Machine Learning Models for the Prediction of Renal Failure in Chronic Kidney Disease: A Retrospective Cohort Study
Abstract
1. Introduction
2. Material and Methods
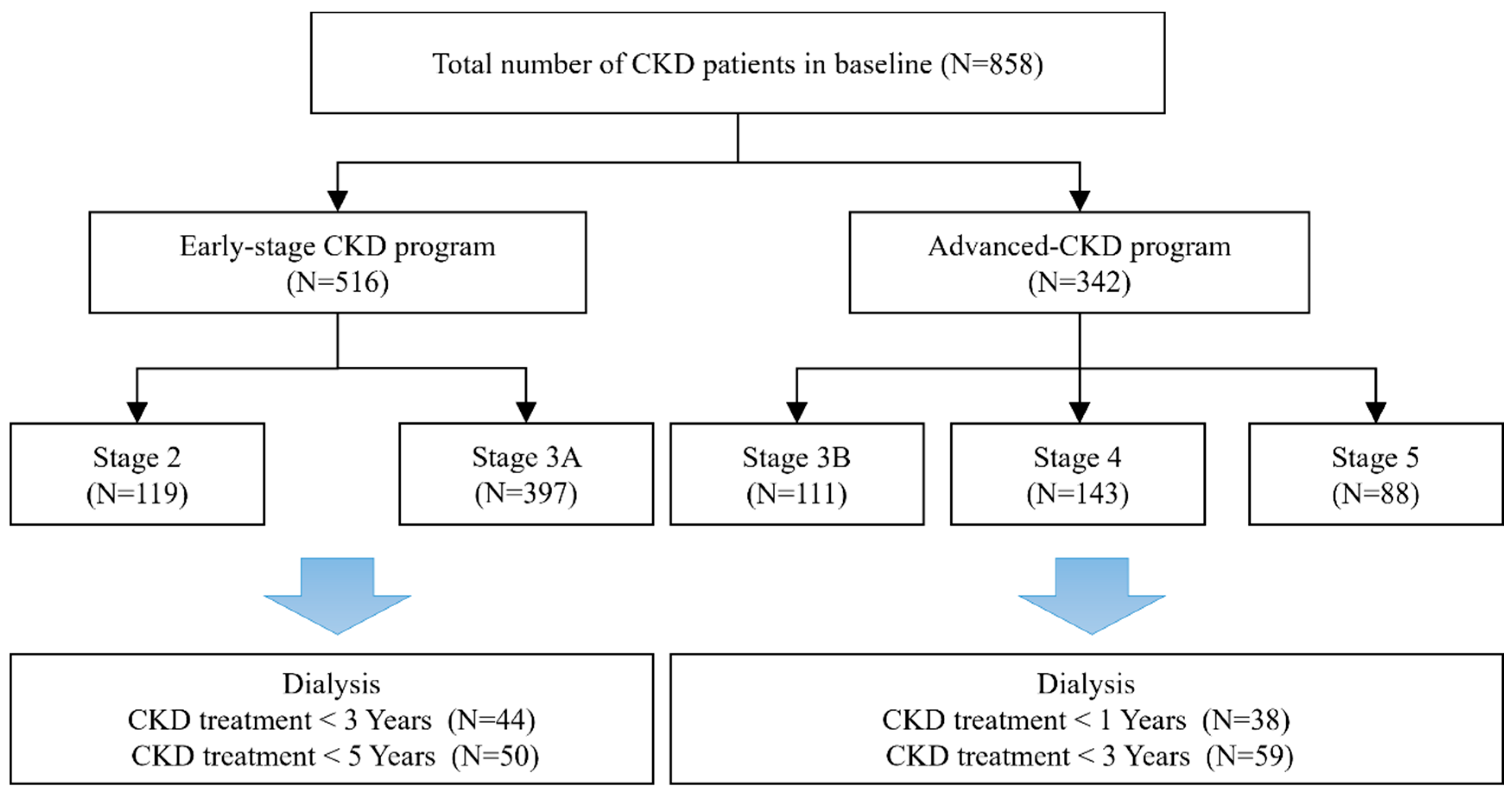
2.1. Patient Population
2.2. Study Design
2.3. Variables
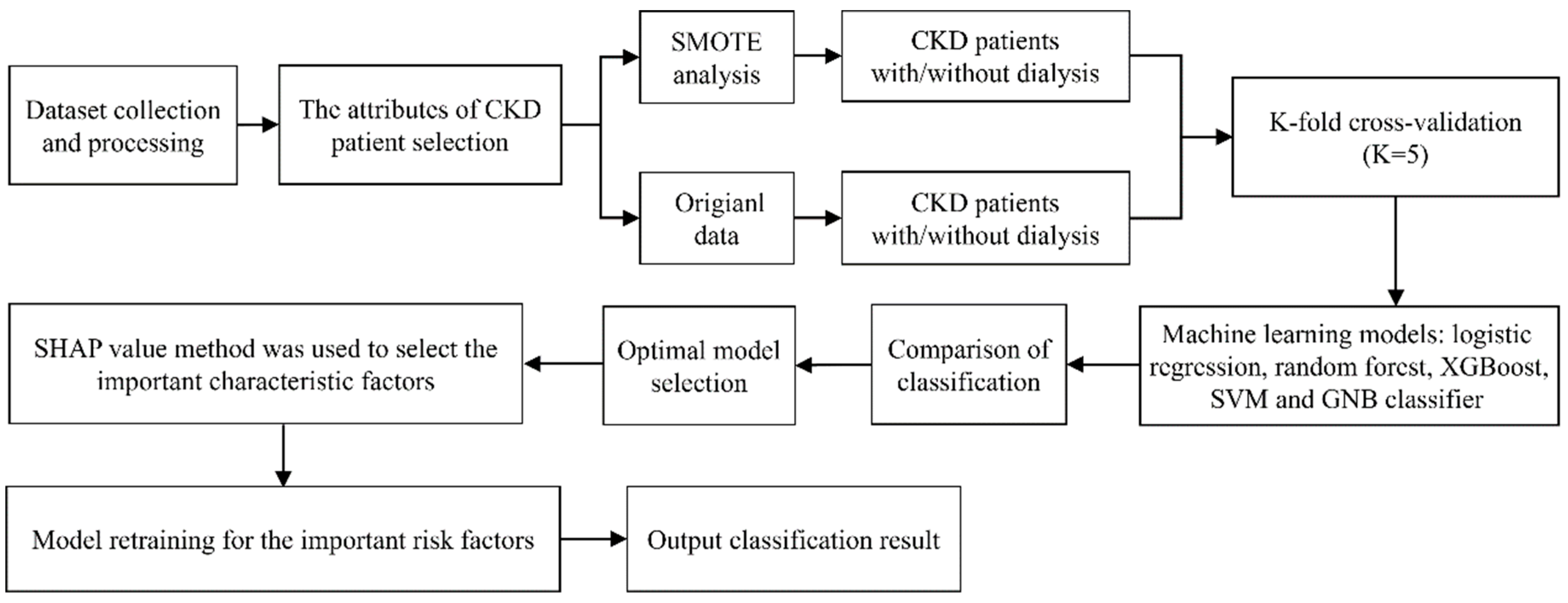
2.4. Statistical Analysis
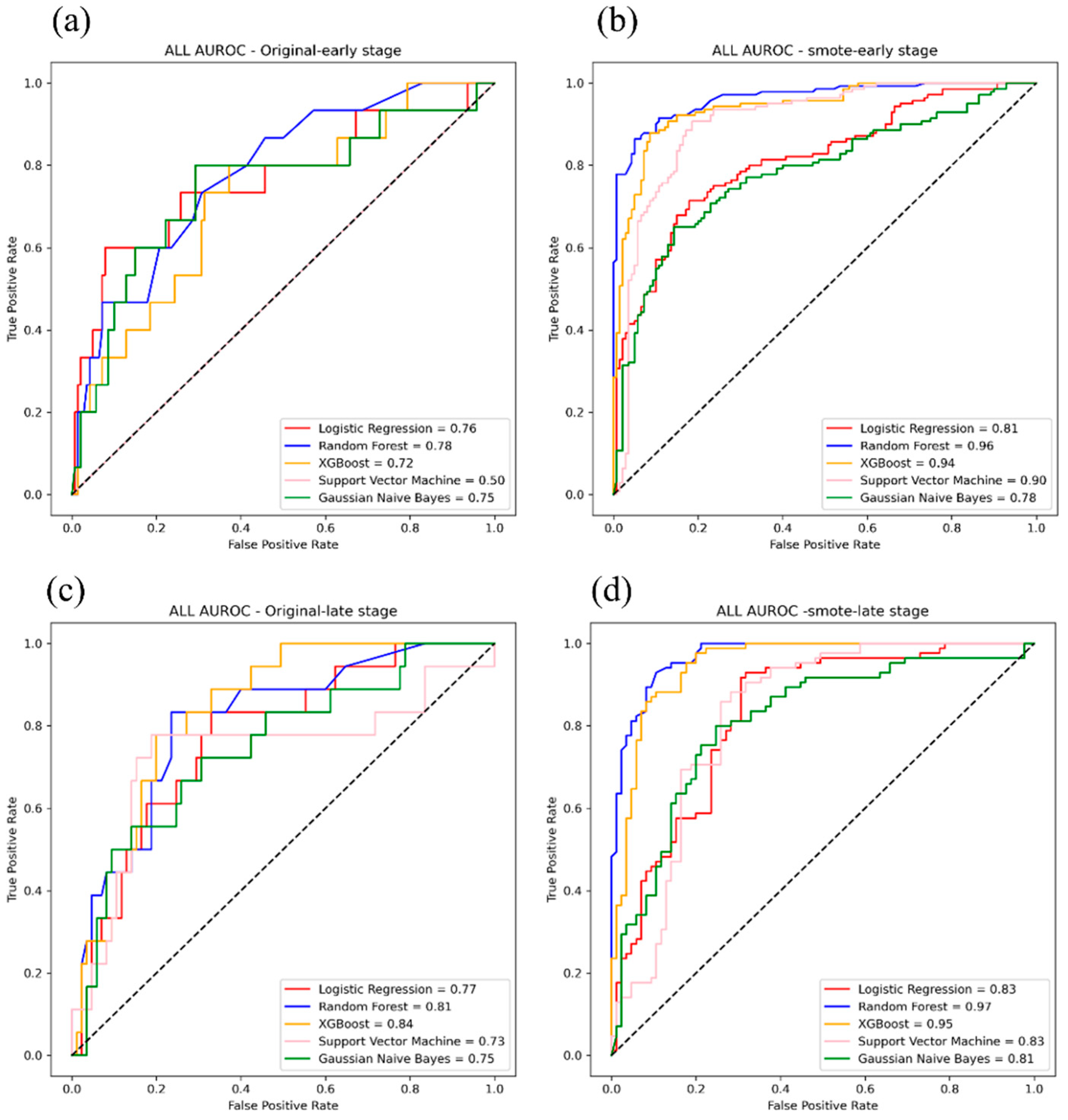
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, B.; Osman, M.; Jafari, M.; Gordon, L.; Tangri, N.; Ferguson, T.W.; Jin, S.; Kappel, J.; Kozakewycz, D. Kidney failure risk equation and cost of care in patients with chronic kidney disease. CJASN 2022, 17, 17–26. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Long, J.; Jiang, W.; Shi, Y.; He, X.; Zhou, Z.; Li, Y.; Yeung, R.O.; Wang, J.; Matsushita, K.; et al. Trends in chronic kidney disease in China. N. Engl. J. Med. 2016, 375, 905–906. [Google Scholar] [CrossRef]
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US renal data system 2019 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2020, 75, A6–A7. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.P.; Morgenstern, H.; Saran, R.; Herman, W.H.; Robinson, B.M. Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol. 2019, 30, 127–135. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsu, C.C.; Lin, M.H.; Chen, K.H.; Wu, I.W. Hospitalization in patients with dialysis in Taiwan: A nationwide population-based observational study. J. Formos. Med. Assoc. 2022, 121, S39–S46. [Google Scholar] [CrossRef]
- Lai, T.S.; Hsu, C.C.; Lin, M.H.; Wu, V.C.; Chen, Y.M. Trends in the incidence and prevalence of end-stage kidney disease requiring dialysis in Taiwan: 2010–2018. J. Formos. Med. Assoc. 2022, 121, S5–S11. [Google Scholar] [CrossRef]
- Johns, T.S.; Yee, J.; Smith-Jules, T.; Campbell, R.C.; Bauer, C. Interdisciplinary care clinics in chronic kidney disease. BMC Nephrol. 2015, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Chertow, G.M.; Yan, B.; Malcolm, E.; Goldhaber-Fiebert, J.D. Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study. PLoS Med. 2018, 15, e1002532. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.; Levin, A.; Levey, A.S. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011, 305, 1553–1559. [Google Scholar] [CrossRef]
- Chang, Y.P.; Liao, C.M.; Wang, L.H.; Hu, H.H.; Lin, C.M. Static and dynamic prediction of chronic renal disease progression using longitudinal clinical data from Taiwan’s national prevention programs. J. Clin. Med. 2021, 10, 3085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Khare, S.; Aggarwal, A. A method to predict diagnostic codes for chronic diseases using machine learning techniques. In Proceedings of the 2016 International Conference on Computing, Communication and Automation (ICCCA), Greater Noida, India, 29–30 April 2016; pp. 281–287. [Google Scholar]
- Ebiaredoh-Mienye, S.A.; Swart, T.G.; Esenogho, E.; Mienye, I.D. A machine learning method with filter-based feature selection for improved prediction of chronic kidney disease. Bioengineering 2022, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Chittora, P.; Chaurasia, S.; Chakrabarti, P.; Kumawat, G.; Chakrabarti, T.; Leonowicz, Z.; Jasinski, M.; Jasinski, L.; Gono, R.; Jasinska, E.; et al. Prediction of chronic kidney disease—A machine learning perspective. IEEE Access 2021, 9, 17312–17334. [Google Scholar] [CrossRef]
- Dovgan, E.; Gradišek, A.; Luštrek, M.; Uddin, M.; Nursetyo, A.A.; Annavarajula, S.K.; Li, Y.-C.; Syed-Abdul, S. Using machine learning models to predict the initiation of renal replacement therapy among chronic kidney disease patients. PLoS ONE 2020, 15, e0233976. [Google Scholar] [CrossRef]
- Subasi, A.; Alickovic, E.; Kevric, J. Diagnosis of chronic kidney disease by using random forest. In CMBEBIH 2017. IFMBE Proceedings, 62; Badnjevic, A., Ed.; Springer: Singapore, 2017; pp. 589–594. [Google Scholar]
- Callahan, A.; Shah, N.H. Machine learning in healthcare. In Key Advances in Clinical Informatics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–291. [Google Scholar]
- Adkins, D.E. Machine learning and electronic health records: A paradigm shift. Am. J. Psychiatry 2017, 174, 93–94. [Google Scholar] [CrossRef]
- Taiwan Society of Nephrology. Taiwan Chronic Kidney Disease Clinical Guidelines. Available online: https://www.tsn.org.tw/ (accessed on 20 June 2021). (In Chinese).
- Ravindra, B.; Sriraam, N.; Geetha, M. Classification of non-chronic and chronic kidney disease using SVM neural networks. Int. J. Eng. Technol. 2018, 7, 191–194. [Google Scholar]
- Dulhare, U.N.; Ayesha, M. Extraction of action rules for chronic kidney disease using Naïve bayes classifier. In Proceedings of the 2016 IEEE International Conference on Computational Intelligence and Computing Research (ICCIC), Tamil Nadu, India, 15–17 December 2016; pp. 1–5. [Google Scholar]
- Seyedzadeh, A.; Tohidi, M.R.; Golmohamadi, S.; Omrani, H.R.; Seyedzadeh, M.S.; Amiri, S.; Hookari, S. Prevalence of renal osteodystrophy and its related factors among end-stage renal disease patients undergoing hemodialysis: Report from Imam Reza Referral Hospital of Medical University of Kermanshah, Iran. Oman Med. J. 2022, 37, e335. [Google Scholar] [CrossRef]
- Mula-Abed, W.A.S.; Al Rasadi, K.; Al Riyami, D. Estimated Glomerular Filtration Rate (eGFR): A serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Med. J. 2012, 27, 108–113. [Google Scholar] [CrossRef]
- Astor, B.C.; Muntner, P.; Levin, A.; Eustace, J.A.; Coresh, J. Association of kidney function with anemia: The third national health and nutrition examination survey (1988–1994). Arch. Intern. Med. 2002, 162, 1401–1408. [Google Scholar] [CrossRef]
- Shaheen, F.A.M.; Souqiyyeh, M.Z.; Al-Attar1, B.A.; Karkar, A.; Jazairi, A.M.H.A.; Badawi, L.S.; Ballut, O.M.; Hakami, A.H.; Naguib, M.; Al-homrany, M.A.; et al. Prevalence of anemia in predialysis chronic kidney disease patients. Saudi J. Kidney Dis. Transplant. 2011, 22, 456–463. [Google Scholar]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. CRIC Study Investigators: Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.P.; Pai, M.F.; Peng, Y.S.; Chiang, C.K.; Ho, T.I.; Hung, K.Y. Serum uric acid levels show a ‘J-shaped’ association with all-cause mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Kawai, R. Low-density lipoprotein (LDL) cholesterol is cross-sectionally associated with preclinical chronic kidney disease (CKD) in Japanese men. Intern. Med. 2010, 49, 713–719. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Cases (Early-Stage CKD Patients) N = 516 | Stage 2 N = 119 | Stage 3A N = 397 | Cases (Advanced-Stage CKD patients) N = 342 | Stage 3B N = 111 | Stage 4 N = 143 | Stage 5 N = 88 | |
|---|---|---|---|---|---|---|---|
| Age (years) | 80.8 ± 11.5 | 79.8 ± 11.2 | 81.1 ± 11.5 | 79.4 ± 0.7 | 79.2 ± 1.1 | 80 ± 1.2 | 78.5 ± 1.4 |
| Sex (%) | |||||||
| Male | 359 (70%) | 84 (71%) | 275 (69%) | 227 (66.4%) | 73 (65.8%) | 103 (72%) | 51 (58%) |
| Female | 157 (30%) | 35 (29%) | 122 (31%) | 115 (33.6%) | 38 (34.2%) | 40 (28%) | 37 (42%) |
| Cardiovascular diseases (%) | |||||||
| None | 214 (41%) | 58 (49%) | 156 (39%) | 318 (93%) | 102 (91.9%) | 132 (92.3%) | 84 (95.5%) |
| Yes | 302 (59%) | 61 (51%) | 241 (61%) | 24 (7%) | 9 (8.1%) | 11 (7.7%) | 4 (4.5%) |
| Diabetes (%) | |||||||
| None | 242 (47%) | 59 (50%) | 183 (46%) | 159 (46.5%) | 59 (53.2%) | 59 (41.3%) | 41 (46.6%) |
| Yes | 274 (53%) | 60 (50%) | 214 (54%) | 183 (53.5%) | 52 (46.8%) | 84 (58.7%) | 47 (53.4%) |
| Hypertension (%) | |||||||
| None | 398 (77%) | 89 (75%) | 309 (78%) | 82 (24%) | 27 (24.3%) | 37 (25.9%) | 18 (20.5%) |
| Yes | 118 (23%) | 30 (25%) | 88 (22%) | 260 (76%) | 84 (75.7%) | 106 (74.1%) | 70 (79.5%) |
| Hemodialysis (%) | |||||||
| None | 466 (90%) | 113 (95%) | 353 (89%) | 283 (82.7%) | 109 (98.2%) | 126 (88.1%) | 48 (54.5%) |
| Yes | 50 (10%) | 6 (5%) | 44 (11%) | 59 (17.3%) | 2 (1.8%) | 17 (11.9%) | 40 (45.5%) |
| BMI (kg/m2) | 26.1 ± 12.6 | 26.9 ± 13.3 | 25.9 ± 12.3 | 24.6 ± 0.2 | 25.2 ± 0.4 | 24.8 ± 0.3 | 23.5 ± 0.5 |
| Systolic blood pressure (mmHg) | 133.4 ± 18.2 | 132.5 ± 18.3 | 133.6 ± 18.2 | 139.1 ± 1.2 | 137.3 ± 0.7 | 139.8 ± 1.8 | 140.4 ± 2.3 |
| Diastolic blood pressure (mmHg) | 72.6 ± 10.9 | 72.3 ± 11.5 | 72.6 ± 10.7 | 73.8 ± 0.9 | 73.6 ± 1.4 | 74.1 ± 1.2 | 73.5 ± 1.5 |
| eGFR (mL/min/1.73 m2) | 53.3 ± 11 | 68.4 ± 8.6 | 48.8 ± 6.9 | 24.3 ± 0.2 | 37.1 ± 0.4 | 23.1 ± 0.4 | 10.3 ± 0.4 |
| Creatinine (mg/dL) | 1.3 ± 0.3 | 1.0 ± 0.2 | 1.4 ± 0.3 | 3.3 ± 0.2 | 1.8 ± 0.1 | 2.6 ± 0.1 | 6.1 ± 0.4 |
| Urine creatinine (mg/dL) | 419.1 ± 717.4 | 309.9 ± 534.7 | 451.8 ± 760.7 | 1973.2 ± 168.9 | 987.2 ± 169.2 | 2067.7 ± 307.6 | 3063.4 ± 331.8 |
| LDL-C (mg/dL) | 101.8 ± 27.7 | 100.1 ± 26.5 | 102.3 ± 28 | 103.2 ± 1.8 | 102.6 ± 2.8 | 105.3 ± 3 | 100.3 ± 3.9 |
| HbA1c (%) | 6.6 ± 1.4 | 6.4 ± 1.2 | 6.6 ± 1.5 | 7.1 ± 0.3 | 7.03 ± 0.5 | 6.7 ± 0.1 | 7.7 ± 0.9 |
| UPCR (mg/g) | 5.3 ± 1.3 | 5.1 ± 1.5 | 5.4 ± 1.3 | 6.6 ± 0.1 | 5.9 ± 0.2 | 6.6 ± 0.2 | 7.4 ± 0.2 |
| Uric Acid (mg/dL) | 7.3 ± 0.11 | 6.8 ± 0.17 | 7.3 ± 0.16 | 7.8 ± 0.24 | |||
| Albumin (g/dL) | 3.6 ± 0.03 | 3.8 ± 0.04 | 3.6 ± 0.04 | 3.3 ± 0.06 | |||
| FPG (mg/dL) | 118.8 ± 2.5 | 102.6 ± 2.8 | 119.1 ± 4.4 | 116.6 ± 4.3 | |||
| Triglyceride (mg/dL) | 142.8 ± 4.8 | 149.7 ± 8.6 | 142.6 ± 0.7.1 | 134.4 ± 9.7 | |||
| Cholesterol (mg/dL) | 178.6 ± 2.5 | 177.1 ± 3.6 | 181.9 ± 3.9 | 175.3 ± 5,4 | |||
| Hemoglobin (gm/dL) | 11.1 ± 0.1 | 12.3 ± 0.2 | 11.2 ± 0.2 | 9.4 ± 0.2 | |||
| Hematocrit (%) | 33.8 ± 0.3 | 37.1 ± 0.6 | 34.1 ± 0.4 | 29.0 ± 0.5 | |||
| BUN (mg/dL) | 47.0 ± 1.4 | 29.9 ± 0.8 | 42.4 ± 1.3 | 76.1 ± 3.3 | |||
| Sodium (Na) (mg/dL) | 140.2 ± 0.2 | 140.6 ± 0.3 | 139.3 ± 0.3 | 140.1 ± 0.5 | |||
| Potassium (K) (mg/dL) | 4.6 ± 0.1 | 4.5 ± 0.1 | 4.7 ± 0.1 | 4.7 ± 0.1 | |||
| Calcium (Ca) (mg/dL) | 8.9 ± 0.1 | 9.3 ± 0.3 | 8.9 ± 0.05 | 8.5 ± 0.1 | |||
| Phosphorus (P) (mg/dL) | 4.1 ± 0.1 | 3.7 ± 0.1 | 4.0 ± 0.1 | 5.0 ± 0.2 | |||
| Algorithm | Year | Dataset | Sensitivity | Specificity | Accuracy | Precision | F1-Score | NPV | AUROC |
|---|---|---|---|---|---|---|---|---|---|
| LR | 3 | Original | 0.92 | 0.72 | 0.74 | 0.23 | 0.37 | 0.99 | 0.83 |
| SMOTE | 0.79 | 0.87 | 0.83 | 0.85 | 0.82 | 0.80 | 0.90 | ||
| 5 | Original | 0.73 | 0.78 | 0.77 | 0.26 | 0.39 | 0.96 | 0.79 | |
| SMOTE | 0.76 | 0.89 | 0.83 | 0.88 | 0.82 | 0.79 | 0.90 | ||
| RF | 3 | Original | 0.92 | 0.33 | 0.90 | 0.99 | 0.95 | 0.61 | 0.73 |
| SMOTE | 0.90 | 0.94 | 0.91 | 0.94 | 0.91 | 0.88 | 0.97 | ||
| 5 | Original | 0.96 | 0.30 | 0.81 | 0.81 | 0.88 | 0.73 | 0.79 | |
| SMOTE | 0.93 | 0.92 | 0.93 | 0.92 | 0.93 | 0.94 | 0.98 | ||
| XGBoost | 3 | Original | 0.95 | 0.21 | 0.79 | 0.81 | 0.87 | 0.54 | 0.65 |
| SMOTE | 0.90 | 0.92 | 0.86 | 0.85 | 0.88 | 0.92 | 0.96 | ||
| 5 | Original | 0.96 | 0.29 | 0.80 | 0.81 | 0.88 | 0.73 | 0.72 | |
| SMOTE | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.91 | 0.98 | ||
| SVM | 3 | Original | 1.00 | 0.00 | 0.92 | 1.00 | 0.96 | 0.00 | 0.50 |
| SMOTE | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.92 | ||
| 5 | Original | 0.90 | 0.00 | 0.90 | 1.00 | 0.95 | 0.00 | 0.50 | |
| SMOTE | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.91 | 0.93 | ||
| GNB | 3 | Original | 0.95 | 0.43 | 0.63 | 0.61 | 0.75 | 0.85 | 0.80 |
| SMOTE | 0.70 | 0.88 | 0.75 | 0.92 | 0.79 | 0.59 | 0.84 | ||
| 5 | Original | 0.94 | 0.39 | 0.88 | 0.92 | 0.93 | 0.47 | 0.75 | |
| SMOTE | 0.76 | 0.79 | 0.78 | 0.80 | 0.78 | 0.74 | 0.84 |
| Algorithm | Year | Dataset | Sensitivity | Specificity | Accuracy | Precision | F1-Score | NPV | AUROC |
|---|---|---|---|---|---|---|---|---|---|
| LR | 1 | Original | 0.90 | 0.69 | 0.72 | 0.26 | 0.41 | 0.98 | 0.84 |
| SMOTE | 0.98 | 0.81 | 0.90 | 0.84 | 0.90 | 0.97 | 0.92 | ||
| 3 | Original | 0.89 | 0.74 | 0.77 | 0.42 | 0.58 | 0.97 | 0.85 | |
| SMOTE | 0.88 | 0.72 | 0.80 | 0.76 | 0.82 | 0.86 | 0.85 | ||
| RF | 1 | Original | 0.99 | 0.42 | 0.85 | 0.85 | 0.91 | 0.91 | 0.92 |
| SMOTE | 0.96 | 0.91 | 0.93 | 0.90 | 0.93 | 0.97 | 0.99 | ||
| 3 | Original | 0.98 | 0.41 | 0.76 | 0.72 | 0.83 | 0.94 | 0.87 | |
| SMOTE | 0.90 | 0.93 | 0.91 | 0.93 | 0.91 | 0.89 | 0.97 | ||
| XGBoost | 1 | Original | 0.99 | 0.37 | 0.82 | 0.81 | 0.89 | 0.91 | 0.92 |
| SMOTE | 0.96 | 0.94 | 0.95 | 0.93 | 0.94 | 0.96 | 0.99 | ||
| 3 | Original | 0.94 | 0.41 | 0.77 | 0.76 | 0.84 | 0.78 | 0.82 | |
| SMOTE | 0.92 | 0.87 | 0.89 | 0.86 | 0.89 | 0.93 | 0.95 | ||
| SVM | 1 | Original | 0.94 | 0.38 | 0.85 | 0.89 | 0.92 | 0.55 | 0.63 |
| SMOTE | 0.88 | 0.94 | 0.91 | 0.95 | 0.91 | 0.89 | 0.94 | ||
| 3 | Original | 0.94 | 0.52 | 0.83 | 0.86 | 0.90 | 0.72 | 0.79 | |
| SMOTE | 0.96 | 0.84 | 0.89 | 0.81 | 0.88 | 0.96 | 0.92 | ||
| GNB | 1 | Original | 1.00 | 0.29 | 0.73 | 0.70 | 0.83 | 1.00 | 0.85 |
| SMOTE | 0.92 | 0.86 | 0.88 | 0.85 | 0.88 | 0.92 | 0.92 | ||
| 3 | Original | 0.97 | 0.41 | 0.75 | 0.73 | 0.83 | 0.89 | 0.85 | |
| SMOTE | 0.85 | 0.74 | 0.78 | 0.68 | 0.76 | 0.88 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-T.; Chang, Y.-P.; Ku, Y.-T.; Lin, C.-M. Machine Learning Models for the Prediction of Renal Failure in Chronic Kidney Disease: A Retrospective Cohort Study. Diagnostics 2022, 12, 2454. https://doi.org/10.3390/diagnostics12102454
Su C-T, Chang Y-P, Ku Y-T, Lin C-M. Machine Learning Models for the Prediction of Renal Failure in Chronic Kidney Disease: A Retrospective Cohort Study. Diagnostics. 2022; 12(10):2454. https://doi.org/10.3390/diagnostics12102454
Chicago/Turabian StyleSu, Chuan-Tsung, Yi-Ping Chang, Yuh-Ting Ku, and Chih-Ming Lin. 2022. "Machine Learning Models for the Prediction of Renal Failure in Chronic Kidney Disease: A Retrospective Cohort Study" Diagnostics 12, no. 10: 2454. https://doi.org/10.3390/diagnostics12102454
APA StyleSu, C.-T., Chang, Y.-P., Ku, Y.-T., & Lin, C.-M. (2022). Machine Learning Models for the Prediction of Renal Failure in Chronic Kidney Disease: A Retrospective Cohort Study. Diagnostics, 12(10), 2454. https://doi.org/10.3390/diagnostics12102454







