Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data and Literature Sources
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analyses
3. Results
3.1. Identification of Studies
3.2. Study Characteristics
| Country | Design | Age (Years) | Number of Patients (Proportion of Female Patients) | Study Length | Study Population | |
|---|---|---|---|---|---|---|
| Sasaki et al., 2022 [34] | Japan | Prospective observation study | 71.5 | 40 (85.0%) | 3 months | Improved group with CS Remained group with CS |
| Kim et al., 2021 [29] | Korea | Retrospective study | CS: 69.4 Non-CS:70 | CS: 102 (86.3%) Non-CS: 320 (89.4%) | 24 months | CS Non-CS |
| Lape et al., 2020 [33] | Korea | Prospective observation study | 66.1 (8.5) | 176 (63.6%) | 12 months | Widespread pain groups (Painful body regions 0 vs. 1–2 vs. ≥3) |
| Koh et al., 2020 [30] | Korea | Retrospective study | 70 (57–83) | Total 222 (91%) CS: 55 (91%) Non-CS:167 (90%) | 24 months | CS Non-CS |
| Dave et al., 2017 [31] | USA | Prospective observation study | Pain site 0: 66.5 Pain sites 1–2: 65.6 Pain sites ≥ 3: 67.2 | Pain site 0: 53 (64.1%) Pain sites 1–2: 121 (55.4%) Pain sites ≥ 3: 67 (67.2%) | 12 months | Widespread pain groups (Painful body regions 0 vs. 1–2 vs. ≥3) Subgroup analysis compared the group with ≥3 painful body regions and the group with 0 painful body regions. |
| Waldy et al., 2015 [36] | England | Additional study using RCT data | 239 (52.3%) | 12 months | Patients who underwent TKA to measure widespread pain sensitivity through QST | |
| Kim et al., 2015 [32] | Korea | Prospective observation study | CS: 69.2 Non-CS: 71.1 | 94 (100%) | 3 months | CS Non-CS |
| Waldy et al., 2013 [35] | England | Prospective cohort (exploratory study) | 68 | 51 (56.9%) | 13 months | Knee OA patients with QST Healthy people without knee pain Comparison of lower QST group and higher QST group in patients with knee OA pain by subgroup analysis |
3.3. Diagnosis of CS
3.4. Clinical Manifestations Based on CS following TKA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lau, R.L.; Gandhi, R.; Mahomed, S.; Mahomed, N. Patient satisfaction after total knee and hip arthroplasty. Clin. Geriatr. Med. 2012, 28, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, D.H.; Lee, Y.S. Is there an optimal age for total knee arthroplasty?: A systematic review. Knee Surg. Relat. Res. 2020, 32, 60. [Google Scholar] [CrossRef] [PubMed]
- Patrick, N.J.; Man, L.L.C.; Wai-Wang, C.; Tim-Yun, O.M.; Wing, C.K.; Hing, C.K.; Yin, C.K.; Ki-Wai, H.K. No difference in long-term functional outcomes or survivorship after total knee arthroplasty with or without computer navigation: A 17-year survivorship analysis. Knee Surg. Relat. Res. 2021, 33, 30. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Kim, K.I.; Bae, D.K.; Park, C.H. Mid-term lifetime survivals of octogenarians following primary and revision total knee arthroplasties were satisfactory: A retrospective single center study in contemporary period. Knee Surg. Relat. Res. 2020, 32, 50. [Google Scholar] [CrossRef]
- Takamura, D.; Iwata, K.; Sueyoshi, T.; Yasuda, T.; Moriyama, H. Relationship between early physical activity after total knee arthroplasty and postoperative physical function: Are these related? Knee Surg. Relat. Res. 2021, 33, 35. [Google Scholar] [CrossRef]
- Gunaratne, R.; Pratt, D.N.; Banda, J.; Fick, D.P.; Khan, R.J.K.; Robertson, B.W. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J. Arthroplast. 2017, 32, 3854–3860. [Google Scholar] [CrossRef]
- Bonnin, M.P.; Basiglini, L.; Archbold, H.A. What are the factors of residual pain after uncomplicated TKA? Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1411–1417. [Google Scholar] [CrossRef]
- Baker, P.N.; van der Meulen, J.H.; Lewsey, J.; Gregg, P.J. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J. Bone Jt. Surg. Br. 2007, 89, 893–900. [Google Scholar] [CrossRef]
- Scott, C.E.; Howie, C.R.; MacDonald, D.; Biant, L.C. Predicting dissatisfaction following total knee replacement: A prospective study of 1217 patients. J. Bone Jt. Surg. Br. 2010, 92, 1253–1258. [Google Scholar] [CrossRef]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986, 3, S226. [Google Scholar]
- Halket, A.; Stratford, P.W.; Kennedy, D.M.; Woodhouse, L.J. Using hierarchical linear modeling to explore predictors of pain after total hip and knee arthroplasty as a consequence of osteoarthritis. J. Arthroplast. 2010, 25, 254–262. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Wylde, V.; Gooberman-Hill, R.; Sayers, A.; Brunton, L.; Beswick, A.D.; Dieppe, P.; Blom, A.W. Trajectories of Pain and Function after Primary Hip and Knee Arthroplasty: The ADAPT Cohort Study. PLoS ONE 2016, 11, e0149306. [Google Scholar] [CrossRef] [PubMed]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef] [PubMed]
- Cottino, U.; Rosso, F.; Pastrone, A.; Dettoni, F.; Rossi, R.; Bruzzone, M. Painful knee arthroplasty: Current practice. Curr. Rev. Musculoskelet Med. 2015, 8, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, S.; Seitlinger, G.; Djahani, O.; Pietsch, M. The painful knee after TKA: A diagnostic algorithm for failure analysis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1442–1452. [Google Scholar] [CrossRef]
- Lim, H.A.; Song, E.K.; Seon, J.K.; Park, K.S.; Shin, Y.J.; Yang, H.Y. Causes of Aseptic Persistent Pain after Total Knee Arthroplasty. Clin. Orthop. Surg. 2017, 9, 50–56. [Google Scholar] [CrossRef]
- Mandalia, V.; Eyres, K.; Schranz, P.; Toms, A.D. Evaluation of patients with a painful total knee replacement. J. Bone Jt. Surg. Br. 2008, 90, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Park, C.N.; White, P.B.; Meftah, M.; Ranawat, A.S.; Ranawat, C.S. Diagnostic Algorithm for Residual Pain After Total Knee Arthroplasty. Orthopedics 2016, 39, e246–e252. [Google Scholar] [CrossRef] [Green Version]
- Piscitelli, P.; Iolascon, G.; Innocenti, M.; Civinini, R.; Rubinacci, A.; Muratore, M.; D’Arienzo, M.; Leali, P.T.; Carossino, A.M.; Brandi, M.L. Painful prosthesis: Approaching the patient with persistent pain following total hip and knee arthroplasty. Clin. Cases Miner Bone Metab. 2013, 10, 97–110. [Google Scholar]
- Preston, S.; Petrera, M.; Kim, C.; Zywiel, M.G.; Gandhi, R. Towards an understanding of the painful total knee: What is the role of patient biology? Curr. Rev. Musculoskelet Med. 2016, 9, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Toms, A.D.; Mandalia, V.; Haigh, R.; Hopwood, B. The management of patients with painful total knee replacement. J. Bone Jt. Surg. Br. 2009, 91, 143–150. [Google Scholar] [CrossRef]
- van Bussel, C.M.; Stronks, D.L.; Huygen, F.J. Complex regional pain syndrome type I of the knee: A systematic literature review. Eur. J. Pain 2014, 18, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Leysen, L.; Adriaenssens, N.; Aguilar Ferrándiz, M.E.; Devoogdt, N.; Tassenoy, A.; Ickmans, K.; Goubert, D.; van Wilgen, C.P.; Wijma, A.J.; et al. Pain following cancer treatment: Guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016, 55, 659–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauw, D.J.; Hassett, A.L. The role of centralised pain in osteoarthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 79–84. [Google Scholar] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Nijs, J.; Leysen, L.; Vanlauwe, J.; Logghe, T.; Ickmans, K.; Polli, A.; Malfliet, A.; Coppieters, I.; Huysmans, E. Treatment of central sensitization in patients with chronic pain: Time for change? Expert Opin. Pharmacother. 2019, 20, 1961–1970. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Carbajal Mejía, J.B.; Wakabayashi, K.; Nakano, T.; Yatani, H. Marginal Bone Loss Around Dental Implants Inserted with Static Computer Assistance in Healed Sites: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 761–775. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Koh, I.J.; Choi, K.Y.; Seo, J.Y.; In, Y. Minimal Clinically Important Differences for Patient-Reported Outcomes After TKA Depend on Central Sensitization. J. Bone Jt. Surg. Am. 2021, 103, 1374–1382. [Google Scholar] [CrossRef]
- Koh, I.J.; Kang, B.M.; Kim, M.S.; Choi, K.Y.; Sohn, S.; In, Y. How Does Preoperative Central Sensitization Affect Quality of Life Following Total Knee Arthroplasty? J. Arthroplast. 2020, 35, 2044–2049. [Google Scholar] [CrossRef]
- Dave, A.J.; Selzer, F.; Losina, E.; Usiskin, I.; Collins, J.E.; Lee, Y.C.; Band, P.; Dalury, D.F.; Iorio, R.; Kindsfater, K.; et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthr. Cartil. 2017, 25, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Yoon, K.B.; Yoon, D.M.; Yoo, J.H.; Ahn, K.R. Influence of Centrally Mediated Symptoms on Postoperative Pain in Osteoarthritis Patients Undergoing Total Knee Arthroplasty: A Prospective Observational Evaluation. Pain Pract. 2015, 15, E46–E53. [Google Scholar] [CrossRef] [PubMed]
- Lape, E.C.; Selzer, F.; Collins, J.E.; Losina, E.; Katz, J.N. Stability of Measures of Pain Catastrophizing and Widespread Pain Following Total Knee Replacement. Arthritis Care Res. 2020, 72, 1096–1103. [Google Scholar] [CrossRef]
- Sasaki, E.; Kasai, T.; Araki, R.; Sasaki, T.; Wakai, Y.; Akaishi, K.; Chiba, D.; Kimura, Y.; Yamamoto, Y.; Tsuda, E.; et al. Central Sensitization and Postoperative Improvement of Quality of Life in Total Knee and Total Hip Arthroplasty: A Prospective Observational Study. Prog. Rehabil. Med. 2022, 7, 20220009. [Google Scholar] [CrossRef]
- Wylde, V.; Palmer, S.; Learmonth, I.D.; Dieppe, P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: An exploratory study. Osteoarthr. Cartil. 2013, 21, 1253–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylde, V.; Sayers, A.; Lenguerrand, E.; Gooberman-Hill, R.; Pyke, M.; Beswick, A.D.; Dieppe, P.; Blom, A.W. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: A cohort analysis. Pain 2015, 156, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Koh, I.J.; Sohn, S.; Kang, B.M.; Kwak, D.H.; In, Y. Central Sensitization Is a Risk Factor for Persistent Postoperative Pain and Dissatisfaction in Patients Undergoing Revision Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Kim, M.S.; Sohn, S.; Song, K.Y.; Choi, N.Y.; In, Y. Duloxetine Reduces Pain and Improves Quality of Recovery Following Total Knee Arthroplasty in Centrally Sensitized Patients: A Prospective, Randomized Controlled Study. J. Bone Jt. Surg. Am. 2019, 101, 64–73. [Google Scholar] [CrossRef]
- Petersen, K.K.; Arendt-Nielsen, L.; Simonsen, O.; Wilder-Smith, O.; Laursen, M.B. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015, 156, 55–61. [Google Scholar] [CrossRef]
- Wylde, V.; Sayers, A.; Odutola, A.; Gooberman-Hill, R.; Dieppe, P.; Blom, A.W. Central sensitization as a determinant of patients’ benefit from total hip and knee replacement. Eur. J. Pain 2017, 21, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Staud, R.; Robinson, M.E.; Price, D.D. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J. Pain 2007, 8, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lluch, E.; Torres, R.; Nijs, J.; Van Oosterwijck, J. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur. J. Pain 2014, 18, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in patients with painful knee osteoarthritis. Pain 2010, 149, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Suokas, A.K.; Walsh, D.A.; McWilliams, D.F.; Condon, L.; Moreton, B.; Wylde, V.; Arendt-Nielsen, L.; Zhang, W. Quantitative sensory testing in painful osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2012, 20, 1075–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [Green Version]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain 2013, 14, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Akinci, A.; Al Shaker, M.; Chang, M.H.; Cheung, C.W.; Danilov, A.; José Dueñas, H.; Kim, Y.C.; Guillen, R.; Tassanawipas, W.; Treuer, T.; et al. Predictive factors and clinical biomarkers for treatment in patients with chronic pain caused by osteoarthritis with a central sensitisation component. Int. J. Clin. Pract. 2016, 70, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.B.; Srinivasan, P.; Spaeth, R.; Tan, Y.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.; et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 2013, 65, 3293–3303. [Google Scholar] [CrossRef]
- Napadow, V.; Kim, J.; Clauw, D.J.; Harris, R.E. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012, 64, 2398–2403. [Google Scholar] [CrossRef] [Green Version]
- Wager, T.D.; Atlas, L.Y.; Lindquist, M.A.; Roy, M.; Woo, C.W.; Kross, E. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013, 368, 1388–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundblad, H.; Kreicbergs, A.; Jansson, K.A. Prediction of persistent pain after total knee replacement for osteoarthritis. J. Bone Jt. Surg. Br. 2008, 90, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.S.; Koh, I.J.; Choi, K.Y.; Ju, G.I.; In, Y. Centrally sensitized patients undergoing total knee arthroplasty have higher expectations than do non-centrally sensitized patients. Knee Surg. Sports Traumatol. Arthrosc. 2021, 30, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.B.; Lorence, D.P.; Ding, J.; McPherson, K.; Black, N.A. The role of expectations in patients’ reports of post-operative outcomes and improvement following therapy. Med. Care 1993, 31, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.A.; Reid, M.C.; Duculan, R.; Girardi, F.P. Improvement in Pain After Lumbar Spine Surgery: The Role of Preoperative Expectations of Pain Relief. Clin. J. Pain 2017, 33, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Koh, I.J.; Sung, Y.G.; Park, D.C.; Yoon, E.J.; In, Y. Influence of increased pain sensitivity on patient-reported outcomes following total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2021, 30, 782–790. [Google Scholar] [CrossRef]
- Louw, A.; Diener, I.; Butler, D.S.; Puentedura, E.J. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch. Phys. Med. Rehabil. 2011, 92, 2041–2056. [Google Scholar] [CrossRef]
- Watson, J.A.; Ryan, C.G.; Cooper, L.; Ellington, D.; Whittle, R.; Lavender, M.; Dixon, J.; Atkinson, G.; Cooper, K.; Martin, D.J. Pain Neuroscience Education for Adults With Chronic Musculoskeletal Pain: A Mixed-Methods Systematic Review and Meta-Analysis. J. Pain 2019, 20, 1140.e1–1140.e22. [Google Scholar] [CrossRef]
- Itoh, N.; Tsuji, T.; Ishida, M.; Ochiai, T.; Konno, S.; Uchio, Y. Efficacy of duloxetine for multisite pain in patients with knee pain due to osteoarthritis: An exploratory post hoc analysis of a Japanese phase 3 randomized study. J. Orthop. Sci. 2021, 26, 141–148. [Google Scholar] [CrossRef]

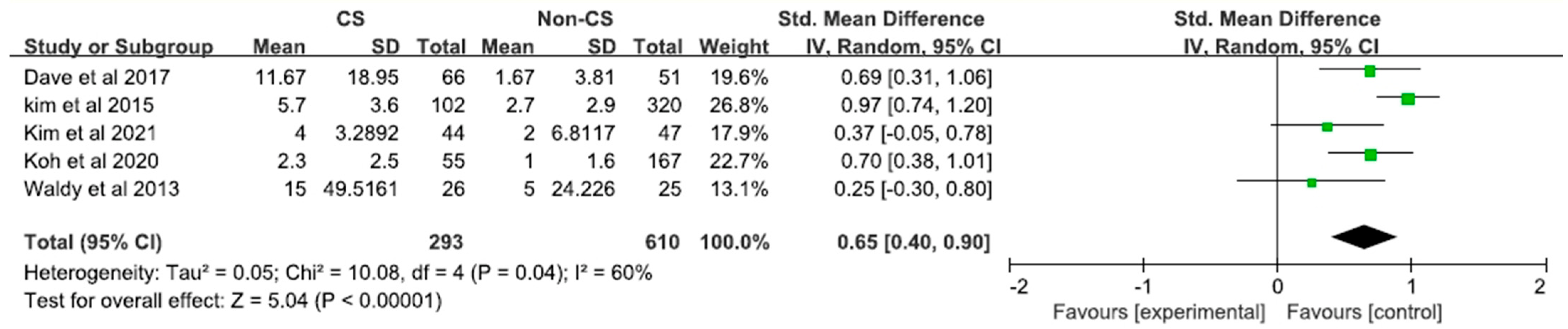
| Study | Proportion of CS at Baseline | Measure of CS | Postoperative Outcome Measure | Important Results and Comments |
|---|---|---|---|---|
| Sasaki et al., 2022 [34] | 19(47.5%) | CSI | KOOS EQ-5D | Preoperative CS was negatively associated with EQ-5D score after TKA (β = −0.44, p = 0.017) Patients who maintained CS before and after surgery had inferior KOOS/EQ-5D results compared to those who improved (all p < 0.05) |
| Kim et al., 2021 [29] | 102 (24.2%) | CSI | WOMAC | The CS group showed significantly inferior preoperative and postoperative WOMAC pain, function, and total scores compared to the non-CS group (all p < 0.05) Preoperative WOMAC total score: CS 61.0 vs. non-CS 57.1 (p < 0.05) Postoperative WOMAC total score: CS 25.8 vs. non-CS 17.4 (p < 0.05) Preoperative WOMAC total score: CS 13.6 vs. non-CS 11.9 (p < 0.05) Postoperative WOMAC total score: CS 5.7 vs. non-CS 2.7 (p < 0.05) |
| Lape et al., 2020 [33] | Whole-body pain diagram (19 sites labeled on the diagram) | WOMAC | There was no significant association between changes in the widespread pain groups and changes in WOMAC pain scores (p > 0.05). | |
| Koh et al., 2020 [30] | 55 (24.8%) | CSI | Pain VAS WOMAC KSS Satisfaction (new KSS) | The CS group showed worse quality of life, functional disability, and dissatisfaction than the non-CS group after TKA (all p < 0.05). Postoperative pain VAS score: CS 2.3 vs. non-CS 1.0 (p < 0.05) Postoperative WOMAC total score: CS 25.2 vs. non-CS 15.4 (p < 0.05) Postoperative KSS total score: CS 165.3 vs. non-CS 177.6 (p < 0.05) |
| Dave et al., 2017 [31] | Whole-body pain diagram (19 sites labeled on the diagram) | WOMAC MCID | Preoperative widespread pain was associated with greater pain at 12 months and failure to reach the MCID (All p < 0.05) Patients with preoperative pain in 3–6 body regions showed higher WOMAC scores at follow-up compared to patients with no painful body regions (median, 10 vs. 0) and were also less likely to achieve MCID (77% vs. 98%) (all p < 0.05) | |
| Waldy et al., 2015 [36] | QST (PPT) | WOMAC | There was no definite association between preoperative PPTs and pain severity at 12 months after TKA in any of the linear regression models (All p < 0.05) | |
| Kim et al., 2015 [32] | 44 (46.8%) | CSI | VAS Satisfaction (pain relief, functional improvement) | Postoperative pain VAS score: CS 4 vs. non-CS 2 (p < 0.05) CS patients reported poor satisfaction regarding pain relief compared to non-CS patients (p < 0.05) |
| Waldy et al., 2013 [35] | QST (PPT and HPT) | WOMAC | When patients were divided into low and high preoperative forearm PPT groups, patients in the low PPT group showed worse 1-year WOMAC pain scores compared to patients in the high PPT group (85 vs. 95, p < 0.05) |
| Quality Assessment of the Studies by the Newcastle–Ottawa Scale | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | ||||||||
| Study | Representative of the Cases | Selection of Control | Ascertainment of Exposure | Outcome of Interest Not Present at the Start of the Study | Comparability of Cohorts | Control for Any Additional Factor | Assessment of Outcomes | Sufficient Follow-Up | Adequacy of Follow-Up | Total 9/9 |
| Sasaki et al. [34] | * | 0 | * | * | 0 | 0 | * | * | 0 | 5 |
| Kim et al. [29]. | * | * | * | * | * | 0 | * | * | 0 | 7 |
| Lape et al. [33] | * | 0 | * | * | 0 | 0 | 0 | * | * | 5 |
| Koh et al. [30] | * | * | * | * | * | 0 | * | * | 0 | 7 |
| Dave et al. [31] | * | * | * | * | * | * | * | * | * | 9 |
| Waldy et al. [36] | * | 0 | * | * | 0 | 0 | * | * | 0 | 5 |
| Kim et al. [32] | * | * | * | * | * | * | * | * | * | 9 |
| Waldy et al. [35] | * | 0 | * | * | * | 0 | * | * | * | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Kim, J.J.; Kang, K.H.; Kim, M.J.; In, Y. Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1248. https://doi.org/10.3390/diagnostics12051248
Kim MS, Kim JJ, Kang KH, Kim MJ, In Y. Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(5):1248. https://doi.org/10.3390/diagnostics12051248
Chicago/Turabian StyleKim, Man Soo, Jae Jung Kim, Ki Ho Kang, Min Jun Kim, and Yong In. 2022. "Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 5: 1248. https://doi.org/10.3390/diagnostics12051248
APA StyleKim, M. S., Kim, J. J., Kang, K. H., Kim, M. J., & In, Y. (2022). Diagnosis of Central Sensitization and Its Effects on Postoperative Outcomes following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Diagnostics, 12(5), 1248. https://doi.org/10.3390/diagnostics12051248








