Computed Tomographic Epidurography in Patients with Low Back Pain and Leg Pain: A Single-Center Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. Statistical Analysis
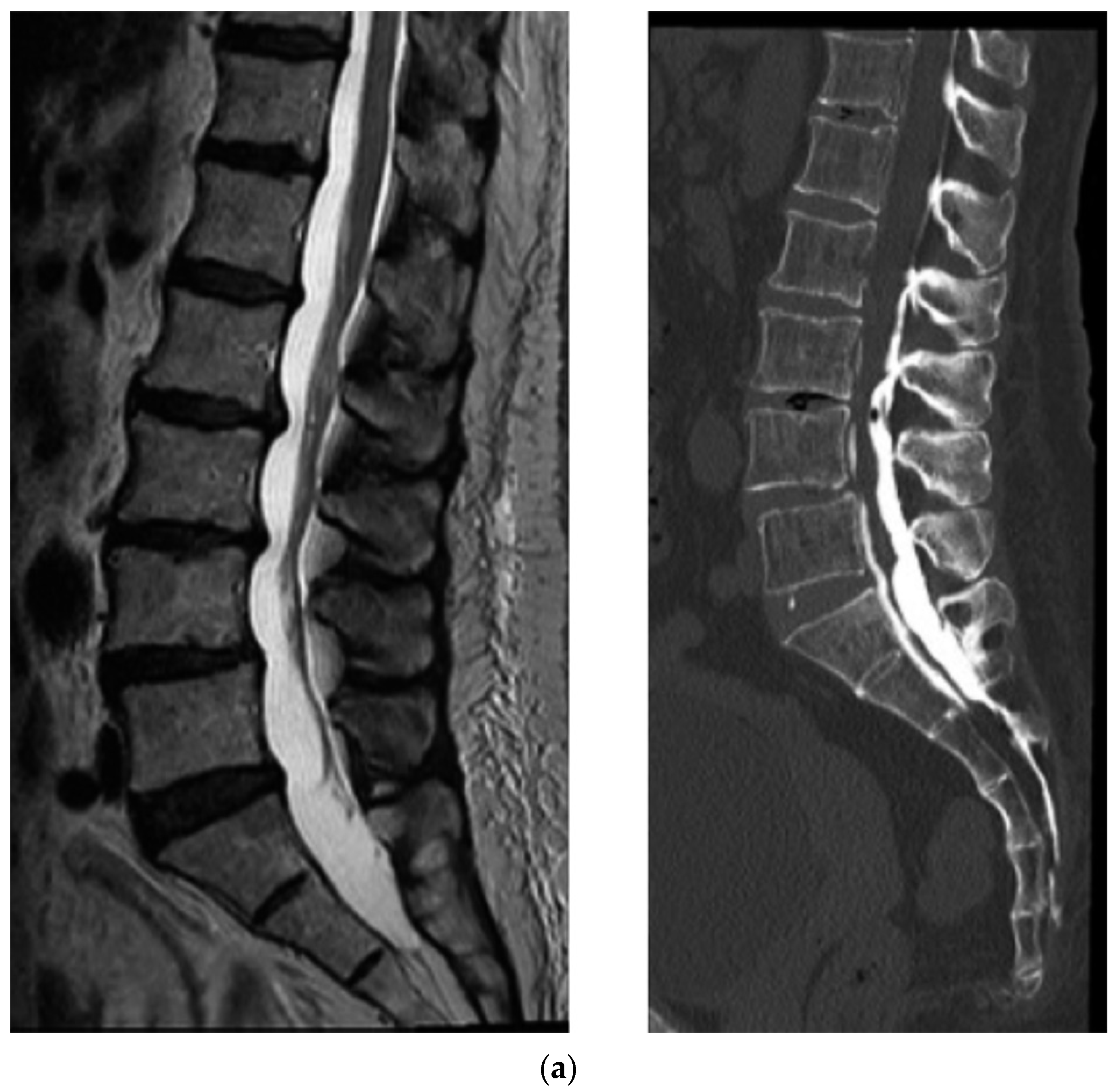
3. Results
4. Discussion
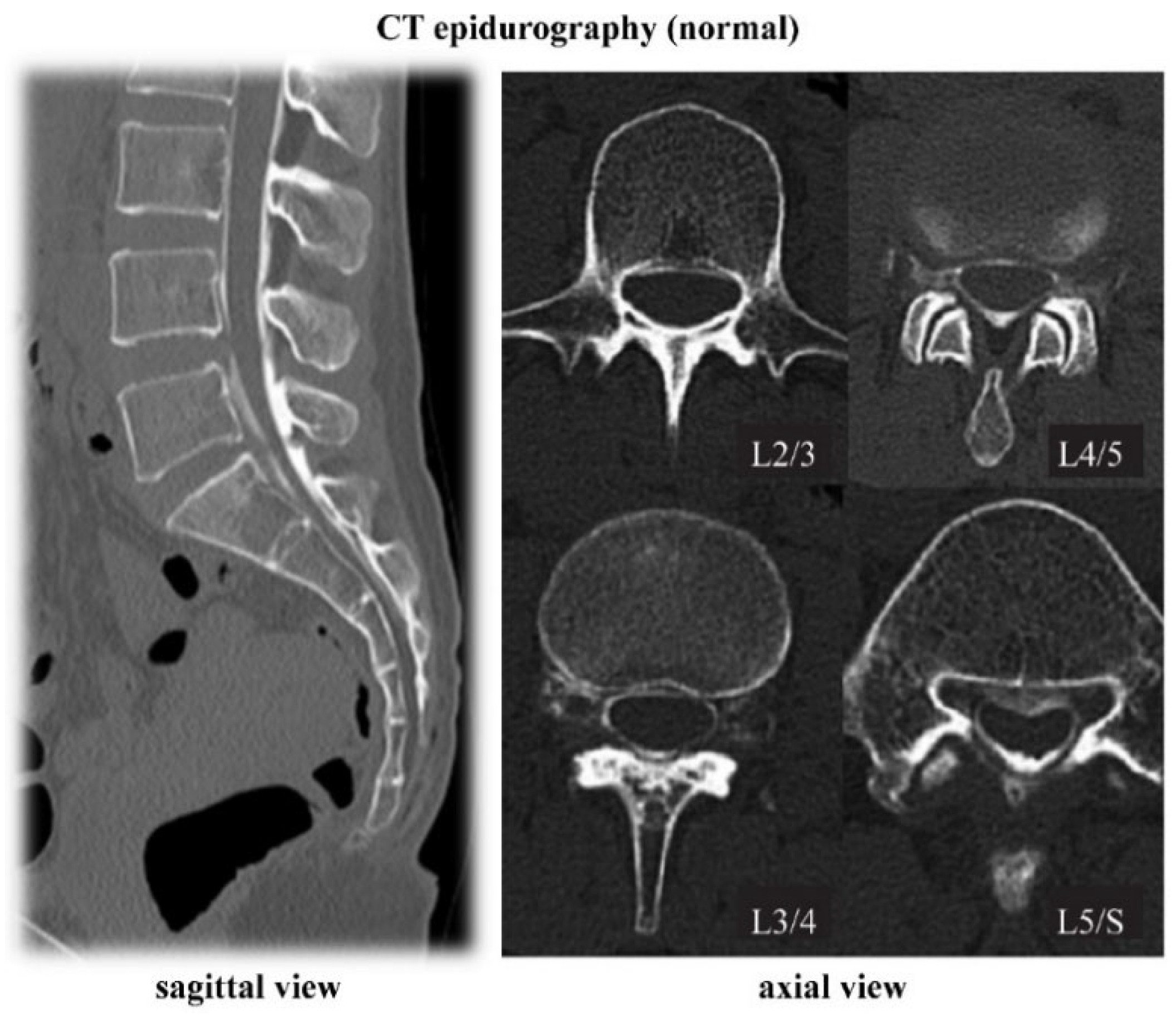
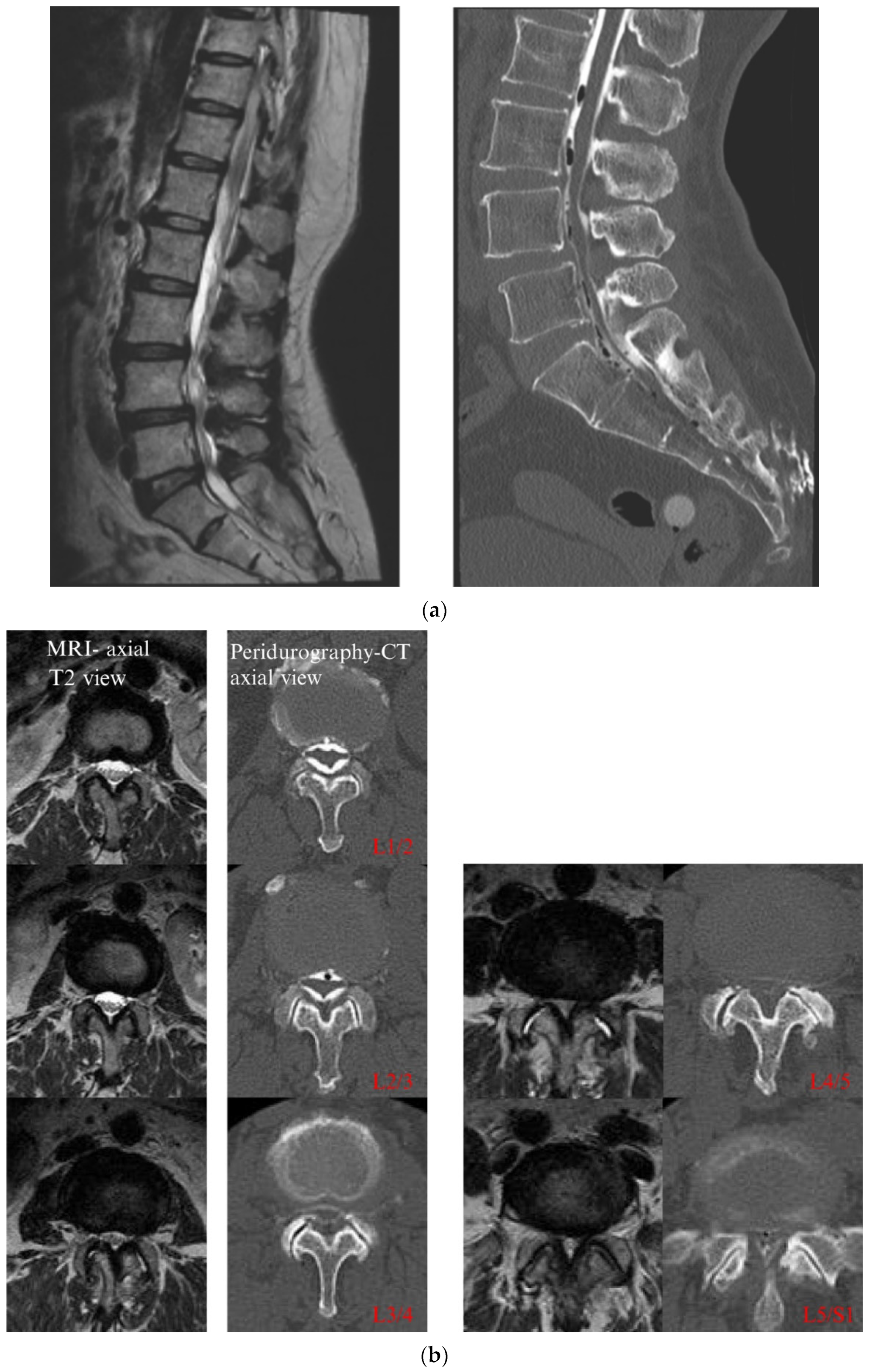
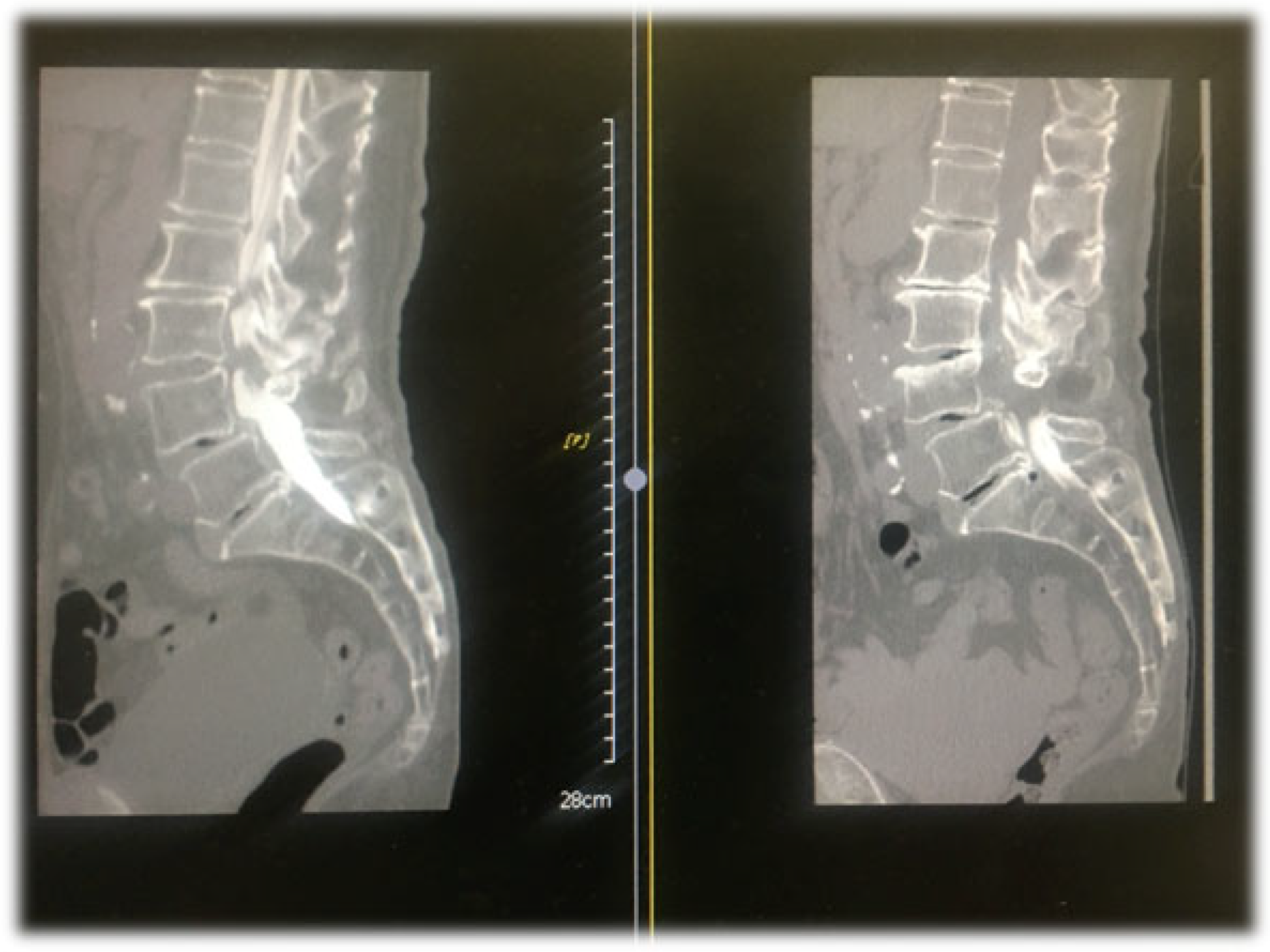
4.1. CT Epidurography
4.2. Presence of Epidural Connective Tissue
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Gallone, M.F.; Mastromauro, L.; Rifino, F.; Pesce, V.; Covelli, I.; Moretti, B. Extracorporeal shockwave therapy versus exercise program in patients with low back pain: Short-term results of a randomised controlled trial. J. Biol. Regul. Homeost. Agents 2018, 32, 385–389. [Google Scholar] [PubMed]
- Andrasinova, T.; Adamova, B.; Buskova, J.; Kerkovsky, M.; Jarkovsky, J.; Bednarik, J. Is there a Correlation between Degree of Radiologic Lumbar Spinal Stenosis and its Clinical Manifestation? Clin. Spine Surg. A Spine Publ. 2018, 31, E403–E408. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Barbieri, M. A comparison of non-endoscopic and endoscopic adhesiolysis of epidural fibrosis. Anaesthesiol. Intensiv. Ther. 2016, 48, 266–271. [Google Scholar] [CrossRef]
- Manchikanti, L.; Bakhit, C.E. Percutaneous lysis of epidural adhesions. Pain Phys. 2000, 3, 46–64. [Google Scholar] [CrossRef]
- Racz, G.B.; Heavner, J.E.; Trescot, A. Percutaneous Lysis of Epidural Adhesions—Evidence for Safety and Efficacy. Pain Pract. 2008, 8, 277–286. [Google Scholar] [CrossRef]
- Helm, S., 2nd; Racz, G.B.; Gerdesmeyer, L.; Justiz, R.; Hayek, S.M.; Kaplan, E.D.; El Terany, M.A.; Knezevic, N.N. Percutaneous and Endoscopic Adhesiolysis in Managing Low Back and Lower Extremity Pain: A Systematic Review and Meta-analysis. Pain Phys. 2016, 19, E245–E282. [Google Scholar] [CrossRef]
- Akbas, M. Percutaneous and Endoscopic Adhesiolysis. Agriculture 2020, 33, 129–141. [Google Scholar] [CrossRef]
- Avellanal, M. Epiduroscopia. Rev. Esp. Anestesiol. Reanim. 2011, 58, 426–433. [Google Scholar] [CrossRef]
- Colosimo, C.; Gaudino, S.; Alexandre, A.M. Imaging in degenerative spine pathology. Acta Neurochir. Suppl. 2011, 108, 9–15. [Google Scholar] [CrossRef]
- Park, S.-H.; Ji, G.Y.; Cho, P.G.; Shin, D.A.; Yoon, Y.S.; Kim, K.N.; Oh, C.H. Clinical Significance of Epidurography Contrast Patterns after Adhesiolysis during Lumbar Percutaneous Epidural Neuroplasty. Pain Res. Manag. 2018, 2018, 6268045. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.Y.; Lee, S.Y.; Min, S.K.; Kim, J.E.; Lee, H.S.; Jeong, H.W.; Park, B.; Choung, J.; Choi, J.B. The effect of additional transforaminal epidural blocks on percutaneous epidural neuroplasty with a wire-type catheterr: A retrospective observational study. Medicine 2019, 98, e18233. [Google Scholar] [CrossRef] [PubMed]
- Seeling, W.; Tomczak, R.; Merk, J.; Mrakovčić, N. CT-epidurography. A comparison of conventional and CT-epidurography with contrast medium injection through a thoracic peridural catheter. Anaesthesist 1995, 44, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Schizas, C.; Theumann, N.; Burn, A.; Tansey, R.; Wardlaw, D.; Smith, F.W.; Kulik, G. Qualitative Grading of Severity of Lumbar Spinal Stenosis Based on the Morphology of the Dural Sac on Magnetic Resonance Images. Spine 2010, 35, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Solaroglu, I.; Okutan, O.; Beskonakli, E. The ATA and Its Surgical Importancee: A newly described ligament lying between the dural sac and the ligamentum flavum at the L5 level. Spine 2011, 36, 1268–1272. [Google Scholar] [CrossRef]
- Miyauchi, A.; Sumida, T.; Kaneko, M.; Manabe, H.; Adachi, N. Morphology and clinical importance of epidural membrane and periradicular fibrous tissue in lumbar spinal stenosis. Eur. Spine J. 2016, 26, 382–388. [Google Scholar] [CrossRef]
- Yamamura, T. A study of the level and localization of disc herniation using epidurography. J. Jpn. Orthop. Assoc. 1967, 41, 991–1008. (In Japanese) [Google Scholar]
- Sasaguri, T.; Uemura, Y.; Hirakawa, N. Comparison between Lumbar MRI and Epiduroscopic Findings. J. Jpn. Soc. Clin. Anest. 2013, 33, 299–306. [Google Scholar] [CrossRef][Green Version]
- Savolaine, E.R.; Pandya, J.B.; Greenblatt, S.H.; Conover, S.R. Anatomy of the Human Lumbar Epidural Space. Anesthesiology 1988, 68, 217–220. [Google Scholar] [CrossRef]
- Stevens, D.S.; Balkany, A.D. Appearance of plica mediana dorsalis during epidurography. Pain Phys. 2006, 9, 268–270. [Google Scholar]
- Shi, B.; Li, X.; Li, H.; Ding, Z. The Morphology and Clinical Significance of the Dorsal Meningovertebra Ligaments in the Lumbosacral Epidural Space. Spine 2012, 37, E1093–E1098. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.J.; Nawaz, S.; Prasad, V.; Mahir, S.; Rattan, R.; Bernard, J.; Adds, P.J. The Posterior Epidural Ligaments: A Cadaveric and Histological Investigation in the Lumbar Region. ISRN Anat. 2013, 2013, 424058. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, R. The dorsomedian connective tissue band in the lumbar epidural space of humans: An anatomical study using epiduroscopy in autopsy cases. Anesth. Analg. 1986, 65, 747–752. [Google Scholar] [CrossRef] [PubMed]

| No. of Patients | 99 |
|---|---|
| Age (years) (range) | 72.7 |
| Sex (M/F) (% male) | 51/48 (51.5%) |
| Average height (cm) | 157.3 |
| Weight (kg) | 60.2 |
| Average BMI (range) | 24.05 |
| Percentage (%) of patients with obesity (BMI > 25%) | 29.70% |
| Diagnosis | n |
|---|---|
| Lumbar spinal canal stenosis (including degenerative spondylolisthesis) | 80 |
| Lumbar disc herniation | 4 |
| Facet joint cyst | 1 |
| Highly degenerate scoliosis | 1 |
| Cause unknown | 13 |
| Total | 99 |
| L1/2 (n) | L2/3 (n) | L3/4 (n) | L4/5 (n) | L5/S1 (n) | Total (n) | |
|---|---|---|---|---|---|---|
| A1 | 48 | 35 | 19 | 26 | 56 | 184 |
| A2 | 3 | 3 | 6 | 5 | 6 | 23 |
| A3 | 5 | 9 | 7 | 5 | 2 | 28 |
| A4 | 4 | 10 | 11 | 7 | 5 | 37 |
| Total | 60 | 57 | 43 | 43 | 69 | 272 |
| B | 4 | 8 | 17 | 13 | 5 | 47 |
| C | 6 | 5 | 13 | 22 | 6 | 52 |
| D | 0 | 0 | 0 | 1 | 1 | 2 |
| Total (n) | 70 | 70 | 73 | 79 | 81 | 373 |
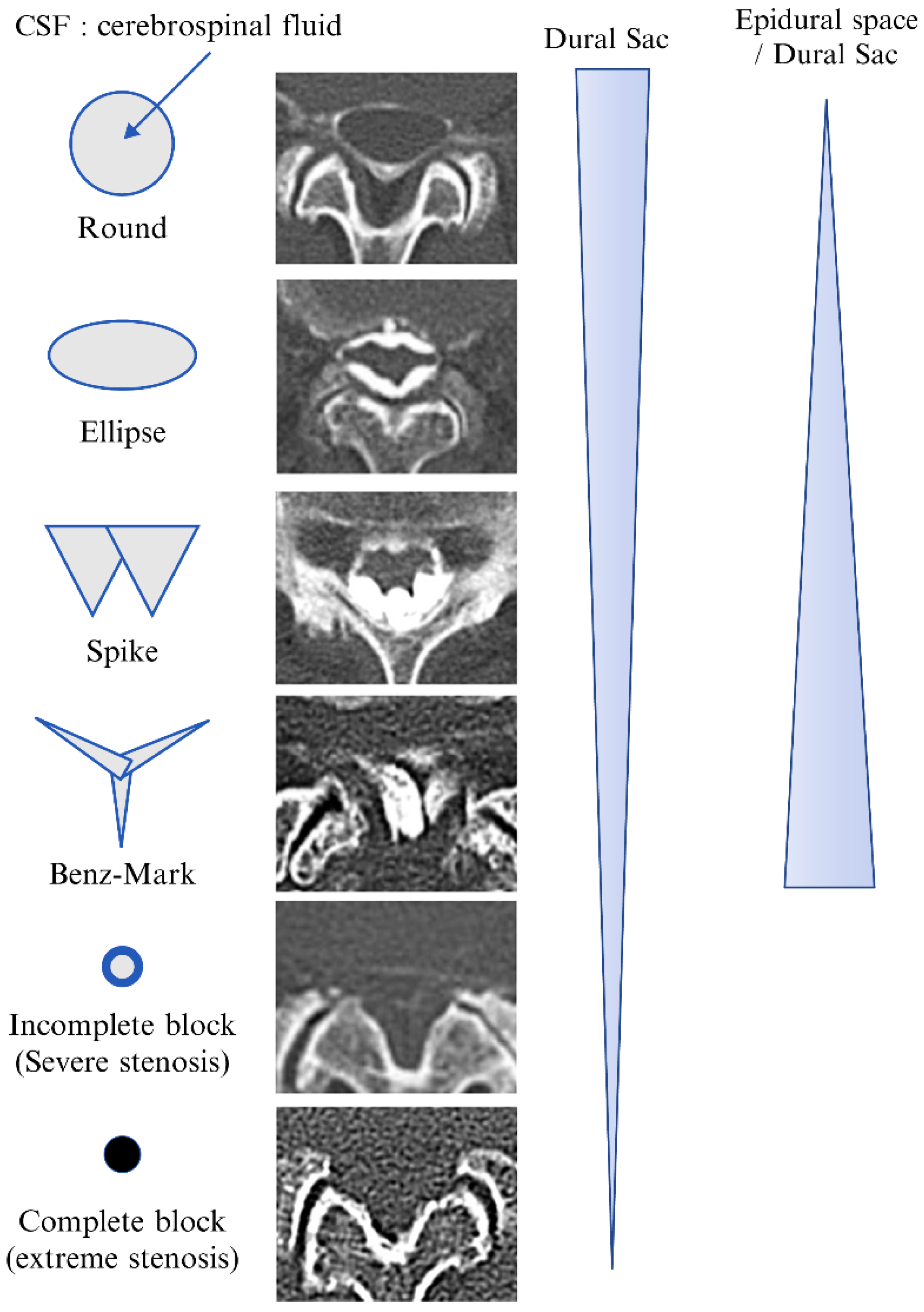
| L1/2 (n) | L2/3 (n) | L3/4 (n) | L4/5 (n) | L5/S1 (n) | Total (n) | |
|---|---|---|---|---|---|---|
| Round | 40 | 26 | 13 | 4 | 5 | 88 |
| Ellipse | 16 | 23 | 25 | 14 | 10 | 88 |
| Spike | 10 | 15 | 20 | 19 | 19 | 83 |
| Benz mark | 0 | 0 | 0 | 22 | 33 | 55 |
| Incomplete block | 4 | 6 | 15 | 18 | 14 | 57 |
| Complete block | 0 | 0 | 0 | 2 | 0 | 2 |
| Total | 70 | 70 | 73 | 79 | 81 | 373 |
| Non-contrast | 29 | 29 | 26 | 20 | 18 | 122 |
| CT Epidurography | Schizas Classification | ||
|---|---|---|---|
| Round and ellipse | 176 | A | 272 |
| Spike and Benz mark | 138 | B | 47 |
| Incomplete block | 57 | C | 52 |
| Complete block | 2 | D | 2 |
| Total | 373 | Total | 373 |
| Non-contrast | 122 | Non-contrast | 122 |
| Type | A (n) | B (n) | Total (n) |
|---|---|---|---|
| Round and ellipse | 171 | 5 | 176 |
| Spike and Benz mark | 101 | 37 | 138 |
| Incomplete block | 0 | 5 | 5 |
| Complete block | 0 | 0 | 0 |
| Total | 272 | 47 | 319 |
| Expected value | |||
| Type | A | B | |
| Round and ellipse | 150.1 | 25.9 | |
| Spike and Benz mark | 64.1 | 21.1 | |
| Incomplete block | 4.26 | 0.74 | |
| Complete block | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokosuka, K.; Sato, K.; Yamada, K.; Yoshida, T.; Shimazaki, T.; Morito, S.; Nishida, K.; Matsuo, A.; Fudo, T.; Shiba, N. Computed Tomographic Epidurography in Patients with Low Back Pain and Leg Pain: A Single-Center Observational Study. Diagnostics 2022, 12, 1267. https://doi.org/10.3390/diagnostics12051267
Yokosuka K, Sato K, Yamada K, Yoshida T, Shimazaki T, Morito S, Nishida K, Matsuo A, Fudo T, Shiba N. Computed Tomographic Epidurography in Patients with Low Back Pain and Leg Pain: A Single-Center Observational Study. Diagnostics. 2022; 12(5):1267. https://doi.org/10.3390/diagnostics12051267
Chicago/Turabian StyleYokosuka, Kimiaki, Kimiaki Sato, Kei Yamada, Tatsuhiro Yoshida, Takahiro Shimazaki, Shinji Morito, Kouta Nishida, Atsushi Matsuo, Takuma Fudo, and Naoto Shiba. 2022. "Computed Tomographic Epidurography in Patients with Low Back Pain and Leg Pain: A Single-Center Observational Study" Diagnostics 12, no. 5: 1267. https://doi.org/10.3390/diagnostics12051267
APA StyleYokosuka, K., Sato, K., Yamada, K., Yoshida, T., Shimazaki, T., Morito, S., Nishida, K., Matsuo, A., Fudo, T., & Shiba, N. (2022). Computed Tomographic Epidurography in Patients with Low Back Pain and Leg Pain: A Single-Center Observational Study. Diagnostics, 12(5), 1267. https://doi.org/10.3390/diagnostics12051267






