Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
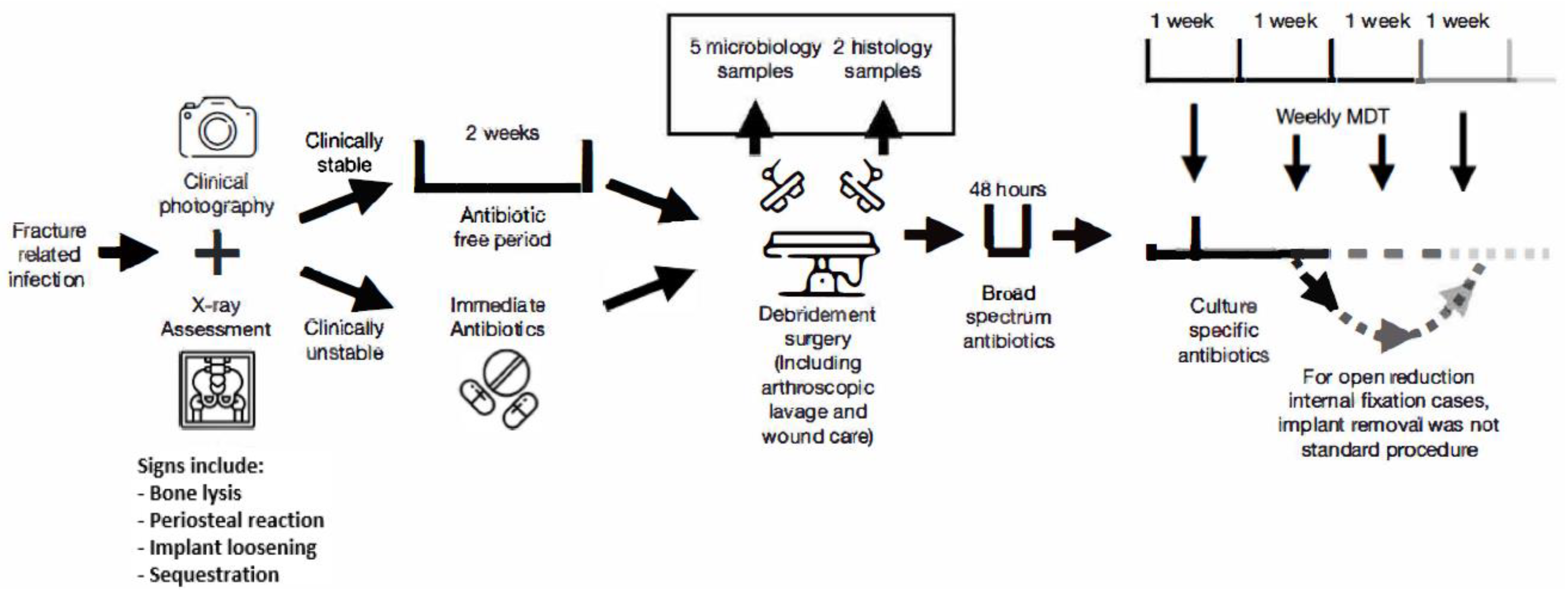
2.2. Treatment Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Polymicrobial Infections
4.2. Obesity
4.3. Diabetes
4.4. Implant Retention
4.5. Gustilo-Anderson (GA)
4.6. FRI Prevention and Characteristics
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birt, M.C.; Anderson, D.W.; Toby, E.B.; Wang, J. Osteomyelitis: Recent advances in pathophysiology and therapeutic strategies. J. Orthop. 2016, 14, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; Obremskey, W.; McNally, M.A.; Metsemakers, W.J.; Verhofstad, M.H.J. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struijs, P.A.A.; Poolman, R.W.; Bhandari, M. Infected Nonunion of the Long Bones. 2007; Volume 21, pp. 507–511. Available online: https://pubmed.ncbi.nlm.nih.gov/17762489/ (accessed on 10 May 2021).
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Lu, V.; Zhang, J.; Krkovic, M. Management of femoral non-union with post-traumatic bone defect using second-generation PRECICE® nail—A case report. Trauma Case Rep. 2021, 37, 100585. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.-A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Kanakaris, N.; Gudipati, S.; Tosounidis, T.; Harwood, P.; Britten, S.; Giannoudis, P.V. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. Bone Jt. J. 2014, 96, 783–788. [Google Scholar] [CrossRef]
- Ktistakis, I.; Giannoudi, M.; Giannoudis, P.V. Infection rates after open tibial fractures: Are they decreasing? Injury 2014, 45, 1025–1027. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.C.; Son, M.S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are We Winning or Losing the Battle with Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Bori, G.; Climent, C.; García-Velez, D.; García-Ramiro, S.; Bosch, J.; Mensa, J.; Soriano, A. KLIC-score for predicting early failure in prosthetic joint infections treated with debridement, implant retention and antibiotics. Clin. Microbiol. Infect. 2015, 21, e9–e786.e17. [Google Scholar] [CrossRef] [Green Version]
- Lu, V.; Zhou, A.; Hassan, H.A.; Thahir, A.; Krkovic, M. Risk factors for septic arthritis and multiple arthroscopic washouts: Minimum 2-year follow-up at a major trauma centre. Clin. Rheumatol. 2022, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- BOAST—Open Fractures. Available online: https://www.boa.ac.uk/static/3b91ad0a-9081-4253-92f7d90e8df0fb2c/29bf80f1-1cb6-46b7-afc761119341447f/openfractures.pdf (accessed on 3 March 2022).
- Gustilo, R.B.; Anderson, J.T. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: Retrospective and prospective analyses. J. Bone Jt. Surg. Am. 1976, 58, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Copes, W.S.; Champion, H.R.; Sacco, W.J.; Lawnick, M.M.; Keast, S.L.; Bain, L.W. The injury severity score revisited. J. Trauma-Inj. Infect. Crit. Care 1988, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.; Morgenstern, M.; McNally, M.A.; Moriarty, F.; McFadyen, I.; Scarborough, M.; Athanasou, N.; Ochsner, P.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.; Verhofstad, M.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BOAST—Fracture Related Infections (FRI). Available online: https://www.boa.ac.uk/static/dee7cba7-5919-4f26-a286033fcf46a458/boast-fracture-related-infections.pdf (accessed on 3 March 2022).
- Van Smeden, M.; De Groot, J.A.H.; Moons, K.G.M.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med. Res. Methodol. 2016, 16, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, T.M.; Rackard, F.A.; Dutton, L.K.; Sparks, M.B.; Harris, M.B.; Gitajn, I.L. Analyzing risk factors for treatment failure in fracture-related infection. Arch. Orthop. Trauma Surg. 2022. [Google Scholar] [CrossRef]
- Horton, S.A.; Hoyt, B.W.; Zaidi, S.M.R.; Schloss, M.G.; Joshi, M.; Carlini, A.R.; Castillo, R.C. Risk factors for treatment failure of fracture-related infections. Injury 2021, 52, 1351–1355. [Google Scholar] [CrossRef]
- Gitajn, I.L.; Heng, M.; Weaver, M.J.; Ehrlichman, L.K.; Harris, M.B. Culture-Negative Infection After Operative Fixation of Fractures. J. Orthop. Trauma 2016, 30, 538–544. [Google Scholar] [CrossRef]
- Choi, H.R.; Kwon, Y.M.; Freiberg, A.A.; Nelson, S.B.; Malchau, H. Periprosthetic joint infection with negative culture results: Clinical characteristics and treatment outcome. J. Arthroplast. 2013, 28, 899–903. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kulkarni, S.S.; Park, J.W.; Kim, J.S.; Oh, H.K.; Rastogi, D. Comparison of infection control rates and clinical outcomes in culture-positive and culture-negative infected total-knee arthroplasty. J. Orthop. 2015, 12 (Suppl. S1), S37–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbari, E.F.; Marculescu, C.; Sia, I.; Lahr, B.D.; Hanssen, A.D.; Steckelberg, J.M.; Gullerud, R.; Osmon, D.R. Culture-negative prosthetic joint infection. Clin. Infect. Dis. 2007, 45, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Ovaska, M.T.; Mäkinen, T.J.; Madanat, R.; Vahlberg, T.; Hirvensalo, E.; Lindahl, J. Predictors of poor outcomes following deep infection after internal fixation of ankle fractures. Injury 2013, 44, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, P.; Leenen, L.P.H.; Hoekstra, M.; Hietbrink, F. Effect of a standardized treatment regime for infection after osteosynthesis. J. Orthop. Surg. Res. 2017, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Silva, M.; Bahk, W.J.; McKellop, H.; Lieberman, J.R. Effect of repeated irrigation and debridement on fracture healing in an animal model. J. Orthop. Res. 2002, 20, 1197–1204. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Wimmer, M.D.; Friedrich, M.J.; Randau, T.M.; Ploeger, M.M.; Schmolders, J.; Strauss, A.A.; Hischebeth, G.T.R.; Pennekamp, P.H.; Vavken, P.; Gravius, S. Polymicrobial infections reduce the cure rate in prosthetic joint infections: Outcome analysis with two-stage exchange and follow-up ≥ two years. Int. Orthop. 2015, 40, 1367–1373. [Google Scholar] [CrossRef]
- Ramsey, M.M.; Rumbaugh, K.P.; Whiteley, M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Cook, L.C.; LaSarre, B.; Federle, M.J. Interspecies communication among commensal and pathogenic streptococci. mBio 2013, 4, e00382-13. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Ikegami, A.; Kuramitsu, H.K. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol. Lett. 2005, 250, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Garcia, J.; Gruenberg, K.; MacDougall, C. Multidrug-Resistant Pseudomonas Infections: Hard to Treat, but Hope on the Horizon? Curr. Infect. Dis. Rep. 2018, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Kavolus, J.J.; Cunningham, D.J.; Rao, S.R.; Wellman, S.S.; Seyler, T.M. Polymicrobial Infections in Hip Arthroplasty: Lower Treatment Success Rate, Increased Surgery, and Longer Hospitalization. J. Arthroplast. 2019, 34, 710–716.e3. [Google Scholar] [CrossRef] [PubMed]
- Löwik, C.A.M.; Zijlstra, W.P.; Knobben, B.A.S.; Ploegmakers, J.J.W.; Dijkstra, B.; De Vries, A.J.; Kampinga, G.A.; Mithoe, G.; Al Moujahid, A.; Jutte, P.C.; et al. Obese patients have higher rates of polymicrobial and Gram-negative early periprosthetic joint infections of the hip than non-obese patients. PLoS ONE 2019, 14, e0215035. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Syrjänen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2012, 37, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Chen, H.L. Obesity and surgical site infections risk in orthopedics: A meta-analysis. Int. J. Surg. 2013, 11, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.P.; Walsh, M.; Sehgal, B.; Preston, C.; DeWal, H.; Di Cesare, P.E. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J. Bone Jt. Surg.-Ser. A 2007, 89, 33–38. [Google Scholar] [CrossRef]
- Böni, L.; Kuster, S.P.; Bartik, B.; Zbinden, R.; Zingg, P.O.; Achermann, Y. Association of cutibacterium avidum colonization in the groin with obesity: A potential risk factor for hip periprosthetic joint infection. Clin. Infect. Dis. 2018, 67, 1878–1882. [Google Scholar] [CrossRef]
- Nave, H.; Beutel, G.; Kielstein, J.T. Obesity-related immunodeficiency in patients with pandemic influenza H1N1. Lancet Infect. Dis. 2011, 11, 14–15. [Google Scholar] [CrossRef]
- Zahr, F.; Genovese, E.; Mathier, M.; Shullo, M.; Lockard, K.; Zomak, R.; McNamara, D.; Toyoda, Y.; Kormos, R.L.; Teuteberg, J.J. Obese patients and mechanical circulatory support: Weight loss, adverse events, and outcomes. Ann. Thorac. Surg. 2011, 92, 1420–1426. [Google Scholar] [CrossRef]
- Roe, J.L.; Fuentes, J.M.; Mullins, M.E. Underdosing of common antibiotics for obese patients in the ED. Am. J. Emerg. Med. 2012, 30, 1212–1214. [Google Scholar] [CrossRef]
- Hall, R.G.; Payne, K.D.; Bain, A.M.; Rahman, A.P.; Nguyen, S.T.; Eaton, S.A.; Busti, A.J.; Vu, S.L.; Bedimo, R. Multicenter Evaluation of Vancomycin Dosing: Emphasis on Obesity. Am. J. Med. 2008, 121, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Lv, T.R.; Zhou, J.C.; Qin, X.D. Effects of obesity on the healing of bone fracture in mice. J. Orthop. Surg. Res. 2018, 13, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.L.; Yukata, K.; Farnsworth, C.W.; Chen, D.-G.; Awad, H.; Hilton, M.J.; O’Keefe, R.J.; Xing, L.; Mooney, R.A.; Zuscik, M.J. Delayed Fracture Healing and Increased Callus Adiposity in a C57BL/6J Murine Model of Obesity-Associated Type 2 Diabetes Mellitus. PLoS ONE 2014, 9, e99656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, B.R.; Hux, J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003, 26, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, K.; Morris, J.; Bridson, T.; Govan, B.; Rush, C.; Ketheesan, N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015, 144, 171. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Anza-Ramírez, C.; Saal-Zapata, G.; illarreal-Zegarra, D.; Zafra-Tanaka, J.H.; Ugarte-Gil, C.; Bernabé-Ortiz, A. Type 2 diabetes mellitus and antibiotic-resistant infections: A systematic review and meta-analysis. J. Epidemiol. Community Health 2021, 76, 75–84. [Google Scholar] [CrossRef]
- Struelens, M.J. The epidemiology of antimicrobial resistance in hospital acquired infections: Problems and possible solutions. BMJ Br. Med. J. 1998, 317, 652. [Google Scholar] [CrossRef] [Green Version]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Davis, T.M.E.; Daly, F.; Walsh, J.; Ilett, K.F.; Beilby, J.P.; Dusci, L.J.; Barrett, P.H. Pharmacokinetics and pharmacodynamics of gliclazide in Caucasians and Australian Aborigines with type 2 diabetes. Br. J. Clin. Pharmacol. 2000, 49, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Hays, R.; Esterman, A.; McDermott, R. Type 2 Diabetes Mellitus Is Associated with Strongyloides stercoralis Treatment Failure in Australian Aboriginals. PLoS Negl. Trop. Dis. 2015, 9, e0003976. [Google Scholar] [CrossRef]
- Hamann, C.; Goettsch, C.; Mettelsiefen, J.; Henkenjohann, V.; Rauner, M.; Hempel, U.; Bernhardt, R.; Fratzl-Zelman, N.; Roschger, P.; Rammelt, S.; et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am. J. Physiol.-Endocrinol. Metab. 2011, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furst, J.R.; Bandeira, L.C.; Fan, W.W.; Agarwal, S.; Nishiyama, K.K.; McMahon, D.J.; Dworakowski, E.; Jiang, H.; Silverberg, S.J.; Rubin, M.R. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2502–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folk, J.W.; Starr, A.J.; Early, J.S. Early wound complications of operative treatment of calcaneus fractures: Analysis of 190 fractures. J. Orthop. Trauma 1999, 13, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Petty, W.; Spanier, S.; Shuster, J.J.; Silvertorne, C. The influence of skeletal implants on incidence of infection. Experiments in a canine model. J. Bone Jt. Surg. Am. 1985, 67, 1236–1244. [Google Scholar] [CrossRef]
- Rightmire, E.; Zurakowski, D.; Vrahas, M. Acute Infections After Fracture Repair: Management with Hardware in Place. Clin. Orthop. Relat. Res. 2008, 466, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Lin, J.; Zhang, C.; Zhu, H.; Zheng, X. Is intracellular Staphylococcus aureus associated with recurrent infection in a rat model of open fracture? Bone Jt. Res. 2020, 9, 71. [Google Scholar] [CrossRef]
- Berkes, M.; Obremskey, W.T.; Scannell, B.; Ellington, J.K.; Hymes, R.A.; Bosse, M. Maintenance of hardware after early postoperative infection following fracture internal fixation. J. Bone Jt. Surg.-Ser. A 2010, 92, 823–828. [Google Scholar] [CrossRef]
- Morgenstern, M.; Kuehl, R.; Zalavras, C.G.; McNally, M.; Zimmerli, W.; Burch, M.A.; Vandendriessche, T.; Obremskey, W.T.; Verhofstad, M.H.J.; Metsemakers, W.J. The influence of duration of infection on outcome of debridement and implant retention in fracture-related infection. Bone Jt. J. 2021, 103-B, 213–221. [Google Scholar] [CrossRef]
- Tennent, D.J.; Shiels, S.M.; Jennings, J.A.; Haggard, W.O.; Wenke, J.C. Local control of polymicrobial infections via a dual antibiotic delivery system. J. Orthop. Surg. Res. 2018, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Kay, H.F.; Sathiyakumar, V.; Yoneda, Z.T.; Lee, Y.M.; Jahangir, A.A.; Ehrenfeld, J.; Obremskey, W.T.; Apfeld, J.; Sethi, M.K. The effects of American society of anesthesiologists physical status on length of stay and inpatient cost in the surgical treatment of isolated orthopaedic fractures. J. Orthop. Trauma 2014, 28, e153–e159. [Google Scholar] [CrossRef]
- del Pozo Garcia, E.; Collazos, J.; Carton, J.A.; Camporro, D.; Asensi, V. Factors predictive of relapse in adult bacterial osteomyelitis of long bones. BMC Infect. Dis. 2018, 18, 635. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.P.; Mathes, S.J.; Alpert, B.S. The muscle flap in the treatment of chronic lower extremity osteomyelitis: Results in patients over 5 years after treatment. Plast. Reconstr. Surg. 1991, 88, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Olesen, U.K.; Juul, R.; Bonde, C.T.; Moser, C.; McNally, M.; Jensen, L.T.; Elberg, J.J.; Eckardt, H. A review of forty five open tibial fractures covered with free flaps. Analysis of complications, microbiology and prognostic factors. Int. Orthop. 2015, 39, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- International Consensus Meeting on Musculoskeletal infection. Part V: Trauma ICM Philly. 2018. Available online: https://icmphilly.com/document/icm-2018-trauma-document/ (accessed on 8 March 2022).
- Willenegger, H.; Roth, B. Behandlungstaktik und Spätergebnisse bei Frühinfekt nach Osteosynthese. Unfallchirurgie 1986, 12, 241–246. [Google Scholar] [CrossRef]
- Kleber, C.; Schaser, K.D.; Trampuz, A. Komplikationsmanagement bei infizierter Osteosynthese: Therapiealgorithmus bei periimplantären Infektionen. Chirurg 2015, 86, 925–934. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37 (Suppl. S2), S59–S66. [Google Scholar] [CrossRef]
- Sandy-Hodgetts, K.; Watts, R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post-surgical wound complications: A systematic review and meta-analysis. BI Database Syst. Rev. Implement. Rep. 2015, 13, 253–303. [Google Scholar] [CrossRef]
- Alfonso, A.R.; Kantar, R.S.; Ramly, E.P.; Daar, D.A.; Rifkin, W.J.; Levine, J.P.; Ceradini, D.J. Diabetes is associated with an increased risk of wound complications and readmission in patients with surgically managed pressure ulcers. Wound Repair Regen. 2019, 27, 249–256. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.-J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- BBosch, P.; Van Den, J.; Plate, J.D.; Ijpma, F.F.; Houwert, R.M.; Huisman, A.; Hietbrink, F.; Leenen, L.P.; Govaert, G.A. Limited Predictive Value of Serum Inflammatory Markers for Diagnosing Fracture-Related Infections: Results of a large retrospective multicenter cohort study. J. Bone Jt. Infect. 2018, 3, 130. [Google Scholar] [CrossRef]
- van den Kieboom, J.; Bosch, P.; Plate, J.D.J.; Ijpma, F.F.A.; Kuehl, R.; McNally, M.A.; Metsemakers, W.-J.; Govaert, G.A.M. Diagnostic accuracy of serum inflammatory markers in late fracture-related infection: A systematic review and meta-analysis. Bone Jt. J. 2018, 100-B, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Dudareva, M.; Govaert, G.; Morgenstern, M.; Metsemakers, W.J. Definition and diagnosis of fracture-related infection. EFORT Open Rev. 2020, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.A.M.; Glaudemans, A.W.J.M. Nuclear medicine imaging of posttraumatic osteomyelitis. Eur. J. Trauma Emerg. Surg. 2016, 42, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, E.; Davenport, R.; Willett, K.; Brohi, K. The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J. Trauma Acute Care Surg. 2014, 76, 730–735. [Google Scholar] [CrossRef]

| Total Population | 102 |
| Male | 66 (64.7%) |
| Female | 36 (35.3%) |
| Mean age at injury in years (range) | 49.71 (18–87) |
| Male | 48.25 (18–84) |
| Female | 52.38 (22–87) |
| BMI (range) | 27.08 (16.12–47.70) |
| Open fracture | 35 (34.3%) |
| Gustilo-Anderson 2 | 6 |
| Gustilo-Anderson 3a | 13 |
| Gustilo-Anderson 3b | 11 |
| Gustilo-Anderson 3c | 5 |
| Closed fracture | 67 (65.7%) |
| Smoking status | |
| Ex-smoker | 33 (32.4%) |
| Non-smoker | 24 (23.5%) |
| Current smoker | 45 (44.1%) |
| Diabetes Mellitus | |
| Yes | 31 (30.4%) |
| No | 71 (69.6%) |
| Fracture Mechanism | |
| RTC (high energy) | 33 (32.4%) |
| Fall from height (high energy) | 35 (34.3%) |
| Trip and fall (low energy) | 27 (26.5%) |
| Crush trauma (low energy) | 6 (5.9%) |
| Gunshot wound (high energy) | 1 (1.0%) |
| Polytrauma (ISS ≥ 16) | |
| Yes | 49 (48.0%) |
| No | 55 (52.0%) |
| Definitive Fixation | |
| Open reduction internal fixation (ORIF) | 87 (85.3%) |
| External Fixation | 15 (14.7%) |
| Reamed * | |
| Yes | 53 (88.3%) |
| No | 7 (11.7%) |
| Humerus Shaft | 5 (4.9%) |
| Radius | 8 (7.8%) |
| Olecranon/Ulna | 9 (8.8%) |
| Hip | 9 (8.8%) |
| Neck of femur | 6 (5.9%) |
| Femoral shaft | 17 (16.7%) |
| Tibial plateau | 5 (4.9%) |
| Tibial shaft | 30 (29.4%) |
| Ankle | 12 (11.8%) |
| Clavicle | 1 (1.0%) |
| Clinical Signs | |
| Fever | 7 (6.9%) |
| Purulent discharge | 78 (76.5%) |
| Wound dehiscence | 58 (56.9%) |
| Dolor (pain) | 96 (94.1%) |
| Rubor (erythema) | 76 (74.5%) |
| Tumor (swelling) | 77 (75.5%) |
| Radiological Signs | |
| Osteomyelitis signs † | 81 (79.4%) |
| Evidence of non-union | 19 (18.6%) |
| Bacteriological Testing | |
| Ultrasound-guided aspiration | 22 (21.6%) |
| 7 cultures taken * | 71 (69.6%) |
| Initial Culture-Specific Antibiotic | ||
| Penicillin | 14 (13.7%) | |
| Cephalosporin | 13 (12.7%) | |
| Tetracycline | 7 (6.9%) | |
| Aminoglycoside | 4 (3.9%) | |
| Macrolide | 2 (2.0%) | |
| Fluoroquinolone | 18 (17.6%) | |
| Glycopeptide | 15 (14.7%) | |
| Carbapenem | 14 (13.7%) | |
| Oxazolidinone | 3 (2.9%) | |
| Lipopeptide | 9 (8.8%) | |
| Lincosamide | 3 (2.9%) | |
| Culprit Bacteria Family | ||
| Polymicrobial (n = 34) | Monomicrobial (n = 63) * | |
| Aeromonas | 1 (2.9%) | 1 (1.6%) |
| Enterobacter | 10 (29.4%) | 10 (15.9%) |
| Pseudomonas | 29 (85.3%) | 10 (15.9%) |
| Enterococcus | 21 (61.8%) | 11 (17.5%) |
| Staphylococcus | 23 (67.6%) | 12 (19.0%) |
| Salmonella | 0 (0%) | 2 (3.2%) |
| Cutibacterium | 5 (14.7%) | 2 (3.2%) |
| Proteus | 2 (5.9%) | 2 (3.2%) |
| Streptococcus | 9 (26.5%) | 6 (9.5%) |
| Corynebacterium | 1 (2.9%) | 3 (4.8%) |
| Others | 6 (17.6%) | 4 (6.3%) |
| One infectious organism | N/A | 63 (100%) |
| Two infectious organisms | 7 (20.6%) | N/A |
| Three infectious organisms | 15 (44.1%) | N/A |
| Four infectious organisms | 12 (35.3%) | N/A |
| Total (n = 102) | Open Fractures (n = 35) | Closed Fractures (n = 67) | |
|---|---|---|---|
| Time from injury to definitive fixation (days) | 10.49 (1–45) | 9.11 (1–43) | 11.25 (1–45) |
| Time from injury to soft tissue cover (hours) a | 49.5 (14–120) | 49.5 (14–120) | N/A |
| Time from injury to FRI diagnosis (days) | 83.1 (12–475) | 63.48 (12–145) | 93.35 (32–475) |
| Time from FRI diagnosis to bone infection team review (days) | 7.68 (0–25) | 6.97 (0–18) | 8.05 (0–25) |
| Acute infection (onset < 6 weeks) | 30 (29.4%) | 21 (60.0%) | 9 (13.4%) |
| Chronic infection (onset > 6 weeks) | 72 (70.6%) | 14 (40.0%) | 58 (86.6%) |
| Recurrent infection | 21 (20.6%) | 7 (20%) | 14 (20.9%) |
| Implant retained | |||
| Yes | 49 (48.0%) | 18 (51.4%) | 31 (46.3%) |
| No | 53 (52.0%) | 17 (48.6%) | 36 (53.7%) |
| Non-union requiring further surgery | 10 (9.8%) | 6 (17.1%) | 4 (6.0%) |
| Signs of systemic sepsis | 16 (15.7%) | 5 (14.3%) | 11 (16.4%) |
| Amputation | 3 (2.9%) | 1 (2.9%) | 2 (3.0%) |
| Elevated ESR b | 63 (61.8%) | 20 (57.1%) | 43 (64.2%) |
| Elevated CRP b | 87 (85.3%) | 30 (85.7%) | 57 (85.1%) |
| Elevated WBC b | 80 (78.4%) | 24 (68.6%) | 56 (83.6%) |
| n = 102 | FRI Treatment Failure | |||||
|---|---|---|---|---|---|---|
| Univariable | Multivariable | |||||
| Treatment Failure (n = 24) | Treatment Success (n = 78) | p-Value | Odds Ratio | 95% CI | p-Value | |
| Age at injury (years) | 48.88 | 51.58 | 0.521 | |||
| Male gender | 15 (54.2%) | 51 (64.1%) | 0.796 | |||
| BMI | 31.88 | 25.86 | 0.186 | |||
| BMI ≥ 30 | 12 (50.0%) | 20 (25.6%) | 0.025 | 2.522 | 0.259–4.816 | 0.006 |
| Smoker | 11 (45.8%) | 34 (43.6%) | 0.847 | |||
| Diabetes Mellitus | 9 (37.5%) | 22 (28.2%) | 0.408 | |||
| Open fracture | 8 (33.3%) | 27 (34.6%) | 0.908 | |||
| Gustilo Anderson | ||||||
| Type 2 | 0 (0%) | 6 (7.7%) | 0.080 | |||
| Type 3a | 5 (20.8%) | 8 (10.3%) | 0.091 | |||
| Type 3b | 5 (20.8%) | 6 (7.7%) | 0.031 | |||
| Type 3c | 3 (12.5) | 2 (2.6%) | 0.033 | 4.683 | 2.037–9.784 | 0.004 |
| Time to definitive fixation (days) | 7.73 | 11.29 | 0.505 | |||
| External fixation as primary management | 5 (20.8%) | 10 (12.8%) | 0.332 | |||
| Culture-negative | 1 (4.2%) | 4 (5.1%) | 0.849 | |||
| Polymicrobial infection | 14 (58.3%) | 20 (25.6%) | 0.011 | |||
| Implant retention a | 14 (77.8%) | 35 (50.7%) | 0.048 | 2.818 | 1.588–7.928 | 0.041 |
| Polytrauma (ISS ≥ 16) | 12 (50.0%) | 37 (47.4%) | 0.826 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, V.; Zhang, J.; Patel, R.; Zhou, A.K.; Thahir, A.; Krkovic, M. Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective. Diagnostics 2022, 12, 1289. https://doi.org/10.3390/diagnostics12051289
Lu V, Zhang J, Patel R, Zhou AK, Thahir A, Krkovic M. Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective. Diagnostics. 2022; 12(5):1289. https://doi.org/10.3390/diagnostics12051289
Chicago/Turabian StyleLu, Victor, James Zhang, Ravi Patel, Andrew Kailin Zhou, Azeem Thahir, and Matija Krkovic. 2022. "Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective" Diagnostics 12, no. 5: 1289. https://doi.org/10.3390/diagnostics12051289
APA StyleLu, V., Zhang, J., Patel, R., Zhou, A. K., Thahir, A., & Krkovic, M. (2022). Fracture Related Infections and Their Risk Factors for Treatment Failure—A Major Trauma Centre Perspective. Diagnostics, 12(5), 1289. https://doi.org/10.3390/diagnostics12051289






