Radiomics Analysis on Gadoxetate Disodium-Enhanced MRI Predicts Response to Transarterial Embolization in Patients with HCC
Abstract
:1. Introduction
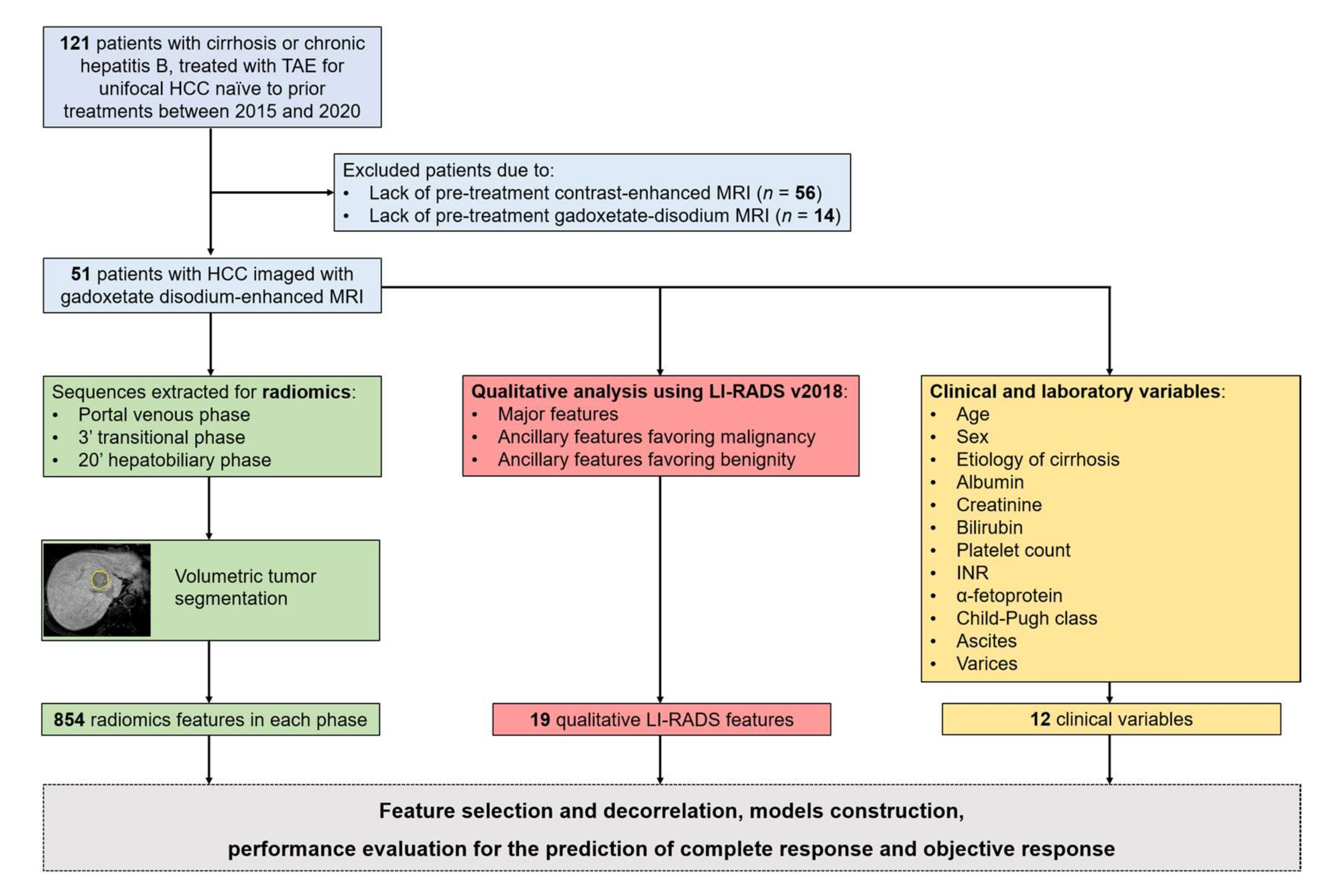
2. Materials and Methods
2.1. Population
2.2. MRI Technique
2.3. MRI Qualitative Analysis
2.4. Segmentation and Radiomics Feature Extraction
2.5. Lesions Outcome
2.6. Statistical Analyses of Qualitative Features
2.7. Radiomics Models Construction
2.8. Radiomics Model 1
2.9. Radiomics Model 2
3. Results
3.1. Population
3.2. Clinical and Qualitative Imaging Analysis
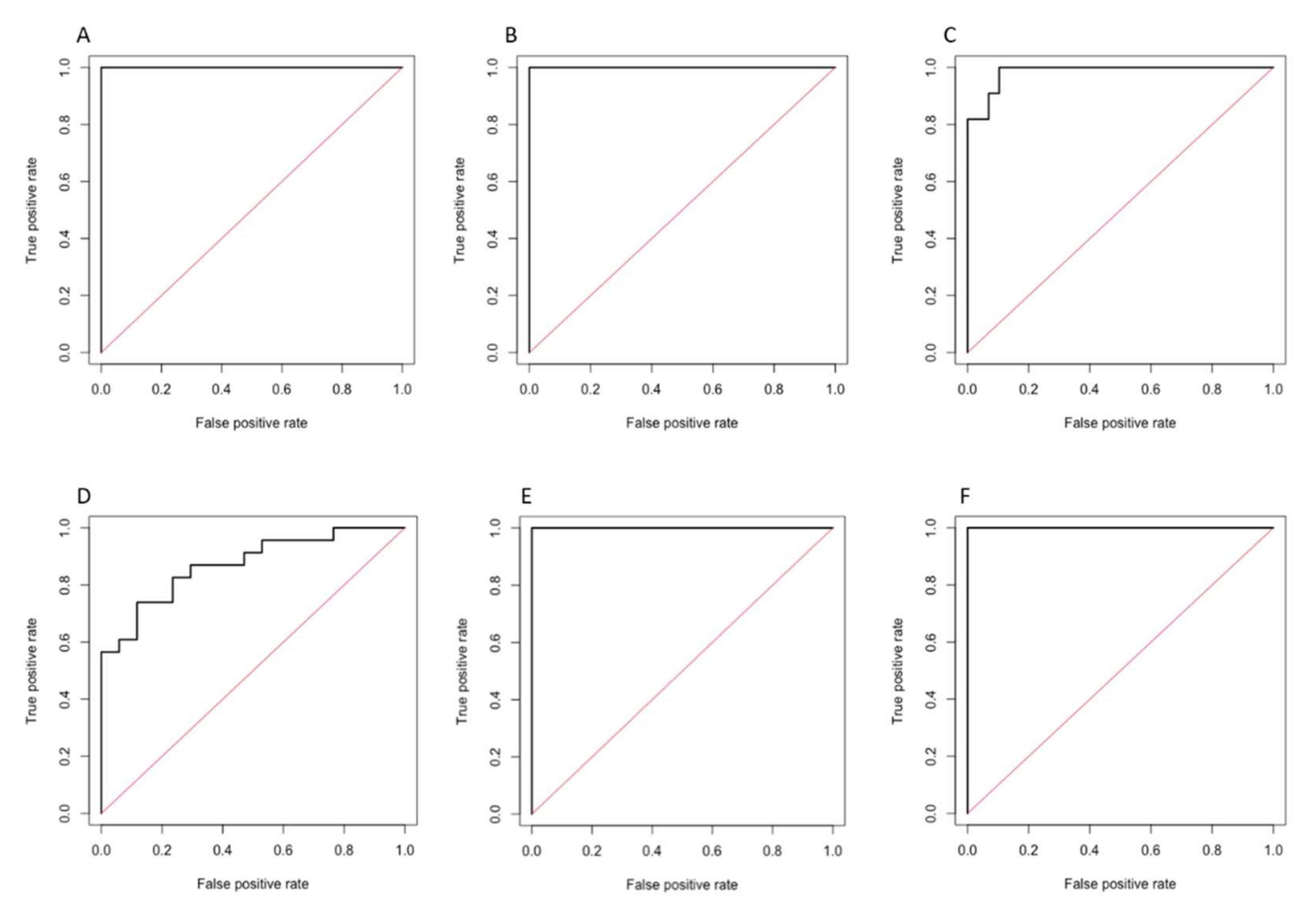
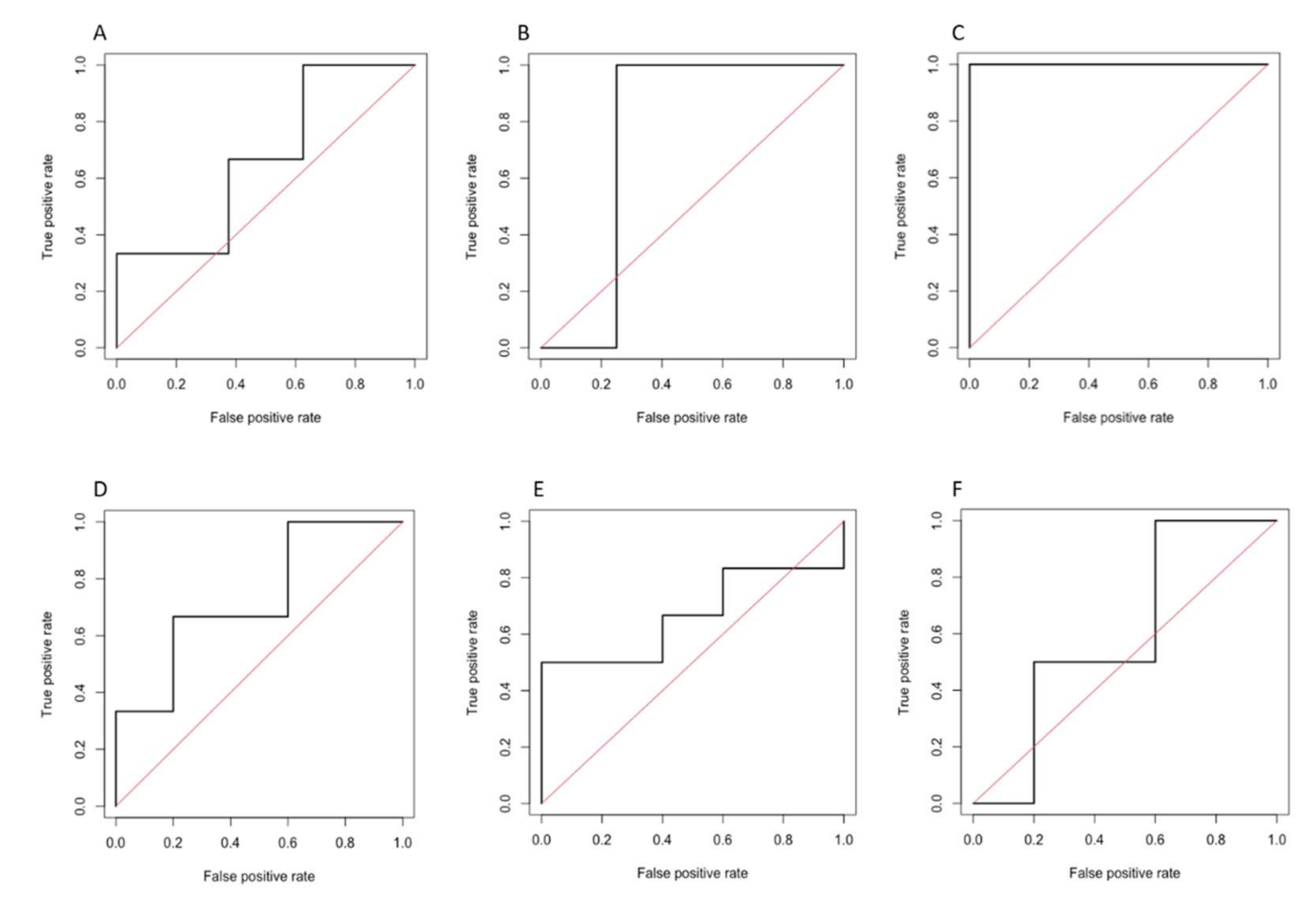
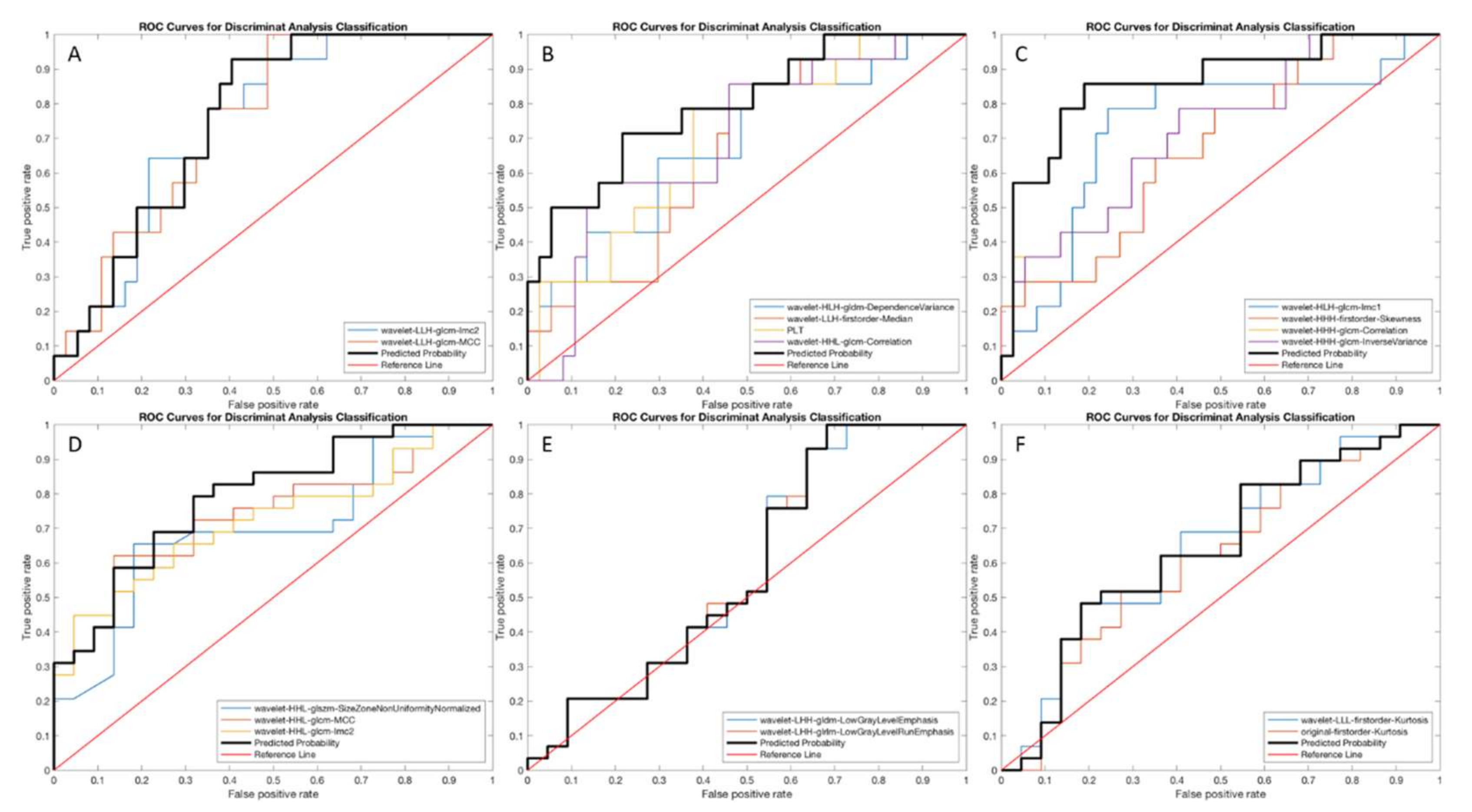
3.3. Performance of Radiomics-Based Models
3.3.1. Radiomics Model 1
3.3.2. Radiomics Model 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380 https://. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabibbo, G.; Genco, C.; Di Marco, V.; Barbara, M.; Enea, M.; Parisi, P.; Brancatelli, G.; Romano, P.; Craxi, A.; Camma’, C. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment. Pharmacol. Ther. 2011, 34, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.O.; Kim, E.B.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Predictive Factors for Complete Response and Recurrence after Transarterial Chemoembolization in Hepatocellular Carcinoma. Gut Liver 2017, 11, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Lee, M.H.; Choi, S.Y.; Yi, B.H.; Lee, H.K. Magnetic resonance imaging features predictive of an incomplete response to transarterial chemoembolization in patients with hepatocellular carcinoma. Medicine 2019, 98, e15592. [Google Scholar] [CrossRef]
- Park, C.; Kim, J.H.; Kim, P.H.; Kim, S.Y.; Gwon, D.I.; Chu, H.H.; Park, M.; Hur, J.; Kim, J.Y.; Kim, D.J. Imaging Predictors of Survival in Patients with Single Small Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Korean J. Radiol. 2021, 22, 213–224. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Vernuccio, F.; Cannella, R.; Comelli, A.; Salvaggio, G.; Lagalla, R.; Midiri, M. Radiomics and artificial intelligence: New frontiers in medicine. Recent Prog. Med. 2020, 111, 130–135. [Google Scholar] [CrossRef]
- Fu, S.; Chen, S.; Liang, C.; Liu, Z.; Zhu, Y.; Li, Y.; Lu, L. Texture analysis of intermediate-advanced hepatocellular carcinoma: Prognosis and patients’ selection of transcatheter arterial chemoembolization and sorafenib. Oncotarget 2016, 8, 37855–37865. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.H.; Choi, S.-y.; Lee, E.S.; Park, S.J.; Byun, J.Y.; Choi, B.I. Prediction of Therapeutic Response of Hepatocellular Carcinoma to Transcatheter Arterial Chemoembolization Based on Pretherapeutic Dynamic CT and Textural Findings. Am. J. Roentgenol. 2017, 209, W211–W220. [Google Scholar] [CrossRef]
- Kloth, C.; Thaiss, W.M.; Kärgel, R.; Grimmer, R.; Fritz, J.; Ioanoviciu, S.D.; Ketelsen, D.; Nikolaou, K.; Horger, M. Evaluation of Texture Analysis Parameter for Response Prediction in Patients with Hepatocellular Carcinoma Undergoing Drug-eluting Bead Transarterial Chemoembolization (DEB-TACE) Using Biphasic Contrast-enhanced CT Image Data: Correlation with Liver Perfusion CT. Acad. Radiol. 2017, 24, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, S.J.; Lee, S.-H.; Lee, H.Y.; Park, H. Predicting Survival Using Pretreatment CT for Patients With Hepatocellular Carcinoma Treated With Transarterial Chemoembolization: Comparison of Models Using Radiomics. Am. J. Roentgenol. 2018, 211, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.P.; Wang, Y.C.; Ju, S.; Lu, C.Q.; Zhong, B.Y.; Ni, C.F.; Zhang, Q.; Yu, Q.; Xu, J.; Ji, J.; et al. Radiomics Analysis on Multiphase Contrast-Enhanced CT: A Survival Prediction Tool in Patients With Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front. Oncol. 2020, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Kang, S.; Ning, Z.; Deng, H.; Shen, J.; Xu, Y.; Zhang, J.; Zhao, W.; Li, X.; Gong, W.; et al. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur. Radiol. 2019, 30, 413–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosshenrich, J.; Zech, C.J.; Heye, T.; Boldanova, T.; Fucile, G.; Wieland, S.; Heim, M.H.; Boll, D.T. Response prediction of hepatocellular carcinoma undergoing transcatheter arterial chemoembolization: Unlocking the potential of CT texture analysis through nested decision tree models. Eur. Radiol. 2020, 31, 4367–4376. [Google Scholar] [CrossRef]
- Sheen, H.; Kim, J.S.; Lee, J.K.; Choi, S.Y.; Baek, S.Y.; Kim, J.Y. A radiomics nomogram for predicting transcatheter arterial chemoembolization refractoriness of hepatocellular carcinoma without extrahepatic metastasis or macrovascular invasion. Abdom. Radiol. 2021, 46, 2839–2849. [Google Scholar] [CrossRef]
- Liu, J.; Pei, Y.; Zhang, Y.; Wu, Y.; Liu, F.; Gu, S. Predicting the prognosis of hepatocellular carcinoma with the treatment of transcatheter arterial chemoembolization combined with microwave ablation using pretreatment MR imaging texture features. Abdom. Radiol. 2021, 46, 3748–3757. [Google Scholar] [CrossRef]
- Song, W.; Yu, X.; Guo, D.; Liu, H.; Tang, Z.; Liu, X.; Zhou, J.; Zhang, H.; Liu, Y.; Liu, X. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J. Magn. Reson. Imaging 2019, 52, 461–473. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, H.; Xia, W.; Wang, D.; Zhou, B.; Zhao, X.; Yang, G.; Xu, L.; Zhang, W.; Liu, P.; et al. Predicting the Outcome of Transcatheter Arterial Embolization Therapy for Unresectable Hepatocellular Carcinoma Based on Radiomics of Preoperative Multiparameter MRI. J. Magn. Reson. Imaging 2020, 52, 1083–1090. [Google Scholar] [CrossRef]
- Kong, C.; Zhao, Z.; Chen, W.; Lv, X.; Shu, G.; Ye, M.; Song, J.; Ying, X.; Weng, Q.; Weng, W.; et al. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur. Radiol. 2021, 31, 7500–7511. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Wu, J.; Zhang, Q.; Lin, T.; Yao, Y.; Chen, Z.; Wang, M.; Sheng, L.; Liu, J.; et al. Radiomics Analysis Based on Contrast-Enhanced MRI for Prediction of Therapeutic Response to Transarterial Chemoembolization in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 582788. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, R.; Jia, P.; Ye, W.; Zhou, R.; Zhu, R.; Wang, J.; Lin, S.; Pang, P.; Ji, W. MRI-Based Radiomics: Nomograms predicting the short-term response after transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma patients with diameter less than 5 cm. Abdom. Radiol. 2021, 46, 3772–3789. [Google Scholar] [CrossRef] [PubMed]
- Kambadakone, A.R.; Fung, A.; Gupta, R.T.; Hope, T.A.; Fowler, K.; Lyshchik, A.; Ganesan, K.; Yaghmai, V.; Guimaraes, A.R.; Sahani, D.V.; et al. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom. Radiol. 2017, 43, 56–74. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. CT/MRI Liver Imaging Reporting and Data System v2018 Core. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018 (accessed on 3 November 2021).
- Furlan, A.; Close, O.; Borhani, A.; Wu, Y.; Heller, M. Respiratory-motion artefacts in liver MRI following injection of gadoxetate disodium and gadobenate dimeglumine: An intra-individual comparative study in cirrhotic patients. Clin. Radiol. 2016, 72, 93.e1–93.e6. [Google Scholar] [CrossRef]
- Shah, M.R.; Flusberg, M.; Paroder, V.; Rozenblit, A.M.; Chernyak, V. Transient arterial phase respiratory motion-related artifact in MR imaging of the liver: An analysis of four different gadolinium-based contrast agents. Clin. Imaging 2017, 41, 23–27. [Google Scholar] [CrossRef]
- Wels, M.G.; Lades, F.; Muehlberg, A.; Suehling, M. General purpose radiomics for multi-modal clinical research. In Medical Imaging 2019: Computer-Aided Diagnosis; SPIE: Washington, DC, USA; Cardiff, UK, 2019; Volume 10950, pp. 1047–1054. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Comelli, A.; Stefano, A.; Coronnello, C.; Russo, G.; Vernuccio, F.; Cannella, R.; Salvaggio, G.; Lagalla, R.; Barone, S. Radiomics: A New Biomedical Workflow to Create a Predictive Model. In Medical Image Understanding and Analysis. MIUA 2020. Communications in Computer and Information Science; Springer: Cham, Switzerland, 2020; Volume 1248, pp. 280–293. [Google Scholar] [CrossRef]
- Barone, S.; Cannella, R.; Comelli, A.; Pellegrino, A.; Salvaggio, G.; Stefano, A.; Vernuccio, F. Hybrid descriptive-inferential method for key feature selection in prostate cancer radiomics. Appl. Stoch. Models Bus. Ind. 2021, 37, 961–972. [Google Scholar] [CrossRef]
- Cilluffo, G.; Sottile, G.; La Grutta, S.; Muggeo, V.M. The Induced Smoothed lasso: A practical framework for hypothesis testing in high dimensional regression. Stat. Methods Med. Res. 2019, 29, 765–777. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sottile, G.; Cilluffo, G.; Muggeo, V.M.R. The R Package Islasso: Estimation and Hypothesis Testing in Lasso Regression; Technical Report; ResearchGate GmbH: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Stefano, A.; Comelli, A.; Bravatà, V.; Barone, S.; Daskalovski, I.; Savoca, G.; Sabini, M.G.; Ippolito, M.; Russo, G. A preliminary PET radiomics study of brain metastases using a fully automatic segmentation method. BMC Bioinform. 2020, 21, 325. [Google Scholar] [CrossRef]
- Russo, G.; Stefano, A.; Alongi, P.; Comelli, A.; Catalfamo, B.; Mantarro, C.; Longo, C.; Altieri, R.; Certo, F.; Cosentino, S.; et al. Feasibility on the Use of Radiomics Features of 11[C]-MET PET/CT in Central Nervous System Tumours: Preliminary Results on Potential Grading Discrimination Using a Machine Learning Model. Curr. Oncol. 2021, 28, 5318–5331. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-H.; Titterington, D.M. Do unbalanced data have a negative effect on LDA? Pattern Recognit 2008, 41, 1558–1571. [Google Scholar] [CrossRef] [Green Version]
- Sieghart, W.; Hucke, F.; Peck-Radosavljevic, M. Transarterial chemoembolization: Modalities, indication, and patient selection. J. Hepatol. 2015, 62, 1187–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tang, W.; Xie, S.; Chen, J.; Zhang, L.; Rong, D.; Kuang, S.; He, B.; Wang, J. The role of lesion hypointensity on gadobenate dimeglumine–enhanced hepatobiliary phase MRI as an additional major imaging feature for HCC classification using LI-RADS v2018 criteria. Eur. Radiol. 2021, 31, 7715–7724. [Google Scholar] [CrossRef]
- Yang, H.; Han, P.; Huang, M.; Yue, X.; Wu, L.; Li, X.; Fan, W.; Li, Q.; Ma, G.; Lei, P. The role of gadoxetic acid-enhanced MRI features for predicting microvascular invasion in patients with hepatocellular carcinoma. Abdom. Radiol. 2022, 47, 948–956. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10-19 mm With Arterial Phase Hyperenhancement. Am. J. Roentgenol. 2019, 213, W57–W65. [Google Scholar] [CrossRef]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- An, C.; Park, S.; Chung, Y.E.; Kim, D.-Y.; Kim, S.-S.; Kim, M.-J.; Choi, J.-Y. Curative Resection of Single Primary Hepatic Malignancy: Liver Imaging Reporting and Data System Category LR-M Portends a Worse Prognosis. Am. J. Roentgenol. 2017, 209, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Lee, S.S.; Park, S.H.; Kim, K.M.; Yu, E.; Park, Y.; Shin, Y.M.; Lee, M.G. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology 2019, 290, 388–397. [Google Scholar] [CrossRef]
- Celsa, C.; Cabibbo, G.; Enea, M.; Battaglia, S.; Rizzo, G.E.M.; Busacca, A.; Giuffrida, P.; Stornello, C.; Brancatelli, G.; Cannella, R.; et al. Are radiological endpoints surrogate outcomes of overall survival in hepatocellular carcinoma treated with transarterial chemoembolization? Liver Int. 2021, 41, 1105–1116. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.-Y.; Shin, J.; Hwang, S.H.; Roh, Y.H.; Chung, Y.E.; Choi, J.-Y. CT and MRI Liver Imaging Reporting and Data System Version 2018 for Hepatocellular Carcinoma: A Systematic Review with Meta-Analysis. J. Am. Coll. Radiol. 2020, 17, 1199–1206. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | CR (n = 14) | PR + SD + PD (n = 37) | p Value | CR + PR (n = 29) | SD + PD (n = 22) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 72.5 (65.0, 75.7) | 74.0 (67.0, 78.5) | 0.526 | 73.0 (65.0, 79.5) | 73.5 (69.5, 77.2) | 0.614 |
| Sex Males Females | 9 (64.3) 5 (35.7) | 28 (75.7) 9 (24.3) | 0.490 | 20 (69.0) 9 (31.0) | 17 (77.3) 5 (22.7) | 0.510 |
| Etiology of cirrhosis Hepatitis C Hepatitis B NAFLD | 12 (85.8) 1 (7.1) 1 (7.1) | 31 (83.8) 5 (13.5) 1 (2.7) | 0.649 | 24 (82.8) 4 (13.8) 1 (3.4) | 19 (86.4) 2 (9.1) 1 (4.5) | 0.864 |
| Albumin (g/dL) | 3.4 (3.3, 3.9) | 3.6 (3.2, 3.9) | 0.767 | 3.4 (3.2, 3.9) | 3.7 (3.4, 4.0) | 0.147 |
| Creatinine (mg/dL) | 0.8 (0.7, 0.9) | 0.8 (0.7, 1.0) | 0.670 | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.803 |
| Bilirubin (mg/dL) | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.2) | 0.983 | 0.8 (0.6, 1.4) | 0.8 (0.6, 1.0) | 0.661 |
| Platelet count (×103/μL) | 115.1 (97.0, 170.7) | 80.0 (59.5, 120.0) | 0.021 | 101.0 (63.0, 130.5) | 86.5 (60.2, 120.7) | 0.697 |
| INR | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.2) | 0.380 | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.2) | 0.985 |
| α-fetoprotein (ng/mL) | 10.3 (3.7, 122.0) | 5.0 (2.9, 47.1) | 0.597 | 8.4 (2.7, 41.7) | 5.0 (2.9, 121.2) | 0.886 |
| Child-Pugh Class A B | 14 (100) 0 (0) | 31 (83.8) 6 (16.2) | 0.170 | 26 (89.7) 3 (10.3) | 19 (86.4) 3 (13.6) | 1.000 |
| Ascites | 0 (0) | 8 (21.6) | 0.088 | 5 (17.2) | 3 (13.6) | 1.000 |
| Varices | 10 (71.4) | 29 (78.4) | 0.715 | 22 (75.9) | 17 (77.3) | 0.906 |
| Characteristics | CR (n = 14) | PR + SD + PD (n = 37) | p Value | CR + PR (n = 29) | SD + PD (n = 22) | p Value |
|---|---|---|---|---|---|---|
| Size (mm) | 23.0 (14.7, 27.0) | 20.0 (16.0, 31.5) | 0.642 | 22.0 (15.5, 28.0) | 20.0 (16.0, 32.0) | 0.593 |
| Nonrim APHE | 13 (92.9) | 36 (97.3) | 0.478 | 27 (93.1) | 22 (100) | 0.500 |
| Nonperipheral “washout” | 11 (78.6) | 32 (86.5) | 0.668 | 22 (75.9) | 21 (95.5) | 0.117 |
| Enhancing “capsule” | 6 (42.9) | 17 (45.9) | 0.843 | 12 (41.4) | 11 (50.0) | 0.581 |
| Threshold growth | 0 (0) | 4 (10.8) | 0.565 | 3 (10.3) | 1 (4.5) | 0.624 |
| US visibility as discrete nodule | 4 (28.6) | 5 (13.5) | 0.236 | 5 (17.2) | 4 (18.2) | 1.000 |
| Subthreshold growth | 5 (35.7) | 9 (24.3) | 0.490 | 6 (20.7) | 8 (36.4) | 0.214 |
| Corona enhancement | 1 (7.1) | 2 (5.4) | 1.000 | 2 (6.9) | 1 (4.5) | 1.000 |
| Fat sparing in solid mass | 1 (7.1) | 3 (8.1) | 1.000 | 3 (10.3) | 1 (4.5) | 0.625 |
| Restricted diffusion | 9 (64.3) | 24 (64.9) | 1.000 | 18 (62.1) | 15 (68.2) | 0.651 |
| Mild-moderate T2 hyperintensity | 7 (50.0) | 20 (54.1) | 0.796 | 13 (44.8) | 14 (63.6) | 0.183 |
| Iron sparing in solid mass | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| Transitional phase hypointensity | 9 (64.3) | 33 (89.2) | 0.093 | 21 (72.4) | 21 (95.5) | 0.060 |
| HBP hypointensity | 12 (85.7) | 32 (86.5) | 1.000 | 24 (82.8) | 20 (90.9) | 0.684 |
| Nonenhancing “capsule” | 0 (0) | 1 (2.7) | 1.000 | 1 (3.4) | 0 (0) | 1.000 |
| Nodule-in-nodule architecture | 1 (7.1) | 3 (8.1) | 1.000 | 2 (6.9) | 2 (9.1) | 1.000 |
| Mosaic architecture | 1 (7.1) | 3 (8.1) | 1.000 | 1 (3.4) | 3 (13.6) | 0.303 |
| Fat in mass, more than adjacent liver | 2 (14.3) | 7 (18.9) | 1.000 | 4 (13.8) | 5 (22.7) | 0.474 |
| Blood products in mass | 1 (7.1) | 0 (0) | 0.275 | 1 (3.4) | 0 (0) | 1.000 |
| Sensitivity | Specificity | Accuracy | AUC (95% CI) | p Value | |
|---|---|---|---|---|---|
| Prediction of complete response | |||||
| PVP | 100 | 100 | 100 | 1.000 (1.000–1.000) | <0.001 |
| 3′ TP | 100 | 100 | 100 | 1.000 (1.000–1.000) | <0.001 |
| HBP | 100 | 90.0 | 92.5 | 0.984 (0.957–1.000) | <0.001 |
| Prediction of objective response | |||||
| PVP | 87.0 | 64.7 | 77.5 | 0.872 (0.765–0.979) | <0.001 |
| 3′ TP | 100 | 100 | 100 | 1.000 (1.000–1.000) | <0.001 |
| HBP | 94.1 | 100 | 97.5 | 1.000 (1.000–1.000) | <0.001 |
| Sensitivity | Specificity | Accuracy | AUC (95% CI) | p Value | |
|---|---|---|---|---|---|
| Prediction of complete response | |||||
| PVP | 87.5 | 33.3 | 72.7 | 0.667 (0.251–1.000) | 0.431 |
| 3′ TP | 75.0 | 33.3 | 63.6 | 0.750 (0.429–1.000) | 0.127 |
| HBP | 100 | 100 | 100 | 1.000 (1.000–1.000) | <0.001 |
| Prediction of objective response | |||||
| PVP | 100 | 40.0 | 72.7 | 0.733 (0.405–1.000) | 0.163 |
| 3′ TP | 40.0 | 66.7 | 54.5 | 0.667 (0.305–1.000) | 0.367 |
| HBP | 20.0 | 100 | 63.6 | 0.600 (0.193–1.000) | 0.630 |
| Sensitivity | Specificity | Accuracy | AUC (95% CI) | p Value | |
|---|---|---|---|---|---|
| Prediction of complete response | |||||
| PVP | 66.6 | 56.6 | 63.8 | 0.757 (0.626–0.888) | 0.002 |
| 3′ TP | 66.1 | 72.8 | 67.9 | 0.795 (0.654–0.936) | 0.024 |
| HBP | 75.5 | 82.8 | 77.5 | 0.861 (0.737–0.984) | 0.010 |
| Prediction of objective response | |||||
| PVP | 71.3 | 61.7 | 65.8 | 0.791 (0.667–0.915) | 0.002 |
| 3′ TP | 54.1 | 65.6 | 60.7 | 0.585 (0.414–0.755) | 0.049 |
| HBP | 58.8 | 90.1 | 76.7 | 0.790 (0.649–0.931) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannella, R.; Cammà, C.; Matteini, F.; Celsa, C.; Giuffrida, P.; Enea, M.; Comelli, A.; Stefano, A.; Cammà, C.; Midiri, M.; et al. Radiomics Analysis on Gadoxetate Disodium-Enhanced MRI Predicts Response to Transarterial Embolization in Patients with HCC. Diagnostics 2022, 12, 1308. https://doi.org/10.3390/diagnostics12061308
Cannella R, Cammà C, Matteini F, Celsa C, Giuffrida P, Enea M, Comelli A, Stefano A, Cammà C, Midiri M, et al. Radiomics Analysis on Gadoxetate Disodium-Enhanced MRI Predicts Response to Transarterial Embolization in Patients with HCC. Diagnostics. 2022; 12(6):1308. https://doi.org/10.3390/diagnostics12061308
Chicago/Turabian StyleCannella, Roberto, Carla Cammà, Francesco Matteini, Ciro Celsa, Paolo Giuffrida, Marco Enea, Albert Comelli, Alessandro Stefano, Calogero Cammà, Massimo Midiri, and et al. 2022. "Radiomics Analysis on Gadoxetate Disodium-Enhanced MRI Predicts Response to Transarterial Embolization in Patients with HCC" Diagnostics 12, no. 6: 1308. https://doi.org/10.3390/diagnostics12061308
APA StyleCannella, R., Cammà, C., Matteini, F., Celsa, C., Giuffrida, P., Enea, M., Comelli, A., Stefano, A., Cammà, C., Midiri, M., Lagalla, R., Brancatelli, G., & Vernuccio, F. (2022). Radiomics Analysis on Gadoxetate Disodium-Enhanced MRI Predicts Response to Transarterial Embolization in Patients with HCC. Diagnostics, 12(6), 1308. https://doi.org/10.3390/diagnostics12061308










