Diagnostic Age, Age at Death and Stage Migration in Men Dying with or from Prostate Cancer in Denmark
Abstract
1. Introduction
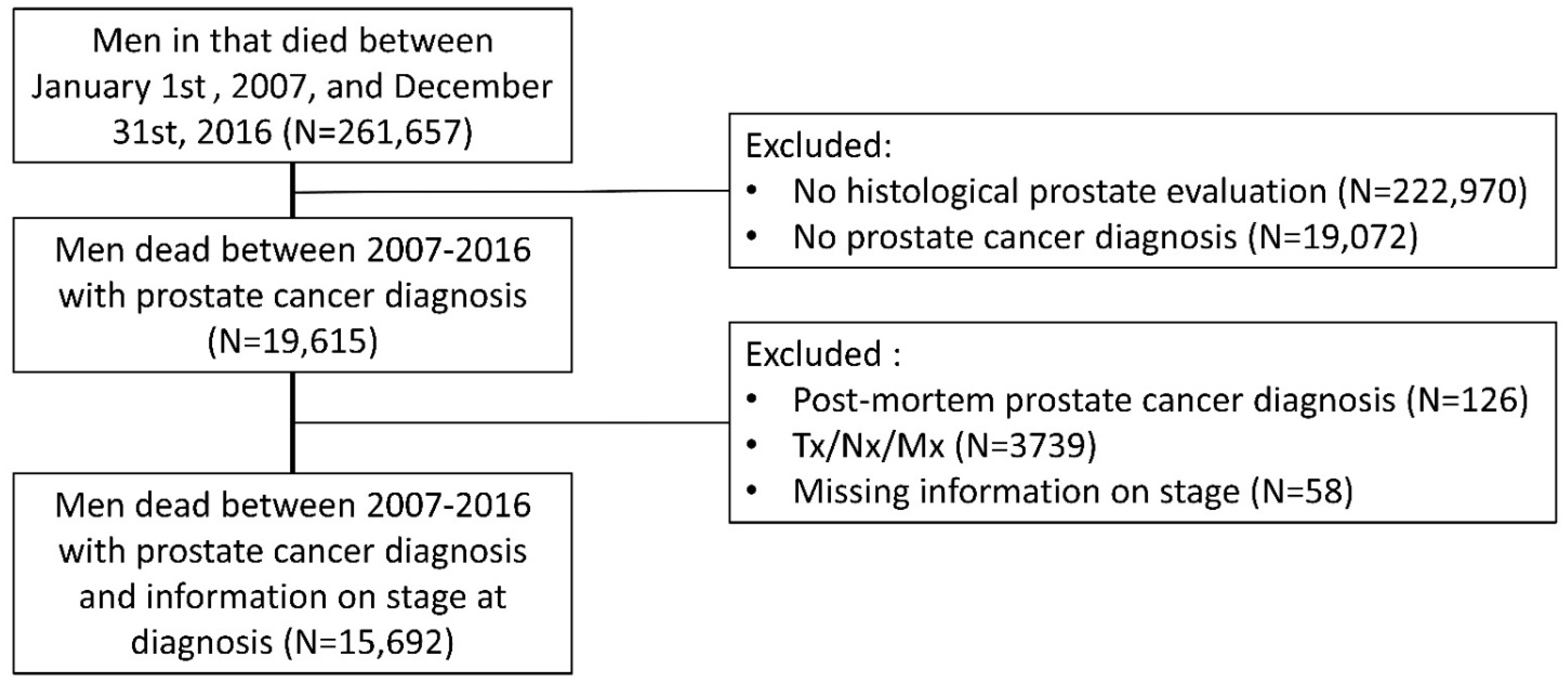
2. Materials and Methods
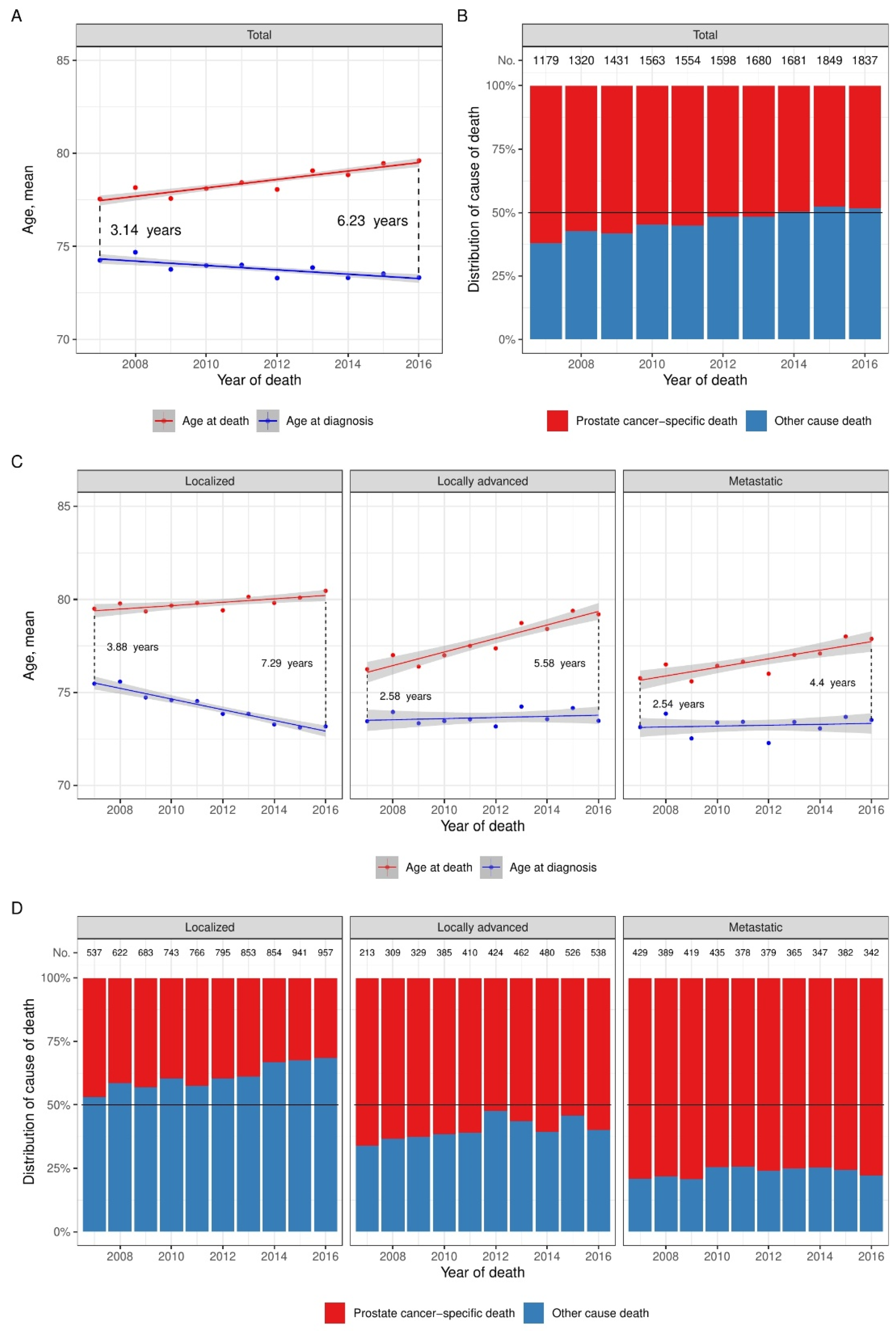
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Spångberg, A.; et al. Radical Prostatectomy or Watchful Waiting in Early Prostate Cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer(Figure presented.). Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.V.; Albertsen, P.C. Better Survival after Curative Treatment for Screen-detected Prostate Cancer Compared with Clinical Diagnosis: A Real Effect or Lead-time Bias? Eur. Urol. 2015, 68, 183–184. [Google Scholar] [CrossRef]
- Finne, P.; Fallah, M.; Hakama, M.; Ciatto, S.; Hugosson, J.; De Koning, H.; Moss, S.; Nelen, V.; Auvinen, A. Lead-time in the european randomised study of screening for prostate cancer. Eur. J. Cancer 2010, 46, 3102–3108. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef]
- Connor, M.J.; Winkler, M.; Ahmed, H.U. Survival in Oligometastatic Prostate Cancer-A New Dawn or the Will Rogers Phenomenon? JAMA Oncol. 2020, 6, 185–186. [Google Scholar] [CrossRef]
- Danckert, B.; Ferlay, J.; Engholm, G.; Hansen, H.; Johannesen, T.; Khan, S.; Køtlum, J.; Olafsdottir, E.; Schmidt, L.; Virtanen, A.; et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2 (26.03.2019). Association of the Nordic Cancer Registries. Danish Cancer Society. Available online: http://www.ancr.nu (accessed on 7 March 2022).
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Brasso, K.; Vainer, B.; Iversen, P. Diagnostic characteristics of lethal prostate cancer. Eur. J. Cancer 2017, 84, 18–26. [Google Scholar] [CrossRef]
- Patrikidou, A.; Loriot, Y.; Eymard, J.C.; Albiges, L.; Massard, C.; Ileana, E.; Di Palma, M.; Escudier, B.; Fizazi, K. Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014, 17, 348–352. [Google Scholar] [CrossRef]
- Helweg-Larsen, K. The Danish register of causes of death. Scand. J. Public Health 2011, 39, 26–29. [Google Scholar] [CrossRef]
- Helgstrand, J.T.; Klemann, N.; Røder, M.A.; Toft, B.G.; Brasso, K.; Vainer, B.; Iversen, P. Danish Prostate Cancer Registry—Methodology and early results from a novel national database. Clin. Epidemiol. 2016, 8, 351–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gjerstorff, M.L. The Danish cancer registry. Scand. J. Public Health 2011, 39, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B. The Danish civil registration system. Scand. J. Public Health 2011, 39, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Varenhorst, E.; Carlsson, P.; Capik, E.; Löfman, O.; Pedersen, K.V. Repeated screening for carcinoma of the prostate by digital rectal examination in a randomly selected population. Acta Oncol. 1992, 31, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Fair, W.R. Screening for carcinoma of the prostate: Efficacy of available screening tests. World J. Surg. 1989, 13, 65–70. [Google Scholar] [CrossRef]
- Pinsky, P.F.; Miller, E.; Prorok, P.; Grubb, R.; Crawford, E.D.; Andriole, G. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int. 2019, 123, 854. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Carlsson, S.; Tammela, T.; Määttänen, L.; Auvinen, A.; Kwiatkowski, M.; Recker, F.; Roobol, M.J. Screening for prostate cancer decreases the risk of developing metastatic disease: Findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur. Urol. 2012, 62, 745–752. [Google Scholar] [CrossRef]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef]
- Culp, M.B.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2019, 77, 38–52. [Google Scholar] [CrossRef]
- Karlsen, R.V.; Larsen, S.B.; Christensen, J.; Brasso, K.; Friis, S.; Tjonneland, A.; Dalton, S.O. PSA testing without clinical indication for prostate cancer in relation to socio-demographic and clinical characteristics in the Danish Diet, Cancer and Health Study. Acta Oncol. 2013, 52, 1609–1614. [Google Scholar] [CrossRef]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of prostatectomy versus observation for early prostate cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Lichtensztajn, D.Y.; Brooks, J.D.; Brasso, K.; Vainer, B.; Iversen, P. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer—A population-based analysis of 2 national cohorts. Cancer 2018, 124, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 chaarted trial. J. Clin. Oncol. 2018, 36, 1080. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.P.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Budäus, L.; Leyh-Bannurah, S.R.; Salomon, G.; Michl, U.; Heinzer, H.; Huland, H.; Graefen, M.; Steuber, T.; Rosenbaum, C. Initial Experience of 68Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur. Urol. 2016, 69, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Soldato, D.; Rubagotti, A.; Zinoli, L.; Zanardi, E.; Barboro, P.; Messina, C.; Castro, E.; Olmos, D.; Boccardo, F. Epidemiological characteristics and survival in patients with de novo metastatic prostate cancer. Cancers 2020, 12, 2855. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Roder, M.A.; Brasso, K.; Rusch, E.; Johansen, J.; Langkilde, N.C.; Hvarness, H.; Carlsson, S.; Jakobsen, H.; Borre, M.; Iversen, P. Length of life gained with surgical treatment of prostate cancer: A population-based analysis. Scand. J. Urol. 2015, 49, 275–281. [Google Scholar] [CrossRef]
- Statistics Denmark. Danmarks Statistik Middellevetiden er Steget; Statistics Denmark: Copenhagen, Denmark, 2021. [Google Scholar]
- Bratt, O.; Folkvaljon, Y.; Eriksson, M.H.; Akre, O.; Carlsson, S.; Drevin, L.; Lissbrant, I.F.; Makarov, D.; Loeb, S.; Stattin, P. Undertreatment of men in their seventies with high-risk nonmetastatic prostate cancer. Eur. Urol. 2015, 68, 53–58. [Google Scholar] [CrossRef]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, Å.; Køtlum, J.E.; Ólafsdttir, E.; Pukkala, E.; Storm, H.H. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.P.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Nielsen, M.; Møller, H.; Tjønneland, A.; Borre, M. Causes of death in men with prostate cancer: Results from the Danish Prostate Cancer Registry (DAPROCAdata). Cancer Epidemiol. 2019, 59, 249–257. [Google Scholar] [CrossRef] [PubMed]


| Variable | Men with Prostate Cancer-Specific Death (n = 8325) | Men with Other Cause Death (n = 7367) |
|---|---|---|
| PSA at diagnosis (ng/mL) 1 | 54 (18–193) | 18 (8.8–45) |
| Missing (number of men) | 3513 | 3153 |
| Diagnostic stage 2 | ||
| Localized | 2958 (35.5%) | 4793 (65.1%) |
| Locally advanced | 2411 (29.0%) | 1665 (22.6%) |
| Metastatic | 2956 (35.5%) | 909 (12.3%) |
| Gleason score 2 | ||
| 6 or below | 966 (11.6%) | 2231 (30.3%) |
| 7 | 2235 (26.8%) | 2359 (32.0%) |
| 8 or above | 4850 (58.3%) | 2503 (34.0%) |
| Other * | 274 (3.3%) | 274 (3.7%) |
| Radical prostatectomy 2 | 213 (2.6%) | 591 (8.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, M.C.M.; Stroomberg, H.V.; Brasso, K.; Helgstrand, J.T.; Røder, A. Diagnostic Age, Age at Death and Stage Migration in Men Dying with or from Prostate Cancer in Denmark. Diagnostics 2022, 12, 1271. https://doi.org/10.3390/diagnostics12051271
Andersen MCM, Stroomberg HV, Brasso K, Helgstrand JT, Røder A. Diagnostic Age, Age at Death and Stage Migration in Men Dying with or from Prostate Cancer in Denmark. Diagnostics. 2022; 12(5):1271. https://doi.org/10.3390/diagnostics12051271
Chicago/Turabian StyleAndersen, Marc Casper Meineche, Hein Vincent Stroomberg, Klaus Brasso, John Thomas Helgstrand, and Andreas Røder. 2022. "Diagnostic Age, Age at Death and Stage Migration in Men Dying with or from Prostate Cancer in Denmark" Diagnostics 12, no. 5: 1271. https://doi.org/10.3390/diagnostics12051271
APA StyleAndersen, M. C. M., Stroomberg, H. V., Brasso, K., Helgstrand, J. T., & Røder, A. (2022). Diagnostic Age, Age at Death and Stage Migration in Men Dying with or from Prostate Cancer in Denmark. Diagnostics, 12(5), 1271. https://doi.org/10.3390/diagnostics12051271






