Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema
Abstract
1. Introduction
2. Materials and Methods
3. Results
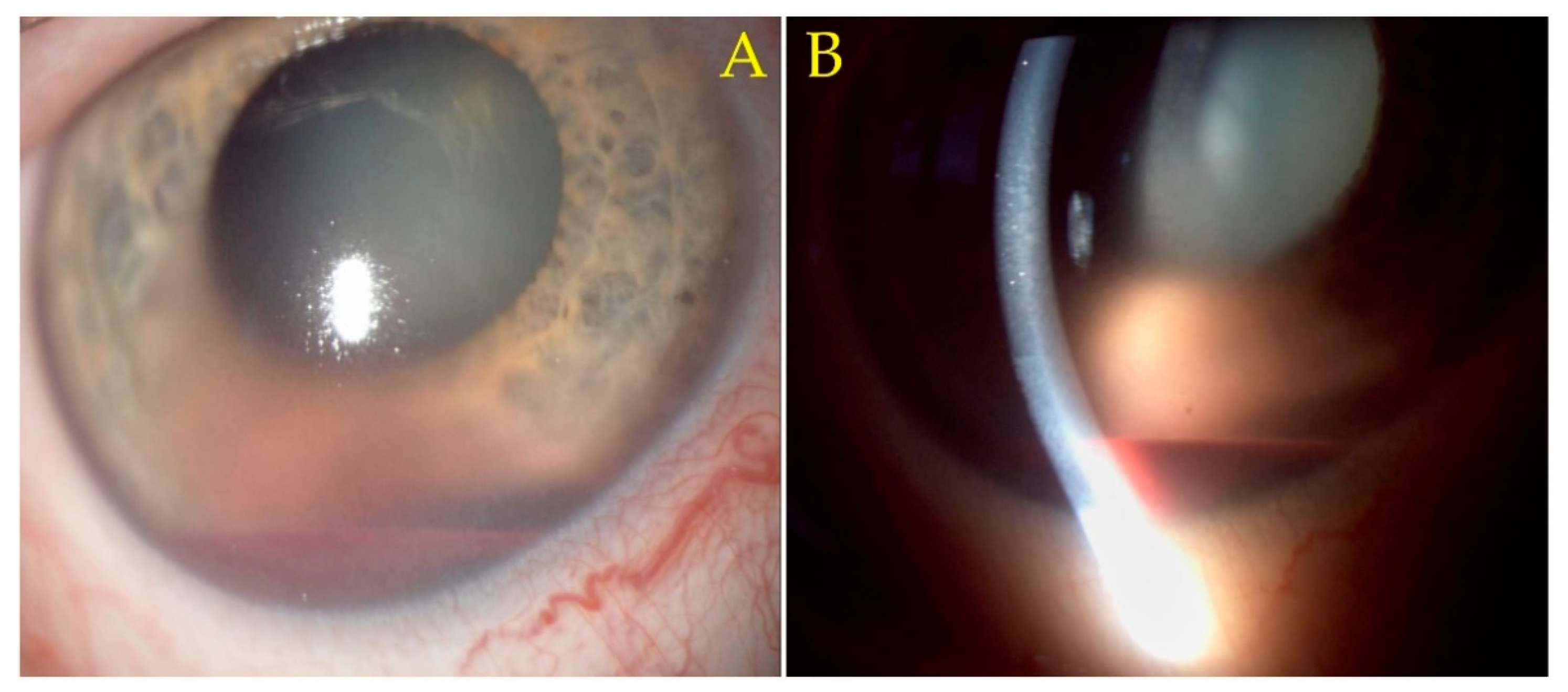
Case Presentation
4. Discussion
5. Conclusions
5.1. What Was Known
5.2. What This Paper Adds
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLL | chronic lymphocytic leukemia |
| Th2 | T-helper 2 (cells) |
| BTK | Bruton’s tyrosine kinase |
| UM | uveal melanoma |
| CTLA-4 | T-lymphocyte-associated antigen 4 |
| PD-1 | programmed cell death-1 (protein) |
| TCR | soluble T cell receptor |
| iv | intravenous |
| NSAID | topical non-steroidal anti-inflammatory drug |
| MPV | mean platelet volume |
| MCV | mean corpuscular volume (of erythrocytes) |
| RDW | red cell distribution width |
| CE-MRI | contrast-enhanced magnetic resonance Imaging |
| FLAIR | fluid attenuated inversion recovery |
| 3D-CISS | three-dimensional constructive interference in steady state |
| DWI | diffusion-weighted imaging (in magnetic resonance imaging) |
| GRE | gradient echo (in magnetic resonance imaging) |
| TIRM | turbo inversion recovery magnitude |
| TSE | turbo spin echo |
| FS | fat-suppressed (in magnetic resonance imaging) |
| VIBE | Volumetric interpolated breath-hold examination |
| MPR | T1 multiplanar reformation/reconstruction |
| CT | computed tomography |
| HE | hematoxylin eosin (stain) |
| AJCC | American Joint Committee of Cancer |
| HMB45 | human melanoma black 45 |
| MART-1 | melanoma-associated antigen recognized by T cells |
| S100 | protein S100 |
| SOX-10 | SRY-box transcription factor 10 |
| COVID-19 | Coronavirus disease 2019 |
| mRNA | messenger ribunucleic acid |
| UBM | ultrasound biomicroscopy |
| PAS | periodic acid Schiff |
| F8 | factor VIII related antigen |
| PD-L1 | programmed cell death ligand 1 |
| CD47 | cluster of differentiation 47 |
| CD200 | cluster of differentiation 200 |
| MHC | major histocompatibility complex |
| PD-L2 | programmed cell death ligand 2 |
| IFN-gamma | interferon gamma |
| CDKN2A | cyclin dependent kinase inhibitor 2A |
| BAP1 | ubiquitin carboxyl-terminal hydrolase (gene) |
| EIF1AX | eukaryotic translation initiation factor 1A X-Linked |
| PC | pseudofollicular proliferative centers |
| TCM | central memory cells |
| ICMJE | International Committee of Medical Journal Editors |
| GDPR | General Data Protection Regulation |
References
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Burger, J.A. Treatment of Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2020, 383, 460–473. [Google Scholar] [CrossRef]
- Bosch, F.; Dalla-Favera, R. Chronic lymphocytic leukaemia: From genetics to treatment. Nat. Rev. Clin. Oncol. 2019, 16, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, J.A.; Powers, J.J.; Gao, Y.; Mariusso, L.F.; Sotomayor, E.M.; Pinilla-Ibarz, J.A. Epigenetic repolarization of T lymphocytes from chronic lymphocytic leukemia patients using 5-aza-2′-deoxycytidine. Leuk. Res. 2011, 35, 1193–1199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Compagno, M.; Wang, Q.; Pighi, C.; Cheong, T.C.; Meng, F.L.; Poggio, T.; Yeap, L.S.; Karaca, E.; Blasco, R.B.; Langellotto, F.; et al. Phosphatidylinositol 3-kinase δ blockade increases genomic instability in B cells. Nature 2017, 542, 489–493. [Google Scholar] [CrossRef]
- Landego, I.; Hewitt, D.; Hibbert, I.; Dhaliwal, D.; Pieterse, W.; Grenier, D.; Wong, R.; Johnston, J.; Banerji, V. PD-1 Inhibition in Malignant Melanoma and Lack of Clinical Response in Chronic Lymphocytic Leukemia in the Same Patients: A Case Series. Curr. Oncol. 2020, 27, 169–172. [Google Scholar] [CrossRef]
- Olsen Catherine, M.; Lane Steven, W.; Green Adèle, C. Increased risk of melanoma in patients with chronic lymphocytic leukaemia. Melanoma Res. 2016, 26, 188–194. [Google Scholar] [CrossRef]
- Turk, T.; Saad, A.M.; Al-Husseini, M.J.; Gad, M.M. The risk of melanoma in patients with chronic lymphocytic leukemia; A population-based study. Curr. Probl. Cancer 2020, 44, 100511. [Google Scholar] [CrossRef]
- Harbour, J.W. The genetics of uveal melanoma: An emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012, 25, 171–181. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, C.A.; Bonzano, C.; Scotto, R.; Iester, M.; Bagnis, A.; Pizzorno, C.; Catti, C.; Traverso, C.E. Moving beyond the Slit-Lamp Gonioscopy: Challenges and Future Opportunities. Diagnostics 2021, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; He, M. Anterior chamber angle assessment techniques. Surv. Ophthalmol. 2008, 53, 250–273. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Damato, B.; Coupland, S.E.; Desjardins, L.; Bechrakis, N.E.; Grange, J.D.; Kivelä, J.-D.G. Staging of ciliary body and choroidal melanomas based on anatomic extent. J. Clin. Oncol. 2013, 31, 2825–2831. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Gao, H.; Zhang, S.; Zhao, C.; Zhou, C.; Wang, C.; Li, Y.; Cai, Z.; Mou, L. Drug repurposing: Ibrutinib exhibits immunosuppressive potential in organ transplantation. Int. J. Med. Sci. 2018, 15, 1118–1128. [Google Scholar] [CrossRef]
- Kondo, K.; Shaim, H.; Thompson, P.A.; Burger, J.A.; Keating, M.; Estrov, Z.; Harris, D.; Kim, E.; Ferrajoli, A.; Daher, M.; et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 2018, 32, 960–970. [Google Scholar] [CrossRef]
- Broggi, G.; Russo, A.; Reibaldi, M.; Russo, D.; Varricchio, S.; Bonfiglio, V.; Spatola, C.; Barbagallo, C.; Foti, P.V.; Avitabile, T.; et al. Histopathology and Genetic Biomarkers of Choroidal Melanoma. Appl. Sci. 2020, 10, 8081. [Google Scholar] [CrossRef]
- Russo, D.; Di Crescenzo, R.M.; Broggi, G.; Merolla, F.; Martino, F.; Varricchio, S.; Ilardi, G.; Borzillo, A.; Carandente, R.; Pignatiello, S.; et al. Expression of P16INK4a in Uveal Melanoma: New Perspectives. Front. Oncol. 2020, 10, 562074. [Google Scholar] [CrossRef]
- Abi-Ayad, N.; Grange, J.D.; Watkin, E.; De Bats, M.; Fleury, J.; Kodjikian, L.; Gambrelle, J. Mélanome annulaire révélé par un hyphéma spontané [Ring melanoma revealed by spontaneous hyphema]. J. Français D’ophtalmologie 2007, 30, 729–732. (In French) [Google Scholar] [CrossRef]
- Demirci, H.; Shields, C.L.; Shields, J.A.; Eagle, R.C., Jr.; Honavar, S. Ring melanoma of the anterior chamber angle: A report of fourteen cases. Am. J. Ophthalmol. 2001, 132, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; McCannel, C.A.; McCannel, T.A. Untreated iris melanoma complicated by hyphema and uncontrolled glaucoma responsive to iodine-125 brachytherapy. Retin. Cases Brief Rep. 2016, 10, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Avitabile, T.; Reibaldi, M.; Bonfiglio, V.; Pignatelli, F.; Fallico, M.; Caltabiano, R.; Broggi, G.; Russo, D.; Varricchio, S.; et al. Iris Melanoma: Management and Prognosis. Appl. Sci. 2020, 10, 8766. [Google Scholar] [CrossRef]
- Fong, A.; Lee, L.; Glasson, W. Pediatric choroidal melanoma in a 13-year-old girl—A clinical masquerade. J. AAPOS 2011, 15, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Leonard, B.C.; Sarin, L.V. Multilobed uveal melanoma masquerading as postoperative choroidal detachment. Br. J. Ophthalmol. 1976, 60, 386–389. [Google Scholar] [CrossRef][Green Version]
- Gangaputra, S.; Kodati, S.; Kim, M.; Aranow, M.; Sen, H.N. Multimodal Imaging in Masquerade Syndromes. Ocul. Immunol. Inflamm. 2017, 25, 160–168. [Google Scholar] [CrossRef]
- Johnson, D.L.; Altaweel, M.M.; Neekhra, A.; Chandra, S.R.; Albert, D.M. Uveal Melanoma Masquerading as Pigment Dispersion Glaucoma. Arch. Ophthalmol. 2008, 126, 866–876. [Google Scholar] [CrossRef][Green Version]
- Vempuluru, V.S.; Jakati, S.; Krishnamurthy, R.; Senthil, S.; Kaliki, S. Glaucoma as the presenting sign of intraocular tumors: Beware of the masquerading sign. Int. Ophthalmol. 2020, 40, 1789–1795. [Google Scholar] [CrossRef]
- Kafkala, C.; Daoud, Y.J.; Paredes, I.; Foster, C.S. Masquerade scleritis. Ocul. Immunol. Inflamm. 2005, 13, 479–482. [Google Scholar] [CrossRef]
- Mehta, A.; Singh, M.; Banerjee, N.; Jain, C.; Kakkar, N.; Gupta, P. Aseptic orbital cellulitis: A master masquerade of intraocular malignancy. Eur. J. Ophthalmol. 2021, 31, NP1–NP4. [Google Scholar] [CrossRef]
- Garmizo, G. Sentinel episcleral vessels. A clue to the diagnosis of asymptomatic ciliary body melanoma. J. Am. Optom. Assoc. 1984, 55, 599–600. [Google Scholar]
- Ossoinig, K.C. Standardized echography: Basic principles, clinical applications, and results. Int. Ophthalmol. Clin. 1979, 19, 127–210. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Minning, C.A., Jr.; Davidorf, F.H. Ossoinig’s angle of ultrasonic absorption and its role in the diagnosis of malignant melanoma. Ann. Ophthalmol. 1982, 14, 564–568. [Google Scholar] [PubMed]
- He, M.; Wang, D.; Jiang, Y. Overview of Ultrasound Biomicroscopy. J. Curr. Glaucoma Pract. 2012, 6, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.M.; Chew, T.; Golchet, P.; Desai, K.; Lin, S.; O’Brien, J. Ultrasound biomicroscopy: Role in diagnosis and management in 130 consecutive patients evaluated for anterior segment tumours. Br. J. Ophthalmol. 2005, 89, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.D.; Gupta, M.; Parsons, A.; Rennie, I.G. Ultrasound biomicroscopy in the management of melanocytoma of the ciliary body with extrascleral extension. Br. J. Ophthalmol. 2005, 89, 14–16. [Google Scholar] [CrossRef]
- Langmann, G.; Pendl, G.; Müllner, K.; Papaefthymiou, G.; Guss, H. Gamma knife radiosurgery for uveal melanomas: An 8-year experience. J. Neurosurg. 2000, 93 (Suppl. S3), 184–188. Available online: https://thejns.org/view/journals/j-neurosurg/93/supplement_3/article-p184.xml (accessed on 5 May 2022). [CrossRef]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef]
- Mirzayev, I.; Gündüz, A.K.; Okçu Heper, A. Partial lamellar sclerouvectomy surgery for anteriorly located uveal tumour resection: A 20-year experience. Eye 2021, 36, 969–977. [Google Scholar] [CrossRef]
- Folberg, R.; Pe’er, J.; Gruman, L.M.; Woolson, R.F.; Jeng, G.; Montague, P.R.; Moninger, T.O.; Yi, H.; Moore, K.C. The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: A matched case-control study. Hum. Pathol. 1992, 23, 1298–1305. [Google Scholar] [CrossRef]
- Folberg, R. Discussion of paper by Foss et al. Br. J. Ophthalmol. 1997, 81, 247–248. [Google Scholar] [CrossRef][Green Version]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Fagone, P.; Longo, A.; Russo, A.; Fallico, M.; Bonfiglio, V.; Nicoletti, F.; Avitabile, T.; Reibaldi, M. Immunobiology of Uveal Melanoma: State of the Art and Therapeutic Targets. Front. Oncol. 2019, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Russo, A.; Mammana, S.; Longo, A.; Bonfiglio, V.; Fallico, M.; Caltabiano, R.; Fagone, P.; Nicoletti, F.; et al. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS ONE 2019, 14, e0210276. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Pramil, E.; Jondreville, L.; Quiney, C.; Nguyen-Khac, F.; Susin, S.A. Activation of Interferon Signaling in Chronic Lymphocytic Leukemia Cells Contributes to Apoptosis Resistance via a JAK-Src/STAT3/Mcl-1 Signaling Pathway. Biomedicines 2021, 9, 188. [Google Scholar] [CrossRef]
- Lv, X.; Ding, M.; Liu, Y. Landscape of Infiltrated Immune Cell Characterization in Uveal Melanoma to Improve Immune Checkpoint Blockade Therapy. Front. Immunol. 2022, 13, 848455. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Kalirai, H.; Sacco, J.J.; Azevedo, R.A.; Duckworth, A.; Slupsky, J.R.; Coulson, J.M.; Coupland, S.E. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J. Pathol. 2020, 250, 420–439. [Google Scholar] [CrossRef]
- Ewens, K.G.; Kanetsky, P.A.; Richards-Yutz, J.; Purrazzella, J.; Shields, C.L.; Ganguly, T.; Ganguly, A. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5160–5167. [Google Scholar] [CrossRef]
- Correia, R.P.; Silva, F.A.; Bacal, N.S.; Campregher, P.V.; Hamerschlak, N.; Amarante-Mendes, G.P. Involvement of memory T-cells in the pathophysiology of chronic lymphocytic leukemia. Rev. Bras. Hematol. Hemoter. 2014, 36, 60–64. (In Portuguese) [Google Scholar] [CrossRef]
- Röth, A.; de Beer, D.; Nückel, H.; Sellmann, L.; Dührsen, U.; Dürig, J.; Baerlocher, G.M. Significantly shorter telomeres in T-cells of patients with ZAP-70+/CD38+ chronic lymphocytic leukaemia. Br. J. Haematol. 2008, 143, 383–386. [Google Scholar] [CrossRef]
- Pochtar, E.V.; Lugovskaya, S.A.; Naumova, E.V.; Dmitrieva, E.A.; Kostin, A.I.; Dolgov, V.V. Specific features of T- and NK-cellular immunity in chronic lymphocytic leukemia. Klin. Lab. Diagn. 2021, 66, 345–352. (In English) [Google Scholar] [CrossRef] [PubMed]
- Elston, L.; Fegan, C.; Hills, R.; Hashimdeen, S.S.; Walsby, E.; Henley, P.; Pepper, C.; Man, S. Increased frequency of CD4+ PD-1+ HLA-DR+ T cells is associated with disease progression in CLL. Br. J. Haematol. 2020, 188, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Fiorcari, S.; Atene, C.G.; Maffei, R.; Debbia, G.; Potenza, L.; Luppi, M.; Marasca, R. Ibrutinib interferes with innate immunity in chronic lymphocytic leukemia patients during COVID-19 infection. Haematologica 2021, 106, 2265–2268. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păsărică, M.A.; Curcă, P.F.; Dragosloveanu, C.D.M.; Tătaru, C.I.; Manole, I.R.; Murgoi, G.E.; Grigorescu, A.C. Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema. Diagnostics 2022, 12, 1312. https://doi.org/10.3390/diagnostics12061312
Păsărică MA, Curcă PF, Dragosloveanu CDM, Tătaru CI, Manole IR, Murgoi GE, Grigorescu AC. Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema. Diagnostics. 2022; 12(6):1312. https://doi.org/10.3390/diagnostics12061312
Chicago/Turabian StylePăsărică, Mihai Adrian, Paul Filip Curcă, Christiana Diana Maria Dragosloveanu, Cătălina Ioana Tătaru, Ioana Roxana Manole, Gabriela Elisabeta Murgoi, and Alexandru Călin Grigorescu. 2022. "Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema" Diagnostics 12, no. 6: 1312. https://doi.org/10.3390/diagnostics12061312
APA StylePăsărică, M. A., Curcă, P. F., Dragosloveanu, C. D. M., Tătaru, C. I., Manole, I. R., Murgoi, G. E., & Grigorescu, A. C. (2022). Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema. Diagnostics, 12(6), 1312. https://doi.org/10.3390/diagnostics12061312






