Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors
Abstract
1. Introduction
2. Contrast-Enhanced EUS
2.1. Contrast Agents
2.2. Contrast-Enhanced Doppler Endoscopic Ultrasound
2.3. Contrast-Enhanced Harmonic Endoscopic Ultrasound
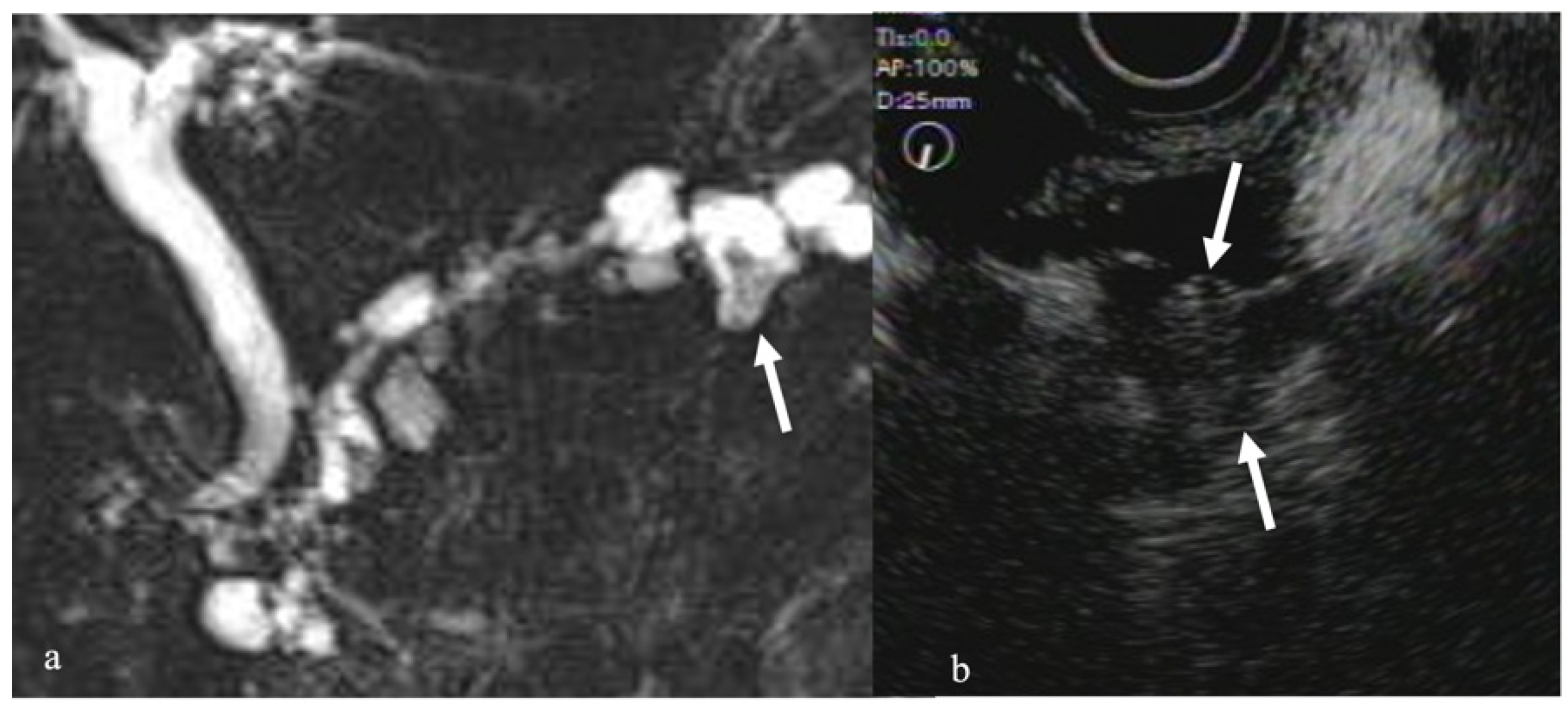
2.4. CEH-EUS for Pancreatic Diseases
3. Pancreatic Tumor
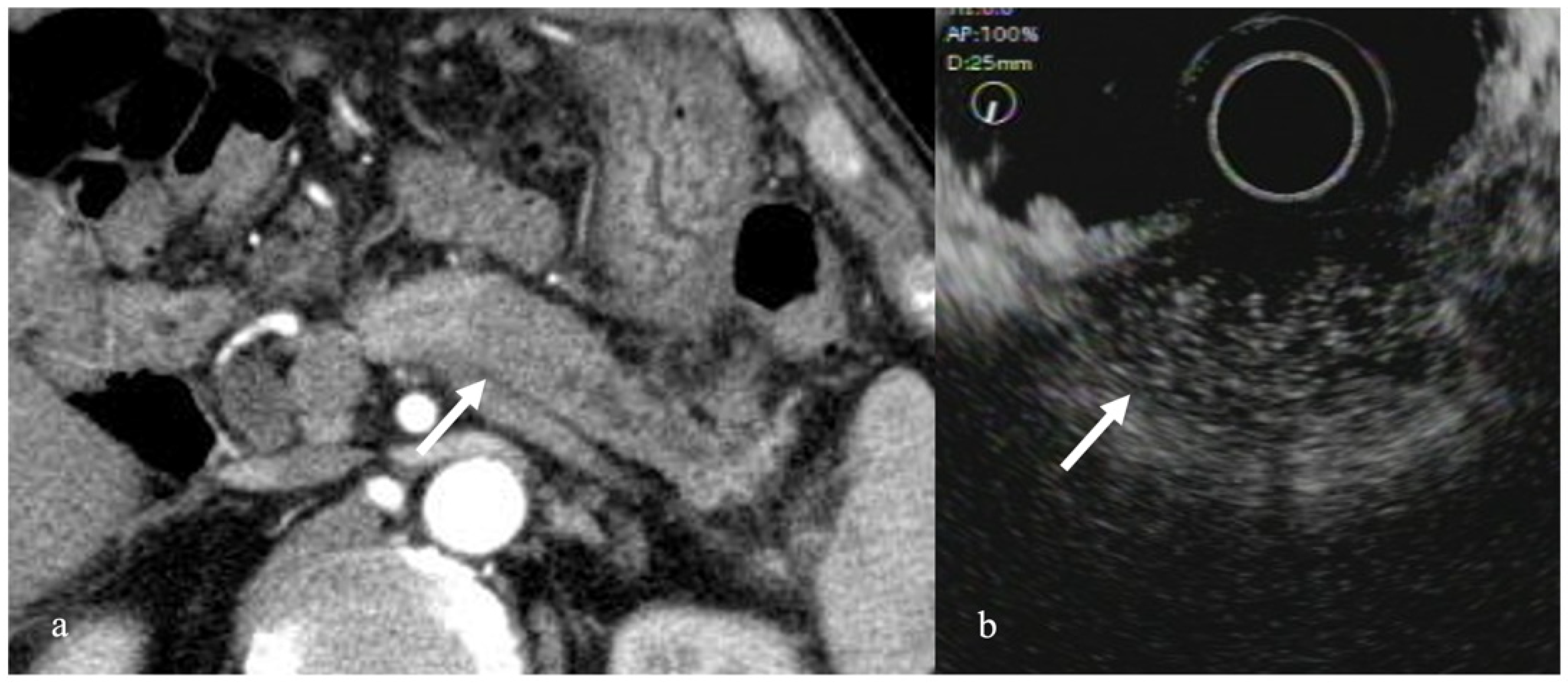
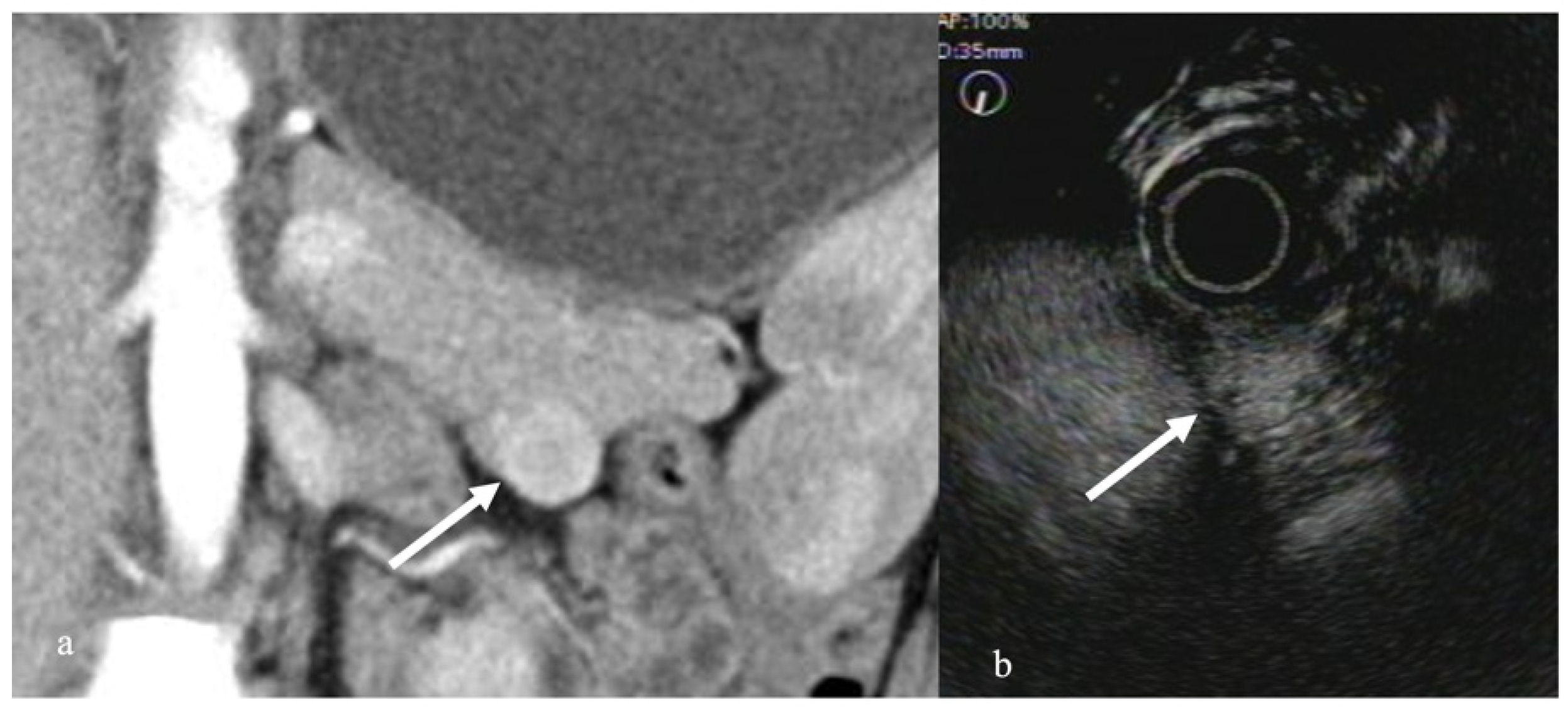
3.1. Solid Pancreatic Tumors
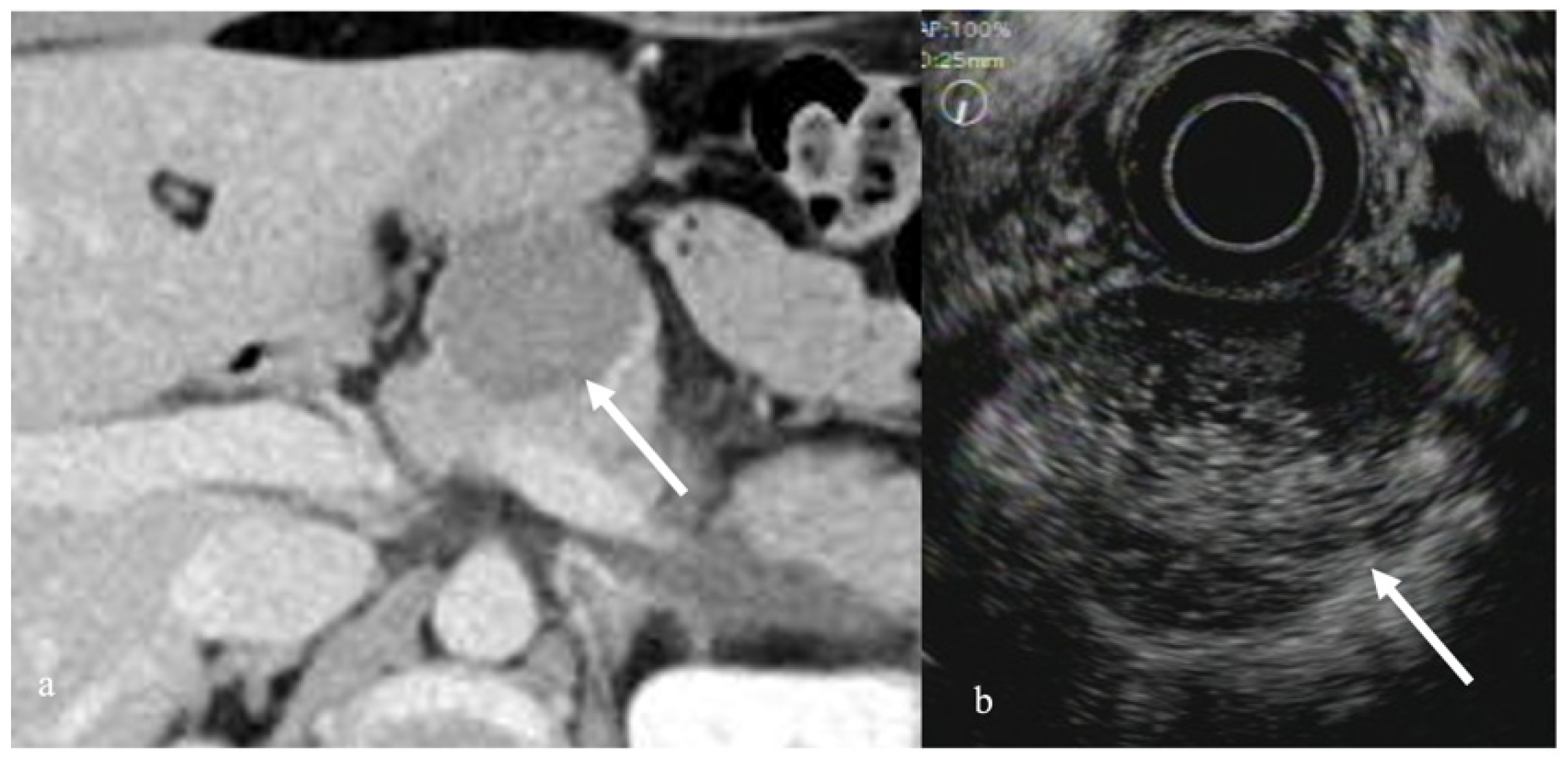
3.2. Autoimmune Pancreatitis
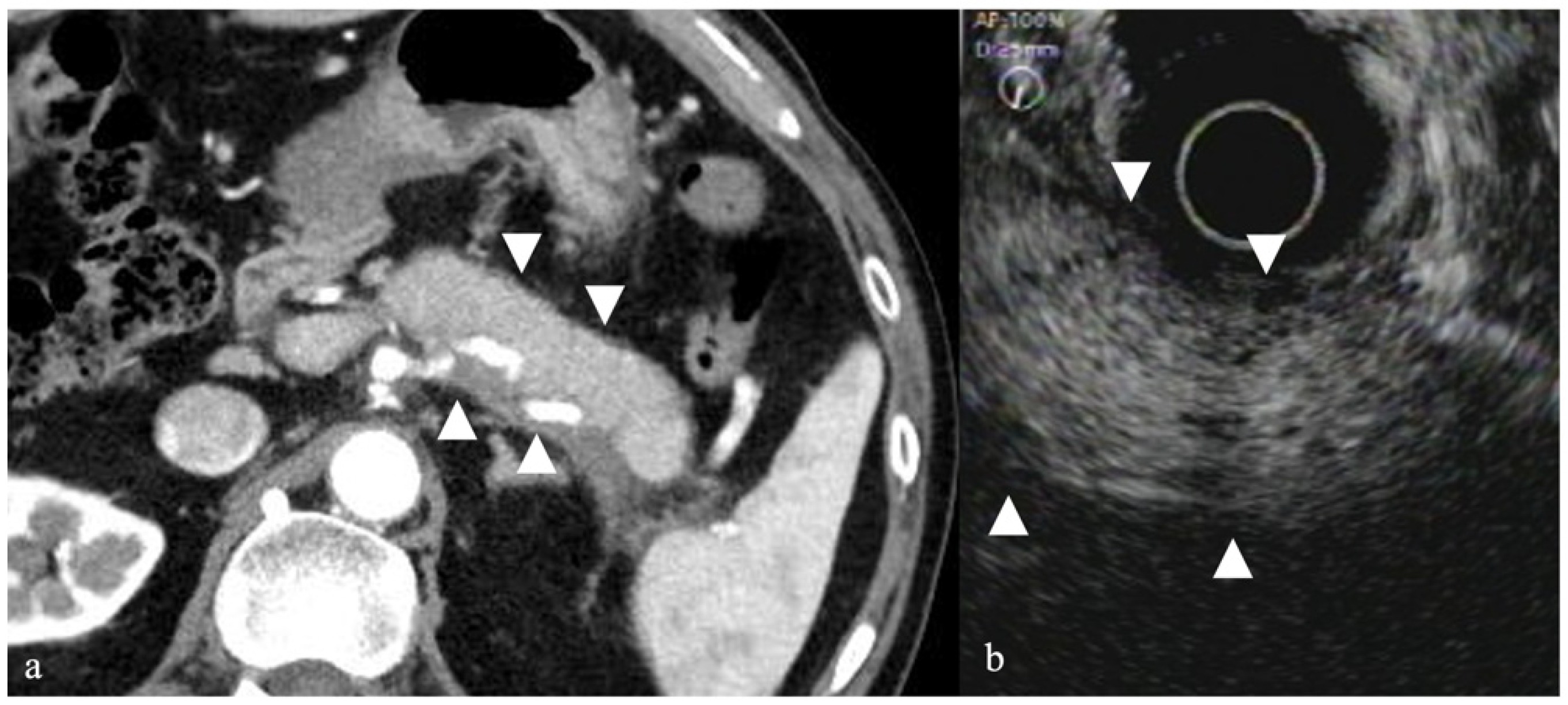
3.3. Pancreatic Cystic Tumors
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Onofrio, M.; Malagò, R.; Zamboni, G.; Falconi, M.; Capelli, P.; Mucelli, R.P. Resectable pancreatic adenocarcinoma: Depiction of tumoral margins at contrast-enhanced ultrasonography. Pancreas 2008, 37, 265–268. [Google Scholar]
- Fritzsch, T.; Hilmann, J.; Kämpfe, M.; Müller, N.; Schöbel, C.; Siegert, J. SHU 508, a transpulmonary echocontrast agent: Initial experience. Investig. Radiol. 1990, 25 (Suppl. 1), S160–S161. [Google Scholar] [CrossRef] [PubMed]
- Schlief, R. Ultrasound contrast agents. Curr. Opin. Radiol. 1991, 3, 198–207. [Google Scholar] [PubMed]
- Faccioli, N.; Crippa, S.; Bassi, C.; D’onofrio, M. Contrast-enhanced ultrasonography of the pancreas. Pancreatology 2009, 9, 560–566. [Google Scholar] [CrossRef][Green Version]
- Recaldini, C.; Carrafiello, G.; Bertolotti, E.; Angeretti, M.G.; Fugazzola, C. Contrast-enhanced ultrasonograpic findings in pancreatic tumors. Int. J. Med. Sci. 2008, 5, 203–208. [Google Scholar] [CrossRef]
- Scialpi, M.; Midiri, M.; Bartolotta, T.V.; Cazzolla, M.P.; Rotondo, A.; Resta, M.C.; Lagalla, R.; Cardinale, A.E. Pancreatic carcinoma versus chronic focal pancreatitis: Contrast-enhanced power Doppler ultrasonography findings. Abdom. Imaging 2005, 30, 222–227. [Google Scholar] [CrossRef]
- Itoh, T.; Hirooka, Y.; Itoh, A.; Hashimoto, S.; Kawashima, H.; Hara, K.; Kanamori, A.; Ohmiya, N.; Niwa, Y.; Goto, H. Usefulness of contrast-enhanced transabdominal ultrasonography in the diagnosis of intraductal papillary mucinous tumors of the pancreas. Am. J. Gastroenterol. 2005, 100, 144–152. [Google Scholar] [CrossRef]
- Kersting, S.; Konopke, R.; Kersting, F.; Volk, A.; Distler, M.; Bergert, H.; Saeger, H.D.; Grützmann, R.; Bunk, A. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology 2009, 137, 1903–1911. [Google Scholar] [CrossRef]
- Săftoiu, A. State-of-the-art imaging techniques in endoscopic ultrasound. World J. Gastroenterol. 2011, 17, 691–696. [Google Scholar] [CrossRef]
- Helmstaedter, L.; Riemann, J.F. Pancreatic cancer–EUS and early diagnosis. Langenbecks Arch. Surg. 2008, 393, 923–927. [Google Scholar] [CrossRef]
- Kato, T.; Tsukamoto, Y.; Naitoh, Y.; Hirooka, Y.; Furukawa, T.; Hayakawa, T. Ultrasonographic and endoscopic ultrasonographic angiography in pancreatic mass lesions. Acta Radiol. 1995, 36, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Maekawa, K.; Suetomi, Y.; Sakamoto, H.; Fukuta, N.; Nakaoka, R.; Kawasaki, T. Dynamic imaging of pancreatic diseases by contrast-enhanced coded phase inversion harmonic ultrasonography. Gut 2004, 53, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Angeli, E.; Carpanelli, R.; Crespi, G.; Zanello, A.; Sironi, S.; Del Maschio, A. Efficacy of SH U 508 A (levovist) in color Doppler ultrasonography of hepatocellular carcinoma vascularization. Radiol. Med. 1994, 87 (Suppl. 1), 24–31. [Google Scholar] [PubMed]
- Masuzaki, R.; Shiina, S.; Tateishi, R.; Yoshida, H.; Goto, E.; Sugioka, Y.; Kondo, Y.; Goto, T.; Ikeda, H.; Omata, M.; et al. Utility of contrast-enhanced ultrasonography with Sonazoid in radiofrequency ablation for hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 759–764. [Google Scholar] [CrossRef]
- Sontum, P.C. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med. Biol. 2008, 34, 824–833. [Google Scholar] [CrossRef]
- Kitano, M.; Sakamoto, H.; Matsui, U.; Ito, Y.; Maekawa, K.; von Schrenck, T.; Kudo, M. A novel perfusion imaging technique of the pancreas: Contrast-enhanced harmonic EUS (with video). Gastrointest. Endosc. 2008, 67, 141–150. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Braden, B.; Barreiros, A.P.; Ott, M.; Hocke, M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin. Gastroenterol. Hepatol. 2008, 6, 590–597.e1. [Google Scholar] [CrossRef]
- Hocke, M.; Ignee, A.; Dietrich, C.F. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy 2011, 43, 163–165. [Google Scholar] [CrossRef]
- Napoleon, B.; Alvarez-Sanchez, M.V.; Gincoul, R.; Pujol, B.; Lefort, C.; Lepilliez, V.; Labadie, M.; Souquet, J.C.; Queneau, P.E.; Scoazec, J.Y.; et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy 2010, 42, 564–570. [Google Scholar] [CrossRef]
- Imazu, H.; Kanazawa, K.; Mori, N.; Ikeda, K.; Kakutani, H.; Sumiyama, K.; Hino, S.; Ang, T.L.; Omar, S.; Tajiri, H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiated autoimmune pancreatitis from pancreatic carcinoma. Scand. J. Gastroenterol. 2012, 47, 853–860. [Google Scholar] [CrossRef]
- Matsubara, H.; Itoh, A.; Kawashima, H.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas 2011, 40, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.T.; Hu, D.M.; Zhu, Q. Contrast-enhanced EUS for differential diagnosis of pancreatic mass lesions: A meta-analysis. Gastrointest. Endosc. 2012, 76, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Imazu, H.; Uchiyama, Y.; Matsunaga, K.; Ikeda, K.-I.; Kakutani, H.; Sasaki, Y.; Sumiyama, K.; Ang, T.L.; Omar, S.; Tajiri, H. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand. J. Gastroenterol. 2010, 45, 732–738. [Google Scholar] [CrossRef]
- Miyata, T.; Kitano, M.; Omoto, S.; Kadosaka, K.; Kamata, K.; Imai, H.; Sakamoto, H.; Nisida, N.; Harwani, Y.; Murakami, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J. Gastroenterol. 2016, 22, 3381–3391. [Google Scholar] [CrossRef]
- Palazzo, M. Role of contrast harmonic endoscopic ultrasonography in other pancreatic solid lesions: Neuroendocrine tumors, autoimmune pancreatitis, and metastases. Endosc. Ultrasound 2016, 5, 373–376. [Google Scholar] [CrossRef]
- Ishikawa, T.; Itoh, A.; Kawashima, H.; Ohno, E.; Matsubara, H.; Nakamura, M.; Miyahara, R.; Ohmiya, N.; Goto, H.; Hirooka, Y. A case of solid-pseudopapillary neoplasm, focusing on contrast-enhanced endoscopic ultrasonography. J. Med. Ultrason 2011, 38, 209. [Google Scholar] [CrossRef]
- Kataoka, K.; Ishikawa, T.; Ohno, E.; Mizutani, Y.; Iida, T.; Furukawa, K.; Nakamura, M.; Honda, T.; Ishigami, M.; Kawashima, H.; et al. Differentiation between solid pseudopapillary neoplasm of the pancreas and nonfunctional pancreatic neuroendocrine neoplasm using endoscopic ultrasound. Pancreas 2022, 51, 106–111. [Google Scholar] [CrossRef]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6260. [Google Scholar] [CrossRef]
- Kobayashi, G.; Fujita, N.; Noda, Y.; Ito, K.; Horaguchi, J.; Koshida, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Michikawa, Y. Vascular image in autoimmune pancreatitis by contrast-enhanced color-Doppler endoscopic ultrasonography: Comparison with pancreatic cancer. Endosc. Ultrasound 2014, 3 (Suppl. 1), S13. [Google Scholar]
- Ohno, E.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Katano, Y.; Ohmiya, N.; Niwa, Y.; Goto, H. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann. Surg. 2009, 249, 628–634. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kato, H.; Tomoda, T.; Matsumoto, K.; Sakakihara, I.; Noma, Y.; Horiguchi, S.; Harada, R.; Tsutsumi, K.; Hori, K.; et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy 2016, 48, 26–34. [Google Scholar] [CrossRef]
- Zhong, L.; Chai, N.; Linghu, E.; Li, H.; Yang, J.; Tang, P. A prospective study on contrast-enhanced endoscopic ultrasound for differential diagnosis of pancreatic cystic neoplasms. Dig. Dis. Sci. 2019, 64, 3616–3622. [Google Scholar] [CrossRef]
- Raut, C.P.; Grau, A.M.; Staerkel, G.A.; Kaw, M.; Tamm, E.P.; Wolff, R.A.; Vauthey, J.N.; Lee, J.E.; Pisters, P.W.; Evans, D.B. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J. Gastrointest. Surg. 2003, 7, 118–128. [Google Scholar] [CrossRef]
- Afify, A.M.; al-Khafaji, B.M.; Kim, B.; Scheiman, J.M. Endoscopic ultrasound-guided fine-needle aspiration of the pancreas. Diagn. Util. Accuracy Acta Cytol. 2003, 47, 341–348. [Google Scholar]
- Eloubeidi, M.A.; Varadarajulu, S.; Desai, S.; Shirley, R.; Heslin, M.J.; Mehra, M.; Arnoletti, J.P.; Eltoum, I.; Wilcox, C.M.; Vickers, S.M. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine-needle aspiration in suspected pancreatic cancer. J. Gastrointest. Surg. 2007, 11, 813–819. [Google Scholar] [CrossRef]
- Tadic, M.; Kujundzic, M.; Stoos-Veic, T.; Kaic, G.; Vukelic-Markovic, M. Role of repeated endoscopic ultrasound-guided fine-needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig. Dis. 2008, 26, 377–382. [Google Scholar] [CrossRef]
- Tsuji, Y.; Yamamoto, H.; Yazumi, S.; Watanabe, Y.; Matsueda, K.; Yamamoto, H.; Chiba, T. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin. Gastroenterol. Hepatol. 2007, 5, 1484–1492. [Google Scholar] [CrossRef]

| Contrast Agent | Composition |
|---|---|
| First generation | |
| Albunex | 5% sonicated serum albumin with stabilized microbubbles |
| Echovist (SHU 454) | Standardized microbubbles with galactose shell |
| Levosist (SHU 508) | Stabilized, standardized microbubbles with galactose, 0.1% palmitic acid shell |
| Myomap | Albumin shell |
| Qantison | Albumin shell |
| Sonavist | Cyanoacrylate shell |
| Second generation | |
| Definity/luminity | C3F8 with lipid stabilizer shell |
| Sonazoid | C4F10 with lipid stabilizer shell |
| Imagent-imavist | C6F14 with lipid stabilizer shell |
| Optison | C3F8 with denatured human albumin shell |
| Bisphere/cardiosphere | Polylactide-coglycolide shell with albumin overcoat |
| Sono Vue | SF6 gas with lipid stabilizer shell |
| AI700/imagify | C4F10 gas core stabilized with polymer shell |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, K.; Kanno, A.; Miwata, T.; Nagai, H.; Ikeda, E.; Ando, K.; Kawasaki, Y.; Tamada, K.; Lefor, A.K.; Yamamoto, H. Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors. Diagnostics 2022, 12, 1311. https://doi.org/10.3390/diagnostics12061311
Yokoyama K, Kanno A, Miwata T, Nagai H, Ikeda E, Ando K, Kawasaki Y, Tamada K, Lefor AK, Yamamoto H. Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors. Diagnostics. 2022; 12(6):1311. https://doi.org/10.3390/diagnostics12061311
Chicago/Turabian StyleYokoyama, Kensuke, Atsushi Kanno, Tetsurou Miwata, Hiroki Nagai, Eriko Ikeda, Kozue Ando, Yuki Kawasaki, Kiichi Tamada, Alan Kawarai Lefor, and Hironori Yamamoto. 2022. "Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors" Diagnostics 12, no. 6: 1311. https://doi.org/10.3390/diagnostics12061311
APA StyleYokoyama, K., Kanno, A., Miwata, T., Nagai, H., Ikeda, E., Ando, K., Kawasaki, Y., Tamada, K., Lefor, A. K., & Yamamoto, H. (2022). Efficacy of Contrast-Enhanced Endoscopic Ultrasonography for the Diagnosis of Pancreatic Tumors. Diagnostics, 12(6), 1311. https://doi.org/10.3390/diagnostics12061311







