COUP-TFII in Kidneys, from Embryos to Sick Adults
Abstract
1. Introduction
2. COUP-TFs
2.1. Overview
2.2. COUP-TFI
2.3. COUP-TFII
3. COUP-TFII in Kidney Organogenesis
3.1. Kidney Development
3.2. Expression of COUP-TFII in the Developing Kidney
3.3. Role of COUP-TFII in Kidney Organogenesis
4. COUP-TFII in AKI
4.1. Expression of COUP-TFII in Adult Kidneys
4.2. AKI
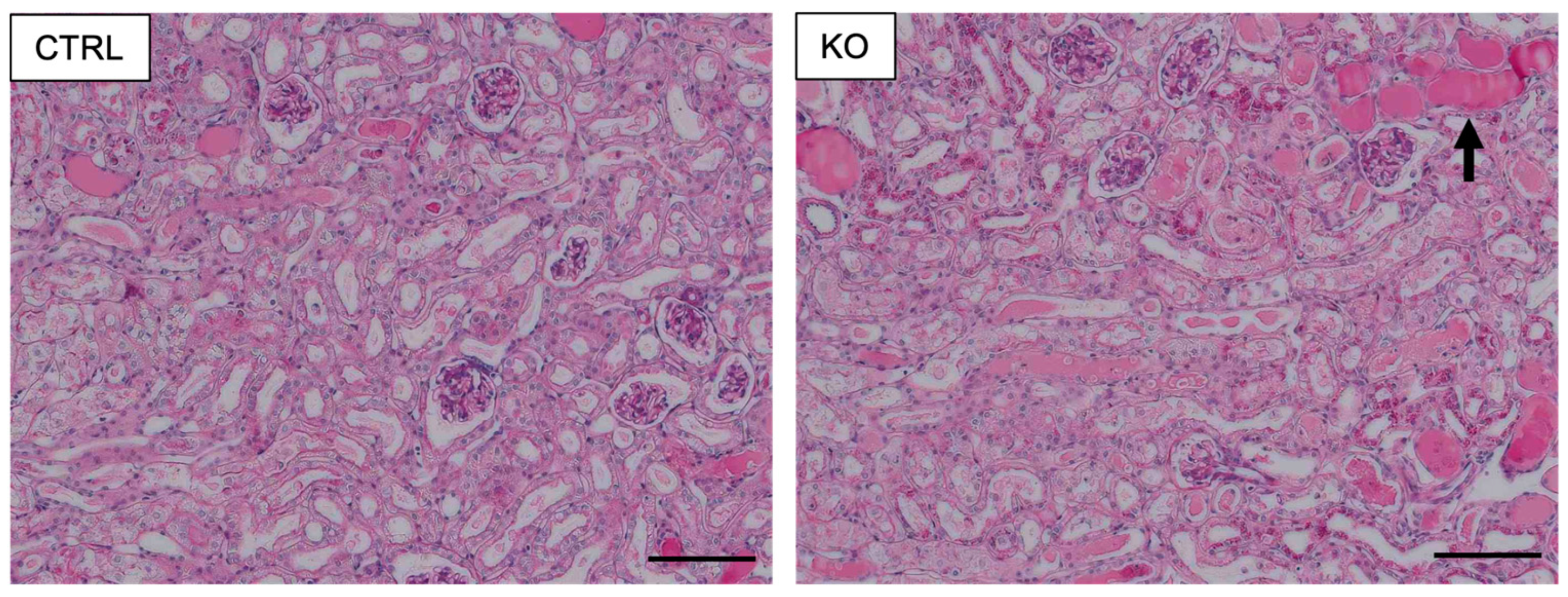
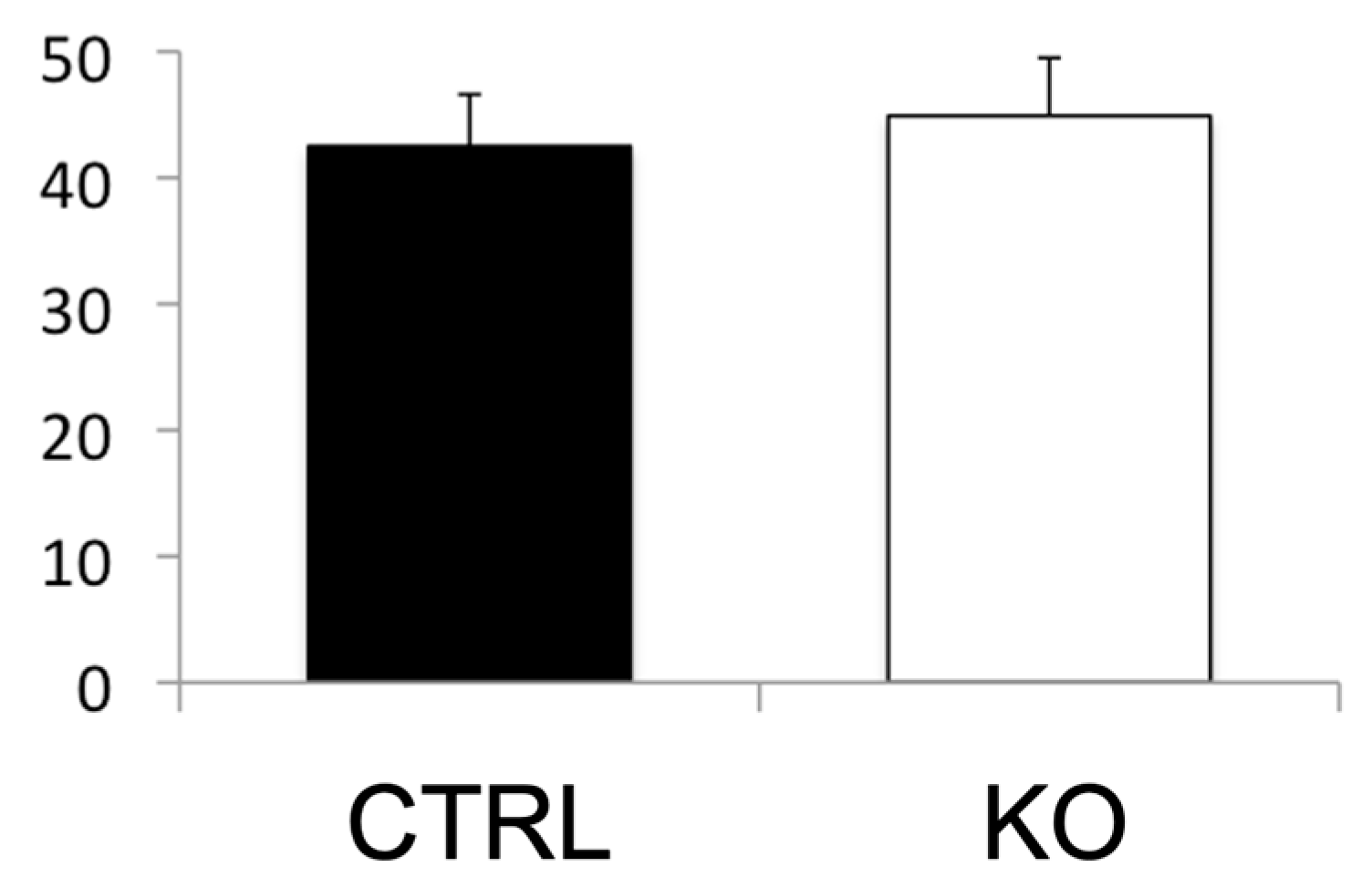
4.3. Role of COUP-TFII in Cisplatin-Induced AKI
5. Potential Roles of COUP-TFII in Kidney
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, E.D.; Carter-SU, C. Principles of Hormone Action. In Williams Textbook of Endocrinology, 14th ed.; Melmed, S., Auchus, R.J., Goldfine, A.B., Koenig, R.J., Rosen, C.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 13–41. [Google Scholar]
- Krust, A.; Green, S.; Argos, P.; Kumar, V.; Walter, P.; Bornert, J.M.; Chambon, P. The chicken oestrogen receptor sequence: Homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986, 5, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu. Rev. Physiol. 2010, 72, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef]
- Tora, L.; White, J.; Brou, C.; Tasset, D.; Webster, N.; Scheer, E.; Chambon, P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 1989, 59, 477–487. [Google Scholar] [CrossRef]
- Berg, J.M. DNA binding specificity of steroid receptors. Cell 1989, 57, 1065–1068. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Moras, D. Nuclear receptors. How to finger DNA. Nature 1995, 375, 190–191. [Google Scholar] [CrossRef]
- Bourguet, W.; Ruff, M.; Chambon, P.; Gronemeyer, H.; Moras, D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature 1995, 375, 377–382. [Google Scholar] [CrossRef]
- Renaud, J.P.; Rochel, N.; Ruff, M.; Vivat, V.; Chambon, P.; Gronemeyer, H.; Moras, D. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature 1995, 378, 681–689. [Google Scholar] [CrossRef]
- Moras, D.; Gronemeyer, H. The nuclear receptor ligand-binding domain: Structure and function. Curr. Opin. Cell Biol. 1998, 10, 384–391. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Perissi, V.; Jepsen, K.; Glass, C.K.; Rosenfeld, M.G. Deconstructing repression: Evolving models of co-repressor action. Nat. Rev. Genet. 2010, 11, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S. The Role of Histone Deacetylase 3 Complex in Nuclear Hormone Receptor Action. Int. J. Mol. Sci. 2021, 22, 9138. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Dispirito, J.R.; Lazar, M.A. The orphan nuclear receptors at their 25-year reunion. J. Mol. Endocrinol. 2013, 51, T115–T140. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Tsai, M.J. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): Coming of age. Endocr. Rev. 1997, 18, 229–240. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef]
- Wang, L.H.; Tsai, S.Y.; Cook, R.G.; Beattie, W.G.; Tsai, M.J.; O’Malley, B.W. COUP transcription factor is a member of the steroid receptor superfamily. Nature 1989, 340, 163–166. [Google Scholar] [CrossRef]
- Miyajima, N.; Kadowaki, Y.; Fukushige, S.; Shimizu, S.; Semba, K.; Yamanashi, Y.; Matsubara, K.; Toyoshima, K.; Yamamoto, T. Identification of two novel members of erbA superfamily by molecular cloning: The gene products of the two are highly related to each other. Nucl. Acids Res. 1988, 16, 11057–11074. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Sagami, I.; Wang, H.; Tsai, M.J.; O’Malley, B.W. Interactions between a DNA-binding transcription factor (COUP) and a non-DNA binding factor (S300-II). Cell 1987, 50, 701–709. [Google Scholar] [CrossRef]
- Wang, L.H.; Ing, N.H.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. The COUP-TFs compose a family of functionally related transcription factors. Gene Expr. 1991, 1, 207–216. [Google Scholar]
- Ladias, J.A.; Karathanasis, S.K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science 1991, 251, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Laudet, V. Evolution of the nuclear receptor superfamily: Early diversification from an ancestral orphan receptor. J. Mol. Endocrinol. 1997, 19, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr. Rev. 1994, 15, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Tsai, S.Y.; Sagami, I.; Tsai, M.J.; O’Malley, B.W. Purification and characterization of chicken ovalbumin upstream promoter transcription factor from HeLa cells. J. Biol. Chem. 1987, 262, 16080–16086. [Google Scholar] [CrossRef]
- Cooney, A.J.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol. Cell. Biol. 1992, 12, 4153–4163. [Google Scholar] [CrossRef]
- Sagami, I.; Tsai, S.Y.; Wang, H.; Tsai, M.J.; O’Malley, B.W. Identification of two factors required for transcription of the ovalbumin gene. Mol. Cell. Biol. 1986, 6, 4259–4267. [Google Scholar] [CrossRef]
- Shibata, H.; Nawaz, Z.; Tsai, S.Y.; O’Malley, B.W.; Tsai, M.J. Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT). Mol. Endocrinol. 1997, 11, 714–724. [Google Scholar] [CrossRef]
- Rohr, O.; Aunis, D.; Schaeffer, E. COUP-TF and Sp1 interact and cooperate in the transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat in human microglial cells. J. Biol. Chem. 1997, 272, 31149–31155. [Google Scholar] [CrossRef]
- Qiu, Y.; Cooney, A.J.; Kuratani, S.; DeMayo, F.J.; Tsai, S.Y.; Tsai, M.J. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: Evidence for a role in segmental patterning of the diencephalon. Proc. Natl. Acad. Sci. USA 1994, 91, 4451–4455. [Google Scholar] [CrossRef]
- Lopes da Silva, S.; Van Horssen, A.M.; Chang, C.; Burbach, J.P. Expression of nuclear hormone receptors in the rat supraoptic nucleus. Endocrinology 1995, 136, 2276–2283. [Google Scholar] [CrossRef]
- Qiu, Y.; Pereira, F.A.; DeMayo, F.J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997, 11, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qiu, Y.; Pereira, F.A.; Crair, M.C.; Tsai, S.Y.; Tsai, M.J. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron 1999, 24, 847–859. [Google Scholar] [CrossRef]
- Zhou, C.; Tsai, S.Y.; Tsai, M.J. COUP-TFI: An intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001, 15, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Armentano, M.; Filosa, A.; Andolfi, G.; Studer, M. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development 2006, 133, 4151–4162. [Google Scholar] [CrossRef] [PubMed]
- Bosch, D.G.; Boonstra, F.N.; Gonzaga-Jauregui, C.; Xu, M.; de Ligt, J.; Jhangiani, S.; Wiszniewski, W.; Muzny, D.M.; Yntema, H.G.; Pfundt, R.; et al. NR2F1 mutations cause optic atrophy with intellectual disability. Am. J. Hum. Genet. 2014, 94, 303–309. [Google Scholar] [CrossRef]
- Chen, C.A.; Bosch, D.G.; Cho, M.T.; Rosenfeld, J.A.; Shinawi, M.; Lewis, R.A.; Mann, J.; Jayakar, P.; Payne, K.; Walsh, L.; et al. The expanding clinical phenotype of Bosch-Boonstra-Schaaf optic atrophy syndrome: 20 new cases and possible genotype-phenotype correlations. Genet. Med. 2016, 18, 1143–1150. [Google Scholar] [CrossRef]
- Tang, K.; Xie, X.; Park, J.I.; Jamrich, M.; Tsai, S.; Tsai, M.J. COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 2010, 137, 725–734. [Google Scholar] [CrossRef]
- Lin, F.J.; Qin, J.; Tang, K.; Tsai, S.Y.; Tsai, M.J. Coup d’Etat: An orphan takes control. Endocr. Rev. 2011, 32, 404–421. [Google Scholar] [CrossRef]
- Pereira, F.A.; Qiu, Y.; Zhou, G.; Tsai, M.J.; Tsai, S.Y. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999, 13, 1037–1049. [Google Scholar] [CrossRef]
- You, L.R.; Lin, F.J.; Lee, C.T.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 2005, 435, 98–104. [Google Scholar] [CrossRef]
- Su, T.; Stanley, G.; Sinha, R.; D’Amato, G.; Das, S.; Rhee, S.; Chang, A.H.; Poduri, A.; Raftrey, B.; Dinh, T.T.; et al. Single-cell analysis of early progenitor cells that build coronary arteries. Nature 2018, 559, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.J.; Chen, X.; Qin, J.; Hong, Y.K.; Tsai, M.J.; Tsai, S.Y. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J. Clin. Investig. 2010, 120, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Tsai, M.J.; Tsai, S.Y. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE 2008, 3, e3285. [Google Scholar] [CrossRef] [PubMed]
- Petit, F.G.; Jamin, S.P.; Kurihara, I.; Behringer, R.R.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc. Natl. Acad. Sci. USA 2007, 104, 6293–6298. [Google Scholar] [CrossRef]
- Kurihara, I.; Lee, D.K.; Petit, F.G.; Jeong, J.; Lee, K.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007, 3, e102. [Google Scholar] [CrossRef]
- Lee, D.K.; Kurihara, I.; Jeong, J.W.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol. Endocrinol. 2010, 24, 930–940. [Google Scholar] [CrossRef]
- Zhao, F.; Franco, H.L.; Rodriguez, K.F.; Brown, P.R.; Tsai, M.J.; Tsai, S.Y.; Yao, H.H. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science 2017, 357, 717–720. [Google Scholar] [CrossRef]
- Li, L.; Xie, X.; Qin, J.; Jeha, G.S.; Saha, P.K.; Yan, J.; Haueter, C.M.; Chan, L.; Tsai, S.Y.; Tsai, M.J. The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab. 2009, 9, 77–87. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, S.; Hsu, C.H.; Eguchi, J.; Rosen, E.D. The orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II is a critical regulator of adipogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 2421–2426. [Google Scholar] [CrossRef]
- Okamura, M.; Kudo, H.; Wakabayashi, K.; Tanaka, T.; Nonaka, A.; Uchida, A.; Tsutsumi, S.; Sakakibara, I.; Naito, M.; Osborne, T.F.; et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 5819–5824. [Google Scholar] [CrossRef]
- Sabra-Makke, L.; Maritan, M.; Planchais, J.; Boutant, M.; Pégorier, J.P.; Even, P.C.; Vasseur-Cognet, M.; Bossard, P. Hypothalamic ventromedial COUP-TFII protects against hypoglycemia-associated autonomic failure. Proc. Natl. Acad. Sci. USA 2013, 110, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, S.; Honda, T.; Aramaki, M.; Hayashi, K.; Kubo, K.; Ishida, M.; Tanaka, D.H.; Kawauchi, T.; Sekine, K.; Kusuzawa, S.; et al. The COUP-TFII/Neuropilin-2 is a molecular switch steering diencephalon-derived GABAergic neurons in the developing mouse brain. Proc. Natl. Acad. Sci. USA 2015, 112, E4985–E4994. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Takamoto, N.; Yan, J.; Tsai, S.Y.; Tsai, M.J. Chicken Ovalbumin Upstream Promoter-Transcription Factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev. Biol. 2009, 326, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Tang, K.; Iida, A.; Inoue, M.; Kodama, T.; Tsai, S.Y.; Tsai, M.J.; Furuta, Y.; Watanabe, S. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J. Neurosci. 2009, 29, 12401–12411. [Google Scholar] [CrossRef]
- Takamoto, N.; You, L.R.; Moses, K.; Chiang, C.; Zimmer, W.E.; Schwartz, R.J.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 2005, 132, 2179–2189. [Google Scholar] [CrossRef]
- Lee, C.T.; Li, L.; Takamoto, N.; Martin, J.F.; Demayo, F.J.; Tsai, M.J.; Tsai, S.Y. The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol. Cell. Biol. 2004, 24, 10835–10843. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Kao, C.Y.; Lin, S.C.; Xu, M.; Xie, X.; Tsai, S.Y.; Tsai, M.J. Dysregulation of nuclear receptor COUP-TFII impairs skeletal muscle development. Sci. Rep. 2017, 7, 3136. [Google Scholar] [CrossRef]
- Al Turki, S.; Manickaraj, A.K.; Mercer, C.L.; Gerety, S.S.; Hitz, M.P.; Lindsay, S.; D’Alessandro, L.C.; Swaminathan, G.J.; Bentham, J.; Arndt, A.K.; et al. Rare variants in NR2F2 cause congenital heart defects in humans. Am. J. Hum. Genet. 2014, 94, 574–585. [Google Scholar] [CrossRef]
- Qiao, X.H.; Wang, Q.; Wang, J.; Liu, X.Y.; Xu, Y.J.; Huang, R.T.; Xue, S.; Li, Y.J.; Zhang, M.; Qu, X.K.; et al. A novel NR2F2 loss-of-function mutation predisposes to congenital heart defect. Eur. J. Med. Genet. 2018, 61, 197–203. [Google Scholar] [CrossRef]
- Upadia, J.; Gonzales, P.R.; Robin, N.H. Novel de novo pathogenic variant in the NR2F2 gene in a boy with congenital heart defect and dysmorphic features. Am. J. Med. Genet. A 2018, 176, 1423–1426. [Google Scholar] [CrossRef]
- High, F.A.; Bhayani, P.; Wilson, J.M.; Bult, C.J.; Donahoe, P.K.; Longoni, M. De novo frameshift mutation in COUP-TFII (NR2F2) in human congenital diaphragmatic hernia. Am. J. Med. Genet. A 2016, 170, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Klaassens, M.; Galjaard, R.J.; Scott, D.A.; Brüggenwirth, H.T.; van Opstal, D.; Fox, M.V.; Higgins, R.R.; Cohen-Overbeek, T.E.; Schoonderwaldt, E.M.; Lee, B.; et al. Prenatal detection and outcome of congenital diaphragmatic hernia (CDH) associated with deletion of chromosome 15q26: Two patients and review of the literature. Am. J. Med. Genet. A 2007, 143A, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, N.; Shanmugam, H.; Baird, L.; Stevens, J.; Byrne, J.L.; Barnhart, D.C.; Rau, C.; Feldkamp, M.L.; Yoder, B.A.; Leppert, M.F.; et al. Germline but not somatic de novo mutations are common in human congenital diaphragmatic hernia. Birth Defects Res. 2018, 110, 610–617. [Google Scholar] [CrossRef] [PubMed]
- You, L.R.; Takamoto, N.; Yu, C.T.; Tanaka, T.; Kodama, T.; Demayo, F.J.; Tsai, S.Y.; Tsai, M.J. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc. Natl. Acad. Sci. USA 2005, 102, 16351–16356. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Tsai, S.Y.; Tsai, M.-J. Role of COUP-TFII in Congenital Diaphragmatic Hernia. In Handbook of Cell Signaling, 2nd ed.; Bradshaw, R.A., Dennis, E.A., Eds.; Academic Press: Burlington, NJ, USA, 2009; Volume 3, pp. 2021–2026. [Google Scholar]
- Qin, J.; Wu, S.P.; Creighton, C.J.; Dai, F.; Xie, X.; Cheng, C.M.; Frolov, A.; Ayala, G.; Lin, X.; Feng, X.H.; et al. COUP-TFII inhibits TGF-β-induced growth barrier to promote prostate tumorigenesis. Nature 2013, 493, 236–240. [Google Scholar] [CrossRef]
- Xie, X.; Tsai, S.Y.; Tsai, M.J. COUP-TFII regulates satellite cell function and muscular dystrophy. J. Clin. Investig. 2016, 126, 3929–3941. [Google Scholar] [CrossRef]
- Lin, S.C.; Li, Y.H.; Wu, M.H.; Chang, Y.F.; Lee, D.K.; Tsai, S.Y.; Tsai, M.J.; Tsai, S.J. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E427–E437. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Ishii, S.; Yamada, M.; Koibuchi, N. Chicken ovalbumin upstream promoter-transcription factor II protects against cisplatin-induced acute kidney injury. Endocr. J. 2020, 67, 283–293. [Google Scholar] [CrossRef]
- Tham, M.S.; Smyth, I.M. Cellular and molecular determinants of normal and abnormal kidney development. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e338. [Google Scholar] [CrossRef]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. Urogenital system. In The Developing Human: Clinically Oriented Embryology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2020; pp. 223–262. [Google Scholar]
- Chan, K.; Li, X. Current Epigenetic Insights in Kidney Development. Genes 2021, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hohenstein, P.; Kuure, S. Embryonic Kidney Development, Stem Cells and the Origin of Wilms Tumor. Genes 2021, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Smyth, I.M.; Cullen-McEwen, L.A.; Caruana, G.; Black, M.J.; Bertram, J.F. Development of the Kidney: Morphology and Mechanisms. In Fetal and Neonatal Physiology, 5th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 953–964. [Google Scholar]
- Oxburgh, L. Kidney Nephron Determination. Annu. Rev. Cell Dev. Biol. 2018, 34, 427–450. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Yu, C.T.; Tang, K.; Tanaka, T.; Kodama, T.; Tsai, M.J.; Tsai, S.Y. The expression profiles of nuclear receptors in the developing and adult kidney. Mol. Endocrinol. 2006, 20, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.T.; Tang, K.; Suh, J.M.; Jiang, R.; Tsai, S.Y.; Tsai, M.J. COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival. Development 2012, 139, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.X.; Adams, J.; Peters, H.; Brown, M.C.; Heaney, S.; Maas, R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999, 23, 113–117. [Google Scholar] [CrossRef]
- Torres, M.; Gómez-Pardo, E.; Dressler, G.R.; Gruss, P. Pax-2 controls multiple steps of urogenital development. Development 1995, 121, 4057–4065. [Google Scholar] [CrossRef]
- Self, M.; Lagutin, O.V.; Bowling, B.; Hendrix, J.; Cai, Y.; Dressler, G.R.; Oliver, G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006, 25, 5214–5228. [Google Scholar] [CrossRef]
- Kreidberg, J.A.; Sariola, H.; Loring, J.M.; Maeda, M.; Pelletier, J.; Housman, D.; Jaenisch, R. WT-1 is required for early kidney development. Cell 1993, 74, 679–691. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Scholz, H.; Boivin, F.J.; Schmidt-Ott, K.M.; Bachmann, S.; Eckardt, K.U.; Scholl, U.I.; Persson, P.B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021, 17, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- KDIGO Work Group. Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Gaudry, S.; Palevsky, P.M.; Dreyfuss, D. Extracorporeal Kidney-Replacement Therapy for Acute Kidney Injury. N. Engl. J. Med. 2022, 386, 964–975. [Google Scholar] [CrossRef]
- Perazella, M.A. Drug-induced acute kidney injury: Diverse mechanisms of tubular injury. Curr. Opin. Crit. Care 2019, 25, 550–557. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef]
- Estrela, G.R.; Wasinski, F.; Felizardo, R.J.F.; Souza, L.L.; Câmara, N.O.S.; Bader, M.; Araujo, R.C. MATE-1 modulation by kinin B1 receptor enhances cisplatin efflux from renal cells. Mol. Cell Biochem. 2017, 428, 101–108. [Google Scholar] [CrossRef]
- Mapuskar, K.A.; Wen, H.; Holanda, D.G.; Rastogi, P.; Steinbach, E.; Han, R.; Coleman, M.C.; Attanasio, M.; Riley, D.P.; Spitz, D.R.; et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox. Biol. 2019, 20, 98–106. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef]
- Landau, S.I.; Guo, X.; Velazquez, H.; Torres, R.; Olson, E.; Garcia-Milian, R.; Moeckel, G.W.; Desir, G.V.; Safirstein, R. Regulated necrosis and failed repair in cisplatin-induced chronic kidney disease. Kidney Int. 2019, 95, 797–814. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 2003, 285, F610–F618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, P.; Li, N.; Wan, C.; Jiang, C.M.; He, J.S.; Wang, D.J.; Zhang, M.; Sun, L.Y. Effects of continuous renal replacement therapy on serum cytokines, neutrophil gelatinase-associated lipocalin, and prognosis in patients with severe acute kidney injury after cardiac surgery. Oncotarget 2017, 8, 10628–10636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Fontnouvelle, C.A.; Greenberg, J.H.; Thiessen-Philbrook, H.R.; Zappitelli, M.; Roth, J.; Kerr, K.F.; Devarajan, P.; Shlipak, M.; Coca, S.; Parikh, C.R.; et al. Interleukin-8 and Tumor Necrosis Factor Predict Acute Kidney Injury After Pediatric Cardiac Surgery. Ann. Thorac. Surg. 2017, 104, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Moeckel, G.; Kashgarian, K.; Racusen, L. Ischemic and Toxic Acute Tubular Injury and Other Ischemic Renal Injuries. In Heptinstall’s Renal Pathology, 7th ed.; Jannette, J., D′Agati, V., Olson, J., Silva, F., Eds.; LWW: Philadelphia, PA, USA, 2014; pp. 1167–1222. [Google Scholar]
- Yan, X.; Qu, X.; Liu, B.; Zhao, Y.; Xu, L.; Yu, S.; Wang, J.; Wang, L.; Su, J. Autophagy-Induced HDAC6 Activity During Hypoxia Regulates Mitochondrial Energy Metabolism Through the β-Catenin/COUP-TFII Axis in Hepatocellular Carcinoma Cells. Front. Oncol. 2021, 11, 742460. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, K.A. Histone Deacetylases in Kidney Physiology and Acute Kidney Injury. Semin. Nephrol. 2020, 40, 138–147. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Shi, Y.; Ma, X.; Zhuang, S.; Liu, N. The Role and Mechanism of Histone Deacetylases in Acute Kidney Injury. Front. Pharmacol. 2021, 12, 695237. [Google Scholar] [CrossRef]
- Libby, A.E.; Jones, B.; Lopez-Santiago, I.; Rowland, E.; Levi, M. Nuclear receptors in the kidney during health and disease. Mol. Asp. Med. 2021, 78, 100935. [Google Scholar] [CrossRef]
- Garg, P. A Review of Podocyte Biology. Am. J. Nephrol. 2018, 47 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- Grahammer, F. New structural insights into podocyte biology. Cell Tissue Res. 2017, 369, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, H.; Fukusumi, Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin. Exp. Nephrol. 2020, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Benzing, T.; Salant, D. Insights into Glomerular Filtration and Albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Eremina, V.; Sood, M.; Haigh, J.; Nagy, A.; Lajoie, G.; Ferrara, N.; Gerber, H.P.; Kikkawa, Y.; Miner, J.H.; Quaggin, S.E. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 2003, 111, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Quaggin, S.E.; Kreidberg, J.A. Development of the renal glomerulus: Good neighbors and good fences. Development 2008, 135, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wassenhove-McCarthy, D.J.; Yamaguchi, Y.; Holzman, L.B.; van Kuppevelt, T.H.; Jenniskens, G.J.; Wijnhoven, T.J.; Woods, A.C.; McCarthy, K.J. Loss of heparan sulfate glycosaminoglycan assembly in podocytes does not lead to proteinuria. Kidney Int. 2008, 74, 289–299. [Google Scholar] [CrossRef]
- Agrawal, S.; He, J.C.; Tharaux, P.L. Nuclear receptors in podocyte biology and glomerular disease. Nat. Rev. Nephrol. 2021, 17, 185–204. [Google Scholar] [CrossRef]
- Liu, M.; Qiao, Z.; Zhang, Y.; Zhan, P.; Yi, F. Histone Deacetylases Take Center Stage on Regulation of Podocyte Function. Kidney Dis. 2020, 6, 236–246. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Renal Physiol. 2003, 284, F1138–F1144. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Roeser, M.; Lachmann, P.; Ishii, S.; Suh, J.M.; Harlander, S.; Desch, M.; Brunssen, C.; Morawietz, H.; Tsai, S.Y.; et al. Chicken ovalbumin upstream promoter transcription factor II regulates renin gene expression. J. Biol. Chem. 2012, 287, 24483–24491. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Arthur, G.; Osborn, J.L.; Yiannikouris, F.B. (Pro)renin receptor in the kidney: Function and significance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R377–R383. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.F.; Tofteng, S.S.; Madsen, K.; Jensen, B.L. Role of the renin-angiotensin system in kidney development and programming of adult blood pressure. Clin. Sci. 2020, 134, 641–656. [Google Scholar] [CrossRef]
- Kruse, S.W.; Suino-Powell, K.; Zhou, X.E.; Kretschman, J.E.; Reynolds, R.; Vonrhein, C.; Xu, Y.; Wang, L.; Tsai, S.Y.; Tsai, M.J.; et al. Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol. 2008, 6, e227. [Google Scholar] [CrossRef]
- Le Guével, R.; Oger, F.; Martinez-Jimenez, C.P.; Bizot, M.; Gheeraert, C.; Firmin, F.; Ploton, M.; Kretova, M.; Palierne, G.; Staels, B.; et al. Inactivation of the Nuclear Orphan Receptor COUP-TFII by Small Chemicals. ACS Chem. Biol. 2017, 12, 654–663. [Google Scholar] [CrossRef]
- Eftekhari, A.; Maleki Dizaj, S.; Ahmadian, E.; Przekora, A.; Hosseiniyan Khatibi, S.M.; Ardalan, M.; Zununi Vahed, S.; Valiyeva, M.; Mehraliyeva, S.; Khalilov, R.; et al. Application of Advanced Nanomaterials for Kidney Failure Treatment and Regeneration. Materials 2021, 14, 2939. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Eftekhari, A.; Mammadova, S.; Ahmadian, E.; Ardalan, M.; Davaran, S.; Nasibova, A.; Khalilov, R.; Valiyeva, M.; Mehraliyeva, S.; et al. Nanomaterials for Chronic Kidney Disease Detection. Appl. Sci. 2021, 11, 9656. [Google Scholar] [CrossRef]
- Pundir, C.S.; Kumar, P.; Jaiwal, R. Biosensing methods for determination of creatinine: A review. Biosens. Bioelectron. 2019, 126, 707–724. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, S.; Koibuchi, N. COUP-TFII in Kidneys, from Embryos to Sick Adults. Diagnostics 2022, 12, 1181. https://doi.org/10.3390/diagnostics12051181
Ishii S, Koibuchi N. COUP-TFII in Kidneys, from Embryos to Sick Adults. Diagnostics. 2022; 12(5):1181. https://doi.org/10.3390/diagnostics12051181
Chicago/Turabian StyleIshii, Sumiyasu, and Noriyuki Koibuchi. 2022. "COUP-TFII in Kidneys, from Embryos to Sick Adults" Diagnostics 12, no. 5: 1181. https://doi.org/10.3390/diagnostics12051181
APA StyleIshii, S., & Koibuchi, N. (2022). COUP-TFII in Kidneys, from Embryos to Sick Adults. Diagnostics, 12(5), 1181. https://doi.org/10.3390/diagnostics12051181






