.4) | 71.7 (25.6) | 74.5 (35.8) | 64.9 (24.1) | 62.5 (30.7) |
| z-Score | N/A | 1.2 (1.0) | 1.3 (1.4) | 0.9 (0.9) | 0.8 (1.2) |
| HS | 1.0 (0.0) | 2.5 (0.6) | 2.3 (0.4) | 2.4 (0.5) | 2.0 (0.7) |
| GSSD | 25.6 (5.0) | 27.8 | 25.1 | 26.5 | 26 |
| SFT, cm | 0.5 (0.1) | 0.6 (0.2) | 0.6 (0.1) | 0.6 (0.2) | 0.5 (0.1) |
| Brachioradialis right | |||||
| GS | 44.4 (10.2) | 74.6 (32.3) | 67.4 (35.2) | 67.4 (31.0) | 62.3 (26.5) |
| z-Score | N/A | 1.2 (1.3) | 0.9 (1.4) | 0.9 (1.3) | 0.7 (1.1) |
| HS | 1.0 (0.0) | 2.6 (0.6) | 2.3 (0.6) | 2.4 (0.7) | 1.9 (0.6) |
| GSSD | 24.4 (3.9) | 27.3 | 25.3 | 25.8 | 27.7 |
| SFT, cm | 0.5 (0.1) | 0.6 (0.2) | 0.6 (0.2) | 0.6 (0.2) | 0.5 (0.2) |
| Rectus femoris of the quadriceps femoris left | |||||
| GS | 56.7 (26.2) | 71.4 (16.7) | 77.6 (31.4) | 73.5 (20.0) | 72.4 (30.8) |
| z-Score | N/A | 0.5 (0.6) | 0.7 (1.1) | 0.6 (0.7) | 0.6 (1.1) |
| HS | 1.3 (0.5) | 2.8 (0.7) | 2.4 (0.5) | 2.6 (0.6) | 2.3 (0.8) |
| GSSD | 28.1 (8.4) | 25.1 | 25.1 | 26.3 | 27.6 |
| SFT, cm | 0.7 (0.2) | 1.1 (0.5) | 0.9 (0.4) | 1.1 (0.5) | 0.9 (0.3) |
| Rectus femoris of the quadriceps femoris right | |||||
| GS | 63.7 (7.7) | 69.8 (21.3) | 71.7 (30.9) | 73.2 (25.5) | 73.4 (27.6) |
| z-Score | N/A | 0.2 (0.7) | 0.3 (1.0) | 0.3 (0.8) | 0.3 (0.9) |
| HS | 1.1 (0.3) | 2.6 (0.7) | 2.4 (0.6) | 2.6 (0.5) | 2.4 (0.6) |
| GSSD | 31.2 (3.8) | 25.4 | 23.6 | 28.4 | 27.4 |
| SFT, cm | 0.7 (0.2) | 1.1 (0.6) | 0.9 (0.4) | 1.1 (0.5) | 0.9 (0.4) |
| Tibialis anterior left | |||||
| GS | 49.1 (14.3) | 74.4 (25.0) | 83.7 (30.5) | 80.6 (36.1) | 75.9 (34.4) |
| z-Score | N/A | 1.0 (1.0) | 1.3 (1.2) | 1.2 (1.4) | 1.0 (1.3) |
| HS | 1.1 (0.3) | 2.9 (0.7) | 2.6 (0.6) | 2.7 (0.5) | 2.5 (0.5) |
| GSSD | 25.1 (6.1) | 26.6 | 25.1 | 26.1 | 29.7 |
| SFT, cm | 0.4 (0.2) | 0.5 (0.3) | 0.4 (0.2) | 0.5 (0.3) | 0.5 (0.3) |
| Tibialis anterior right | |||||
| GS | 46.8 (13.9) | 85.9 (27.7) | 76.4 (29.8) | 77.7 (31.8) | 79.9 (30.1) |
| z-Score | N/A | 1.4 (1.0) | 1.1 (1.1) | 1.1 (1.2) | 1.2 (1.1) |
| HS | 1.2 (0.4) | 2.7 (0.8) | 2.7 (0.8) | 2.9 (0.5) | 2.3 (0.6) |
| GSSD | 27.4 (4.0) | 29 | 27.6 | 27 | 28.1 |
| SFT, cm | 0.4 (0.2) | 0.5 (0.3) | 0.4 (0.2) | 0.6 (0.3) | 0.4 (0.2) |
| Healthy Controls | ICU-AW+ Day 3 | ICU-AW− Day 3 | ICU-AW+ Day 10 | ICU-AW− Day 10 | |
|---|---|---|---|---|---|
| MGS | 49.5 (12.8) | 75.5 (21.8) | 73.2 (30.0) | 72.1 (24.2) | 69.2 (27.5) |
| MZS | N/A | 1.0 (0.8) | 0.9 (1.1) | 0.8 (0.9) | 0.7 (1.0) |
| MHS | 1.1 (0.1) | 2.6 (0.5) | 2.4 (0.4) | 2.6 (0.4) | 2.2 (0.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klawitter, F.; Walter, U.; Patejdl, R.; Endler, J.; Reuter, D.A.; Ehler, J. Sonographic Evaluation of Muscle Echogenicity for the Detection of Intensive Care Unit-Acquired Weakness: A Pilot Single-Center Prospective Cohort Study. Diagnostics 2022, 12, 1378. https://doi.org/10.3390/diagnostics12061378
Klawitter F, Walter U, Patejdl R, Endler J, Reuter DA, Ehler J. Sonographic Evaluation of Muscle Echogenicity for the Detection of Intensive Care Unit-Acquired Weakness: A Pilot Single-Center Prospective Cohort Study. Diagnostics. 2022; 12(6):1378. https://doi.org/10.3390/diagnostics12061378
Chicago/Turabian StyleKlawitter, Felix, Uwe Walter, Robert Patejdl, Josefine Endler, Daniel A. Reuter, and Johannes Ehler. 2022. "Sonographic Evaluation of Muscle Echogenicity for the Detection of Intensive Care Unit-Acquired Weakness: A Pilot Single-Center Prospective Cohort Study" Diagnostics 12, no. 6: 1378. https://doi.org/10.3390/diagnostics12061378
APA StyleKlawitter, F., Walter, U., Patejdl, R., Endler, J., Reuter, D. A., & Ehler, J. (2022). Sonographic Evaluation of Muscle Echogenicity for the Detection of Intensive Care Unit-Acquired Weakness: A Pilot Single-Center Prospective Cohort Study. Diagnostics, 12(6), 1378. https://doi.org/10.3390/diagnostics12061378






