Abstract
Objective: The aim of the present study was to analyze the progression of non-motor symptoms (NMS) burden in Parkinson’s disease (PD) patients regarding the development of motor fluctuations (MF). Methods: PD patients without MF at baseline, who were recruited from January 2016 to November 2017 (V0) and evaluated again at a 2-year follow-up (V2) from 35 centers of Spain from the COPPADIS cohort, were included in this analysis. MF development at V2 was defined as a score ≥ 1 in the item-39 of the UPDRS-Part IV, whereas NMS burden was defined according to the Non-motor Symptoms Scale (NMSS) total score. Results: Three hundred and thirty PD patients (62.67 ± 8.7 years old; 58.8% males) were included. From V0 to V2, 27.6% of the patients developed MF. The mean NMSS total score at baseline was higher in those patients who developed MF after the 2-year follow-up (46.34 ± 36.48 vs. 34.3 ± 29.07; p = 0.001). A greater increase in the NMSS total score from V0 to V2 was observed in patients who developed MF (+16.07 ± 37.37) compared to those who did not develop MF (+6.2 ± 25.8) (p = 0.021). Development of MF after a 2-year follow-up was associated with an increase in the NMSS total score (β = 0.128; p = 0.046) after adjustment to age, gender, years from symptoms onset, levodopa equivalent daily dose (LEDD) and the NMSS total score at baseline, and the change in LEDD from V0 to V2. Conclusions: In PD patients, the development of MF is associated with a greater increase in the NMS burden after a 2-year follow-up.
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder causing motor and non-motor symptoms (NMS) that result in disability, loss of patient autonomy, and diminished quality of life (QoL) [1]. From a pathophysiological point of view, motor symptoms in PD are attributed to the degeneration of the dopaminergic nigrostriatal system [2]. Nevertheless, increasing evidence has shown that PD is a multisystem disorder characterized also by the degeneration of the mesocortical dopaminergic system, the noradrenergic system of the locus coeruleus, the serotonergic system of the dorsal raphe nuclei, and the cholinergic system of the nucleus basalis of Meynert, as well as the histaminergic, peptidergic, and olfactory-related systems [3]. This explains the complexity in management of NMS in PD and why many therapeutic strategies are based on correcting the deficit of neurotransmitters other than dopamine [4]. However, NMS can be related to dopamine as well. Increasing dopamine activity not only in the striatum but also in other areas of the brain could improve some NMS such as attention, executive functions, apathy, depression, anxiety, restless legs and periodic limb movements, urinary urgency, nocturia, dribbling of saliva, constipation, pain, or fatigue [5,6,7,8,9]. Moreover, NMS can be related to dopamine changes in brain and blood [10]. Thus, some patients can suffer from non-motor fluctuations (NMF) (i.e., NMS that fluctuate during the day) [11] or can experience motor fluctuations (MF) with the development of NMS during the OFF episodes (e.g., pain associated with dystonia) [12]. The close connection of NMF and MF strongly suggests that the strategies used to treat motor complications—namely, continuous dopaminergic stimulation—also apply for the therapy of NMF. Thus, a dopaminergic treatment reducing the daily OFF time can improve some NMS [9,13,14] or even the global NMS burden [15,16]. In line with this, we demonstrated recently in a cross-sectional study conducted in Spain that MF are frequent and associated with a greater NMS burden even during the first 5 years of disease duration [17]. This is of great importance because NMS burden is associated with a worse QoL and is also an independent predictor of clinically significant QoL impairment in PD [18,19].
In this context, we hypothesized that PD patients who develop MF in the short-term will increase their NMS burden compared with those patients who do not. Understanding this potential association is of interest because, in clinical practice, to detect MF is an essential point for the application of management strategies in PD [20]. The aim of the present study was to analyze the progression of NMS burden in PD patients from a Spanish cohort regarding the development of MF after a 2-year follow-up. Moreover, the change in health-related quality of life (HR-QoL) and global QoL (GQoL) was analyzed as well.
2. Material and Methods
PD patients without MF at baseline, who were recruited from 35 centers of Spain from the COPPADIS cohort [21] from January 2016 to November 2017 and evaluated again at 2-year follow-up, were included in the study. Methodology about COPPADIS-2015 study can be consulted in https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0548-9 accessed on 25 February 2016 [22]. This is a multicenter, observational, longitudinal-prospective, 5-year follow-up study designed to analyze disease progression in a Spanish population of PD patients. All patients included were diagnosed according to UK PD Brain Bank criteria [22].
In PD subjects, information on sociodemographic aspects, factors related to PD, comorbidity, and treatment was collected at baseline (visit V0) and at 2 years ± 1 month (visit V2). V0 and V2 evaluations included motor assessment (Hoenh & Yahr [H&Y], Unified Parkinson’s Disease Rating Scale [UPDRS] part III and part IV, Freezing of Gait Questionnaire [FOGQ]), NMS (Non-Motor Symptoms Scale [NMSS], Parkinson’s Disease Sleep Scale [PDSS], Visual Analog Scale-Pain [VAS-Pain], Visual Analog Fatigue Scale [VAFS]), cognition (PD-CRS), mood and neuropsychiatric symptoms (Beck Depression Inventory-II [BDI-II], Neuropsychiatric Inventory [NPI], Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale [QUIP-RS]), disability (Schwab & England Activities of Daily Living Scale [ADLS]), and QoL (the 39-item Parkinson’s disease Questionnaire [PDQ-39], the EUROHIS-QOL 8-item index [EUROHIS-QOL8]) [22]. In all the scales/questionnaires, a higher score indicates a more severe affectation except for PD-CRS, PDSS, ADLS, and EUROHIS-QOL8, where it is opposite.
MF were defined according to the Unified Parkinson’s Disease Rating Scale–Part IV (UPDRS-IV) [23]. Patients with a score = 0 on item-39 of the UPDRS-IV (UPDRS-IV-39) were considered as without MF whereas those with a UPDRS-IV-39 score ≥ 1 were defined as with MF. For this study, patients from the COPPADIS cohort who presented with MF (i.e., UPDRS-IV-39 ≥ 1) at baseline were excluded. In patients with MF, the motor assessment was made during the OFF state (without medication in the last 12 h) and during the ON state. On the other hand, the assessment was only performed without medication in patients without MF. Other data about motor complications were obtained from the UPDRS-IV.
The NMS burden was defined according to the NMSS total score [24]. The NMSS includes 30 items, each with a different non-motor symptom. The symptoms refer to the 4 weeks prior to assessment. The total score for each item is the result of multiplying the frequency (0, never; 1, rarely; 2, often; 3, frequent; 4, very often) × severity (1, mild; 2, moderate; 3, severe) and will vary from 0 to 12 points. The scale score ranges from 0 to 360 points. The items are grouped into 9 different domains: (1) Cardiovascular (items 1 and 2; score, 0 to 24); (2) Sleep/fatigue (items 3, 4, 5, and 6; score, 0 to 48); (3) Mood/apathy (items 7, 8, 9, 10, 11, and 12; score, 0 to 72); (4) Perceptual problems/hallucinations (items 13, 14, and 15; score, 0 to 36); (5) Attention/memory (items 16, 17, and 18; score, 0 to 36); (6) Gastrointestinal symptoms (items 19, 20, and 21; score 0 to 36); (7) Urinary symptoms (items 22, 23, and 24; score, 0 to 36); (8) Sexual dysfunction (items 25 and 26; score 0 to 24); (9) Miscellaneous (items 27, 28, 29, and 30; score, 0 to 48). Regarding the NMS burden, different groups were defined: mild (NMSS 1–20); moderate (NMSS 21–40); severe (NMSS 41–70); very severe (NMSS > 70) [25].
The PDQ-39 [26] and EUROHIS-QOL8 [27] were used to assess the HRQoL and GQoL, respectively. The PDQ-39 includes 39 items grouped into 8 domains: (1) Mobility (items 1 to 10); (2) Activities of daily living (ADL) (items 11 to 16); (3) Emotional well-being (items 17 to 22); (4) Stigma (items 23 to 26); (5) Social support (items 27 to 29); (6) Cognition (items 30 to 33); (7) Communication (items 34 to 36); (8) Pain and discomfort (items 37 to 39). For each item, the score may range from 0 (never) to 4 (always). The symptoms refer to the 4 weeks prior to assessment. Domain total scores are expressed as a percentage of the corresponding maximum possible score and a Summary Index is obtained as average of the domain scores. The EUROHIS-QOL8 is an 8-item GQoL questionnaire (quality of life, health status, energy, autonomy for ADL, self-esteem, social relationships, economic capacity, and habitat) derived from the WHOQOL-BREF. For each item, the score ranges from 0 (not at all) to 5 (completely). The total score is expressed as the mean of the individual scores. A higher score indicates a better QoL.
3. Data Analysis
Data were processed using SPSS 20.0 for Windows. Only PD patients from the COPPADIS cohort with data of the UPDRS-IV and NMSS total score collected at both visits, V0 and V2, were included in the analysis. For comparisons between patients with vs. without MF at V2, the Student’s t-test, Mann–Whitney U test, Chi-square test, or Fisher test were used as appropriate (distribution for variables was verified by one-sample Kolmogorov-Smirnov test). Spearman’s or Pearson’s correlation coefficient, as appropriate, were used for analyzing the relationship between the change from V0 to V2 in continuous variables (NMSS, PDQ-39SI, EUROHIS-QOL8). Correlations were considered weak for coefficient values ≤ 0.29, moderate for values between 0.30 and 0.59, and strong for values ≥0.60. Marginal homogeneity tests were applied for comparing the frequency distribution of groups (NMS burden; from mild to very severe) between V0 and V2.
General linear model (GLM) repeated measure was used to test whether the mean differences of the total score and each domain of the NMSS, PDQ-39SI, and EUROHIS-QOL8 between the two visits (V0 and V2) were significant. The Bonferroni method was used as a post-hoc test after ANOVA. Cohen’s d formula was applied for measuring the effect size; it was considered as follows: <0.2—Negligible; 0.2–0.49—Small; 0.50–0.79—Moderate; ≥0.80—Large. Age, gender, years from symptoms onset, H&Y stage, levodopa equivalent daily dose (LEDD) and the NMSS total score at baseline, and the change in LEDD from V0 to V2 were included as covariates in the model. The total score of each scale at V0 (NMSS, PDQ-39SI, and EUROHIS-QOL8) was included as covariate for the analysis of their domains.
With the aim to investigate if the development of MF from V0 to V2 was an independent factor associated with an increase in the NMS burden and impairment in the QoL, linear regression models with the change from V0 to V2 in the total score of the NMSS, PDQ-39SI, and EUROHIS-QOL8 (these variables as dependent variable in each model) were conducted. In all cases, the analysis was adjusted to age, gender, years from symptoms onset, H&Y stage, LEDD and the NMSS total score at baseline, and the change in LEDD from V0 to V2. The p-value was considered significant when it was <0.05.
4. Standard Protocol Approvals, Registrations, and Patient Consents
For this study, we received approval from the Comité de Ética de la Investigación Clínica de Galicia in Spain (2014/534; 02/DEC/2014). Written informed consent was obtained from all participants in this study. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Postauthorization Prospective Follow-up study with the code COH-PAK-2014-01.
5. Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
6. Results
Three hundred and thirty PD patients (62.67 ± 8.7 years old; 58.8% males) without MF at baseline were included. From V0 to V2, 27.6% of the patients (91/330) developed MF. In the group of patients with MF at V2, OFF episodes were predictable in 89% of the cases and unpredictable in 15.4%; early morning dystonia was reported by 25.3% of the patients; and the proportion of the waking day during the OFF state was 82.4% from 1 to 25%, 16.5% from 26 to 50%, and only 1 patient with >50%. Thirty-six out of 91 patients who developed MF (39.6%) presented dyskinesia as well, being disabling in 15 patients (15/36; 41.7%).
Compared with those patients who did not develop MF from V0 to V2, at V0, patients who presented with MF at V2 were younger (60.75 ± 9.06 vs. 63.41 ± 8.46 years old; p = 0.012), had a longer disease duration (5.36 ± 3.51 vs. 3.65 ± 3.09 years from symptoms onset; p < 0.0001); were receiving more dopaminergic medication; and had a worse status in terms of motor symptoms, NMS, QoL, and autonomy for ADL (Table 1). The mean NMSS total score at baseline was higher in those patients who developed MF after the 2-year follow-up than in those who did not develop MF (46.34 ± 36.48 vs. 34.3 ± 29.07; p = 0.001) (Table 1 and Figure 1). At V0, the frequency of severe and very severe NMS burden was higher in those patients who developed MF at V2 compared with those who did not (27.5% vs. 18% and 18.7% vs. 12.1%, respectively; p = 0.011) (Figure 2).

Table 1.
Different PD-related variables in PD patients who developed MF at V2 (MF at V2; N = 91) compared with those patients who did not develop MF at V2 (nonMF at V2; N = 239).
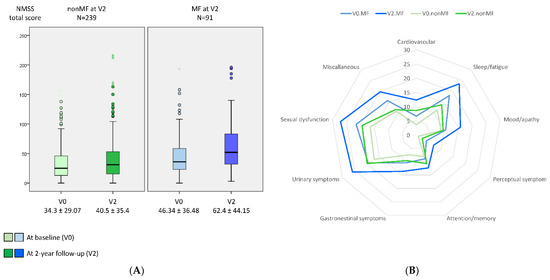
Figure 1.
(A) NMSS total score (y-axis) at baseline (V0) and after a 2-year follow-up (V2) in PD patients who developed MF at V2 (MF at V2 (PD-MFV2); N = 91) and those patients who did not develop MF at V2 (nonMF at V2 (PD-nonMFV2); N = 239). NMSS total score at V0, PD-MFV2 vs. PD-nonMFV2, p = 0.001; NMSS total score at V2, PD-MFV2 vs. PD-nonMFV2, p < 0.0001; change in the NMSS total score from V0 to V2 in PD-MFV2, p < 0.0001; change in the NMSS total score from V0 to V2 in PD-nonMFV2, p < 0.0001; comparison between the change in the NMSS total score from V0 to V2 in PD-MFV2 vs. PD-nonMFV2, p = 0.021. Data are presented as box plots, with the box representing the median and the two middle quartiles (25–75%). (B) Mean score on each domain of the NMSS at V0 and at V2 in both groups, PD-MFV2 and PD-nonMFV2. At V0, the difference was significant between both groups in NMSS-1 (Cardiovascular) (p = 0.001), NMSS-2 (Sleep/fatigue) (p = 0.001), NMSS-4 (Perceptual symptoms) (p < 0.0001), and NMSS-9 (Miscellaneous) (p = 0.005). At V2, the difference was significant between both groups in all domains (p values from 0.024 to <0.0001) except in NMSS-5 (Attention/memory) (p = 0.364). p values were computed using the Kolmogorov–Smirnov, Mann–Whitney, and Wilcoxon tests. Mild outliers (O) are data points that are more extreme than Q1 − 1.5 * IQR or Q3 + 1.5 * IQR.
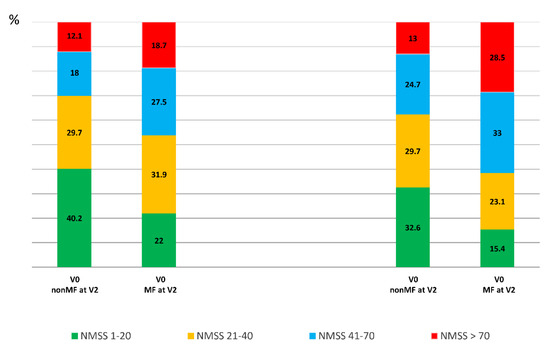
Figure 2.
Frequency of patients with mild (NMSS 1–20), moderate (NMSS 21–40), severe (NMSS 41–70), and very severe (NMSS > 70) NMS burden at V0 and at V2 considering two groups: patients who developed MF at V2 (MF at V2 (PD-MFV2); N = 91) and those who did not developed MF at V2 (nonMF at V2 (PD-nonMFV2); N = 239). PD-nonMFV2 vs. PD-MFV2 at V0, p = 0.011; PD-nonMFV2 vs. PD-MFV2 at V2, p < 0.0001; change in PD-nonMFV2 from V0 to V2, p = 0.003; change in PD-MFV2 from V0 to V2, p = 0.001. p values were computed using the Chi-square and marginal homogeneity test.
A greater increase in the NMSS total score from V0 to V2 was observed in those patients who developed MF at V2 (+16.07 ± 37.37) compared with those who did not develop MF (+6.2 ± 25.8) (p = 0.021) (Table 1 and Figure 1). Two-hundred and two out of 330 patients (64.2%) presented at V2 a NMSS total score higher than at V0, but no differences between patients who developed MF vs. those who did not develop MF after the 2-year follow-up were observed (68.1% vs. 62.1%; p = 0.218). However, after the 2-year follow-up, the frequency of severe and very severe NMS burden was significantly higher in the group who developed MF (p < 0.0001) (Figure 2). Applying GLM repeated measure and after adjustment to covariates (age, gender, years from symptoms onset, H&Y stage, LEDD and the NMSS total score at baseline, and the change in LEDD from V0 to V2), a significantly greater increase (34.6% vs. 17.9%; p = 0.005) in the NMSS total score was observed in patients who developed MF at V2 (from 46.34 ± 36.48 to 62.37 ± 44.15; Cohen’s effect size = 0.57; p = 0.003) compared with those who did not develop MF (from 34.3 ± 29.07 vs. 40.5 ± 35.4; Cohen’s effect size = 0.33; p < 0.0001) (Table 2). An increase in the score of different domains from V0 to V2 was significant in both groups, with and without MF at V2, but there were no significant differences between them (Table 2 and Figure 1). Regarding QoL, the increase in the PDQ-39SI and decrease in EUROHIS-QOL8 total score indicating a QoL impairment between both visits, V0 and V2, was significantly greater in the group of patients who developed MF (PDQ-39SI, +35% vs. +26.5% (p = 0.002); EUROHIS-QOL8, −29.9% vs. −0.7% (p = 0.030)) (Table 2). By domain and after adjustment to covariates including the PSQ-39SI score at V0, the increase on the score of “pain and discomfort” domain in the group who developed MF at V2 (from 28.55 ± 20.01 to 32.87 ± 24.33; Cohen’s effect size = 0.30; p = 0.015) was significantly higher (p = 0.039) compared with patients who did not develop MF (from 20.65 ± 18.82 to 23.97 ± 22.12; Cohen’s d effect size = 0.16; p = 0.071) (Table 2). The mean score on all domains of the PDQ-39SI was the highest in patients who developed MF after the 2-year follow-up at V2 and the lowest in patients who did not develop MF, at V0 (Figure 3). A moderate correlation was observed between the change from V0 to V2 in the NMSS total score and the change in the PDQ-39SI in the whole cohort (N = 320; r = 0.402; p < 0.0001) and in both groups, patients with (N = 91; r = 0.328; p = 0.002) and without MF (N = 239; r = 0.433; <0.0001) at V2. However, the correlation between the change in the total score of the NMSS and the EUROHIS-QOL8 was only significant in patients who developed MF at V2 (N = 91; r = −0.277; p = 0.009) but not in patients who did not develop MF at V2 (N = 239; r = −0.111; p = 0.088).

Table 2.
Changes in non-motor symptoms and quality of life in PD patients who developed MF at V2 (MF at V2; N = 91) compared with those patients who did not develop MF at V2 (nonMF at V2; N = 239).
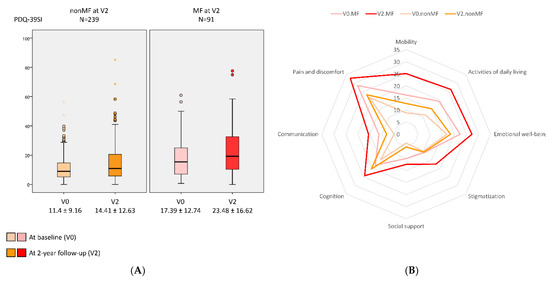
Figure 3.
(A) QoL (PDQ-39SI) (y-axis) at baseline (V0) and after a 2-year follow-up (V2) (x-axis) in PD patients who developed MF at V2 (MF at V2 (PD-MFV2); N = 91) and those patients who did not developed MF at V2 (nonMF at V2 (PD-nonMFV2); N = 239). PDQ-39SI at V0, PD-MFV2 vs. PD-nonMFV2, p < 0.0001; PDQ-39SI at V2, PD-MFV2 vs. PD-nonMFV2, p < 0.0001; change in the PDQ-39SI from V0 to V2 in PD-MFV2, p < 0.0001; change in the PDQ-39SI from V0 to V2 in PD-nonMFV2, p < 0.0001; comparison between the change in the PDQ-39SI from V0 to V2 in PD-MFV2 vs. PD-nonMFV2, p = 0.005. (B) Mean score on each domain of the PDQ-39SI at V0 and at V2 in both groups, PD-MFV2 and PD-nonMFV2. At V0, the difference was significant between both groups in all domains (p values from 0.023 to <0.0001) except in PDQ-39SI-4 (Stigmatization) (p = 0.169) and PDQ-39SI-6 (Cognition) (p = 0.097). At V2, the difference was significant between both groups in all domains (p values from 0.005 to <0.0001) except in PDQ-39SI-6 (Cognition) (p = 0.319). PDQ-39 is expressed as a Summary Index (PDQ-39SI). Data are presented as box plots, with the box representing the median and the two middle quartiles (25–75%). p values were computed using the Kolmogorov–Smirnov, Mann–Whitney, and Wilcoxon tests. Mild outliers (O) are data points that are more extreme than Q1 − 1.5 * IQR or Q3 + 1.5 * IQR.
To develop MF after a 2-year follow-up was associated with an increase in the NMSS total score without controlling for other factors (β = 0.148; 95% CI, 2.69–16.98; p = 0.007) but also after adjustment to age, gender, years from symptoms onset, LEDD and the NMSS total score at baseline, and the change in LEDD from V0 to V2 as well (β = 0.128; 95% CI, 0.17–16.86; p = 0.046). However, when time on levodopa and the H&Y stage were included in the model as covariates, it was not significant (with time on levodopa therapy, p = 0.062; with H&Y, p = 0.167; both variables, p = 0.212). Development of MF was associated with an increase in the PDQ39SI from V0 to V2 (β = 0.135; 95% CI, 0.61–5.55; p = 0.015) but not with the change in the EUROHIS-QOL8 total score (p = 0.207). However, it was not significant after controlling for other covariates (age, gender, years from symptoms onset, LEDD and the PDQ-39SI at baseline, and the change in LEDD from V0 to V2) (p = 0.094).
7. Discussion
The present study observes that MF are frequent in PD, appearing in a cohort of 330 patients with a mean of 4 years from symptoms onset in one of every 4 subjects after a 2-year follow-up, and also that they are related to NMS. Specifically, NMS burden was greater at baseline in PD patients who 2 years later developed MF, and the increase in the NMS burden after the 2-year follow-up was double in this group as well. Moreover, similar results were obtained in terms of QoL. Importantly, all patients at baseline were without MF and this is the first time that NMS burden progression is specifically analyzed regarding the development of MF in a PD cohort.
MF are frequent in PD [28,29,30,31]. In the COPPADIS cohort, of 690 patients with a mean disease duration of 5.5 years (DS 4.37), 33.9% had MF [17]. This percentage was 18.1% in the subgroup of patients with ≤5 years of disease duration (N = 396), with a mean disease duration of 2.7 years (DS 1.5) from symptoms onset [17]. The frequency will depend in part on the methods used—from an interview to wearable tools—and how sensitive we can be to detect them [32]. Stocchi et al. analyzed wearing-off (WO) in 617 PD patients with a mean disease duration of 6.6 years (DS 4.6) and observed that neurologist identified the presence of WO with an interview in 56.9% of the patients, whereas the percentage was 67.3% when the self-rated 19-question Wearing-Off Questionnaire (WOQ-19) was administered [33]. Identifying fluctuations is important in PD patients for two reasons. Firstly, their presence is associated with a worse status in terms of motor, NMS, QoL, and autonomy for ADL [17]. Secondly, the therapeutic strategy is conditioned by their presence to the point that there are several drugs marketed with indication to be only for patients with MF [34].
MF (either early or advanced) can significantly add to the NMS burden in PD [35,36]. However, few studies specifically focused on the NMS prevalence in motor-fluctuating PD patients [17,37,38]. Recent data published of 1589 PD patients from the SYNAPSIS study support the high prevalence of NMS in PD patients with MF in real-life condition, thus reinforcing the need for assessing them for diagnostic accuracy and for delivering holistic care [37]. Using the NMSS, we previously observed a greater NMS burden in the group with MF in a cross-sectional study conducted in PD patients from the COPPADIS cohort. In particular, 28 out of the 30 NMS included in the NMSS were significantly more frequent in patients with MF compared with those who did not have MF, and the mean score of all domains of the NMSS except urinary symptoms and sexual dysfunction was significantly higher in the group with MF [17]. Watanabe et al. recently explored the changes in NMS and QoL during 52 weeks in 996 Japanese PD patients exhibiting MF using the Movement Disorder Society Unified PD Rating Scale (MDS-UPDRS) Part I and the 8-item PD Questionnaire (PDQ-8), respectively [38]. They detected that changes in MDS-UPDRS Part I scores were variable and related to changes in HRQoL and identified 3 separate groups: unchanged (63.8%); deteriorated (20.1%); improved (16.2%). However, very importantly, all patients included in this study had MF. To our knowledge, our study is the first one to prospectively analyze the change in the NMS burden in relation to the development of MF in PD patients who initially did not have MF. As we previously reported in this cohort [39], about 6 out of 10 patients increased the NMSS total score after a 2-year follow-up. Although there were no differences in the percentage between the two groups—patients who developed MF and patients who did not develop MF—a greater NMS burden increase was observed in the first group. We did not analyze specifically if NMS fluctuated (e.g., NMS-MDS [40]), but this finding would support the relationship between NMS and the presence of OFF episodes with an increase in NMS perception during the OFF episodes. Importantly, the effect of MF on NMS burden persisted after adjustment to some variables related to NMS in PD such as age, gender, disease duration, or even dopaminergic medication [35,41,42]. However, NMS in PD are related to motor stage as well [17,18,25,42], and after the inclusion of the H&Y stage in the model, the effects disappeared. The same happened when time on levodopa therapy was included as covariate in the model. It is well-known that both aspects are related to the development of MF [30,31]. A more advanced H&Y stage is related to a greater degree of denervation of the striatal nucleus with more sensitivity to the development of MF [43]. On the other hand, a longer time on levodopa could imply a longer disease duration but also more time exposed to certain causative mechanisms (presynaptic and postsynaptic changes and pharmacokinetic and pharmacodynamic factors) [30,31]. The data as a whole indicate that PD patients who will develop MF in the short-term are patients with a more advanced disease with a greater NMS burden and patients with an increased risk of developing more severe NMS burden. To detect NMS burden progression is relevant because it is associated with a worse QoL [18,28]; importantly, in this context, MF development was associated with a greater worsening of both HRQoL and GQoL in the present analysis. To reduce NMS burden in PD patients has been demonstrated to be associated with an improvement in QoL [14,15]. In summary, our findings reinforce the idea that there is a close relationship between motor and NMS and that dopaminergic treatment can be helpful in some cases [5,10].
The present study has some limitations. The sample size of the group of patients with MF at V2 was smaller (N = 91) compared with the group without MF (N = 231), and the information about NMS burden follow-up was recorded in 330 patients of 462 (71.4%) without MF at baseline from the COPPADIS cohort. This is a limitation observed in other prospective studies, with percentages ranging from 61.9% to 89.8% [39,42,44]. We used the NMSS to assess the NMS burden progression, but some studies suggest that a battery of separate NMS scales is more sensitive to change than the NMSS [45]. Our sample was not fully representative of the PD population due to inclusion and exclusion criteria (i.e., age limit, no dementia, no severe comorbidities, no second line therapies, etc.). For some variables, the information was not collected in all cases (the smallest sample size was for the change in NPI (N = 255) since it was covered by the caregiver and not all had a primary caregiver). On the contrary, the strengths of our study include a very thorough assessment, a prospective longitudinal follow-up design, and the extensive clinical and demographic information recorded.
In conclusion, we demonstrated for the first time in a prospective study that, in PD, the development of MF is associated with a greater NMS burden increase in the short-term. In practice, it is essential to detect MF early and ask about NMS, especially in patients with a greater disease severity and a longer time on levodopa.
Author Contributions
D.S.-G.: conception, organization, and execution of the project; statistical analysis; writing of the first draft of the manuscript; recruitment and/or evaluation of participants; C.C.B. and M.J.F.P.: Collaboration in the preparation of the manuscript. Review and critique; C.M.M.: review and critique; T.d.D.F., E.S.C., H.C., S.J., M.A., P.P., L.P., M.C., J.G.C., N.C., I.L., J.H.-V., I.C., L.L.M., I.G.A., M.A.Á.R., V.G.M., V.N., V.P., J.D.G.-S., C.B., B.S.V., M.Á.S., L.V., S.E., E.C., F.C.P., J.C.M.C., P.S.A., M.G.A.L., N.L.A., I.G., J.K., M.B.E., M.S., J.R.M., C.V., M.K., O.d.F., J.G.A., R.A.R., C.O., L.M.L.D., P.M.: review and critique; recruitment and/or evaluation of participants; D.M.: review and critique; review of English style; P.M.-M.: review and critique; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Santos García D. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, and Teva. De Deus Fonticoba T.: None. Cores Bartolomé C. has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma. Feal Painceiras M. J.: None. Martínez Miró C.: None. Suárez Castro E.: None. Canfield H.: None. Jesús S. has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018). Aguilar M.: UCB and Schwabe with assistance to a Congress; Nutricia with assistance to a Congress and payment of lecture. Pastor P.: None. Planellas LL.: None. Cosgaya M.: None. García Caldentey J. has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva. Caballol N. has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva, and KRKA and sponsorship from Zambon, TEVA, and Abbvie for attending medical conferences. Legarda I. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Hernández Vara J. has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi-Genzyme. Cabo I. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. López Manzanares L.: Compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon. González Aramburu I.: None. Ávila Rivera MA. has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences. Gómez Mayordomo V.: None. Nogueira V.: None. Puente V. has served as consultant for Abbvie and Zambon; has received grant/research from Abbvie. Dotor García-Soto J.: Compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi-Genzyme, Allergan, Biogen, Roche, UCB, and Novartis. Borrué C.: None. Solano Vila B. has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, Bial. Álvarez Sauco M. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Vela L. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Escalante S. has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial. Cubo E.; travel grants from Abbvie, Allergan, and Boston; and lecturing honoraria from Abbvie, International Parkinson’s disease Movement Disorder Society. Carrillo Padilla F. has received honoraria from Zambon (SEN Congress assistance). Martínez Castrillo JC. has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie; he has also received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz. Sánchez Alonso P. has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Alonso Losada M. G. has received honoraria for educational presentations and advice service by Zambon and Bial. López Ariztegui N. has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial. Gastón I. has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon. Kulisevsky J.: (1) Consulting fees: Roche, Zambon; (2) Stock/allotment: No; (3) Patent royalties/licensing fees: No; (4) Honoraria (e.g., lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g., trips, travel, or gifts): No. Blázquez Estrada M. has received honoraria for educational presentations and advice service by Abbvie, Abbott, UCB Pharma, Allergan, Zambon, Bial, and Qualigen. Seijo M. has received honoraria for educational services from KRKA, UCB, Zambon, and Bial and travel grants from Daiichi and Roche. Ruiz Martínez J. has received honoraria for educational presentations, attending medical conferences, and advice service by Abbvie, UCB Pharma, Zambon, Italfarmaco, Bial, and Teva. Valero C. has received honoraria for educational services from Zambon, Abbvie and UCB. Kurtis M. has received honoraria from Bial, the Spanish Neurology Society, and the International and Movement Disorders Society. de Fábregues O. has received honoraria for educational presentations and advice service by Bial, Zambon, Abbvie, KRKA, and Teva. González Ardura J. has received honoraria for speaking from italofarma, Krka, Genzyme, UCB, Esteve, Psyma iberica marketing research SL, and Ferrer; a course grant from Teva; and travel grant from Merck. Alonso Redondo R.: None. Ordás C.: None. López Díaz L. M. has received honoraria from UCB, Lundbeck, and KRKA. McAfee D.: None. Martínez-Martin P. has received honoraria from National School of Public Health (ISCIII), Editori-al Viguera and Takeda Pharmaceuticals for lecturing in courses, and from the International Parkinson and Movement Disorder Society (MDS) for management of the Program on Rating Scales. Mir P. has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and has received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] cofounded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [ PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, and the Fundación Mutua Madrileña.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ap-proved by Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 2/DEC/2014).
Informed Consent Statement
Written informed consent from all participants in this study were obtained before the start of the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. No computer coding was used in the completion of the current manuscript.
Acknowledgments
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación DEGEN (https://fundaciondegen.org/ accessed on 15 April 2022), Alpha Bioresearch (www.alphabioresearch.com accessed on 15 April 2022), and other institutions helping us.
Conflicts of Interest
The authors have no conflict of interest to report.
Abbreviations
ADLS, Schwab and England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; EUROHIS-QOL8, EUROHIS-QOL 8-item index; FOG-Q, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent daily dose; MF, motor fluctuations; NMF, non-motor fluctuations; NMS, non-motor symptoms; NMSS, Non-motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD, Parkinson’s disease; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39SI, 39-item Parkinson’s Disease Quality of Life Questionnaire Summary Index; PDSS, Parkinson’s Disease Sleep Scale; QoL, quality of life; QUIP-RS, Questionnaire for Impulsive–Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS–Pain, Visual Analog Scale–Pain.
Appendix A
Coppadis Study Group: Adarmes AD, Almeria M, Alonso Losada MG, Alonso Cánovas A, Alonso Frech F, Alonso Redondo R, Álvarez I, Álvarez Sauco M, Aneiros Díaz A, Arnáiz S, Arribas S, Ascunce Vidondo A, Aguilar M, Ávila MA, Bernardo Lambrich N, Bejr-Kasem H, Blázquez Estrada M, Botí M, Borrue C, Buongiorno MT, Cabello González C, Cabo López I, Caballol N, Cámara Lorenzo A, Canfield Medina H, Carrillo F, Carrillo Padilla FJ, Casas E, Catalán MJ, Clavero P, Cortina Fernández A, Cosgaya M, Cots Foraster A, Crespo Cuevas A, Cubo E, de Deus Fonticoba T, de Fábregues-Boixar O, Díez-Fairen M, Dotor García-Soto J, Erro E, Escalante S, Estelrich Peyret E, Fernández Guillán N, Gámez P, Gallego M, García Caldentey J, García Campos C, García Moreno JM, Gastón I, Gómez Garre MP, Gómez Mayordomo V, González Aloy J, González-Aramburu I, González Ardura J, González García B, González Palmás MJ, González Toledo GR, Golpe Díaz A, Grau Solá M, Guardia G, Hernández Vara J, Horta-Barba A, Idoate Calderón D, Infante J, Jesús S, Kulisevsky J, Kurtis M, Labandeira C, Labrador MA, Lacruz F, Lage Castro M, Lastres Gómez S, Legarda I, López Ariztegui N, López Díaz LM, López Domínguez D, López Manzanares L, López Seoane B, Lucas del Pozo S, Macías Y, Mata M, Martí Andres G, Martí MJ, Martínez Castrillo JC, Martinez-Martin P, McAfee D, Meitín MT, Mendoza Plasencia Z, Menéndez González M, Méndez del Barrio C, Mir P, Miranda Santiago J, Morales Casado MI, Moreno Diéguez A, Nogueira V, Novo Amado A, Novo Ponte S, Ordás C, Pagonabarraga J, Pareés I, Pascual-Sedano B, Pastor P, Pérez Fuertes A, Pérez Noguera R, Planas-Ballvé A, Planellas L, Prats MA, Prieto Jurczynska C, Puente V, Pueyo Morlans M, Puig Daví A, Redondo Rafales N, Rodríguez Méndez L, Rodríguez Pérez AB, Roldán F, Ruíz De Arcos M, Ruíz Martínez J, Sánchez Alonso P, Sánchez-Carpintero M, Sánchez Díez G, Sánchez Rodríguez A, Santacruz P, Santos García D, Segundo Rodríguez JC, Seijo M, Sierra Peña M, Solano Vila B, Suárez Castro E, Tartari JP, Valero C, Vargas L, Vela L, Villanueva C, Vives B.
| Name (Last Name, First Name) | Location | Role | Contribution |
| Astrid Adarmes, Daniela | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Almeria, Marta | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Alonso Losada, Maria Gema | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Alonso Cánovas, Araceli | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Frech, Fernando | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Redondo, Ruben | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Aneiros Díaz, Ángel | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Álvarez, Ignacio | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Álvarez Sauco, María | Hospital General Universitario de Elche, Elche, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Arnáiz, Sandra | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Arribas, Sonia | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Ascunce Vidondo, Arancha | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Aguilar, Miquel | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Ávila Rivera, Maria Asunción | Consorci Sanitari Integral, Hospital General de L’Hospitalet, L’Hospitalet de Llobregat, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Bernardo Lambrich, Noemí | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator | Evaluation of participants and/or data management |
| Bejr-Kasem, Helena | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Blázquez Estrada, Marta | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator | Evaluation of participants and/or data management |
| Botí González, Maria Ángeles | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Borrué, Carmen | Hospital Infanta Sofía, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Buongiorno, Maria Teresa | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Cabello González, Carolina | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Scheduling of evaluations |
| Cabo López, Iria | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Caballol, Nuria | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain. | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Cámara Lorenzo, Ana | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Canfield Medina, Héctor | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo, Fátima | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo Padilla, Francisco José | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Casas, Elena | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Catalán, Maria José | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Clavero, Pedro | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cortina Fernández, A | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Coordination of blood extractions |
| Cosgaya, Marina | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cots Foraster, Anna | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Crespo Cuevas, Ane | Hospital del Mar, Barcelona, Spain. | Site investigator | Evaluation of participants and/or data management |
| Cubo, Esther | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| De Deus Fonticoba, Teresa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Nurse study coordinator Evaluation of participants and/or data management |
| De Fábregues-Boixar, Oriol | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Díez Fairen, M | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Dotor García-Soto, Julio | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| Erro, Elena | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Escalante, Sonia | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Estelrich Peyret, Elena | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Fernández Guillán, Noelia | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Gámez, Pedro | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Gallego, Mercedes | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| García Caldentey, Juan | Centro Neurológico Oms 42, Palma de Mallorca, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| García Campos, Cristina | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| García Moreno, Jose Manuel | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI (until MAR/21) | Coordination at the center Evaluation of participants and/or data management |
| Gastón, Itziar | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Gómez Garre, María del Pilar | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Genetic studies coordination |
| Gómez Mayordomo, Víctor | Hospital Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aloy, Javier | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aramburu, Isabel | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| González Ardura, Jessica | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI (until FEB/21) | Evaluation of participants and/or data management |
| González García, Beatriz | Hospital La Princesa, Madrid, Spain | Site investigator | Nurse study coordinator |
| González Palmás, Maria Josefa | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| González Toledo, Gabriel Ricardo | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Golpe Díaz, Ana | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Laboratory analysis coordination |
| Grau Solá, Mireia | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Guardia, Gemma | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Hernández Vara, Jorge | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Horta Barba, Andrea | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Idoate Calderón, Daniel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigaor | neuropsychologist; evaluation of participants |
| Infante, Jon | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Jesús, Silvia | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Kulisevsky, Jaime | Hospital de Sant Pau, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Kurtis, Mónica | Hospital Ruber Internacional, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Labandeira, Carmen | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator | Evaluation of participants and/or data management |
| Labrador Espinosa, Miguel Ángel | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging data analysis |
| Lacruz, Francisco | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Lage Castro, Melva | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| Lastres Gómez, Sonia | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Legarda, Inés | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| López Ariztegui, Nuria | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| López Díaz, Luis Manuel | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| López Domínguez, Daniel | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| López Manzanares, Lydia | Hospital La Princesa, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| López Seoane, Balbino | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Lucas del Pozo, Sara | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Macías, Yolanda | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Mata, Marina | Hospital Infanta Sofía, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí Andres, Gloria | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí, Maria José | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Martínez Castrillo, Juan Carlos | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Martinez-Martin, Pablo | Centro Nacional de Epidemiología y CIBERNED, Instituto de Salud Carlos III. Madrid | Collaborator in statistical and methods analysis | Methods and statistical reviewer |
| McAfee, Darrian | University of Pennsylvania, Philadelphia | Collaborator in English style | English style reviewer |
| Meitín, Maria Teresa | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| Menéndez González, Manuel | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Méndez del Barrio, Carlota | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Mendoza Plasencia, Zebenzui | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Mir, Pablo | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Miranda Santiago, Javier | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Morales Casado, Maria Isabel | Complejo Hospitalario de Toledo, Toledo, Spain. | Site investigator | Evaluation of participants and/or data management |
| Moreno Diéguez, Antonio | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Nogueira, Víctor | Hospital Da Costa de Burela, Lugo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Novo Amado, Alba | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Novo Ponte, Sabela | Hospital Universitario Puerta de Hierro, Madrid, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ordás, Carlos | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain. | Site investigator | Evaluation of participants and/or data management |
| Pagonabarraga, Javier | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pareés, Isabel | Hospital Ruber Internacional, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Pascual-Sedano, Berta | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pastor, Pau | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pérez Fuertes, Aída | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Pérez Noguera, Rafael | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Planas-Ballvé, Ana | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Planellas, Lluís | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator (until DEC/19) | Evaluation of participants and/or data management |
| Prats, Marian Ángeles | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prieto Jurczynska, Cristina | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Puente, Víctor | Hospital del Mar, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Pueyo Morlans, Mercedes | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Puig Daví, Arnau | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Redondo, Nuria | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Rodríguez Méndez, Luisa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Rodríguez Pérez, Amparo Belén | Hospital General Universitario de Elche, Elche, Spain | Site investigator | Evaluation of participants and/or data management |
| Roldán, Florinda | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging studies |
| Ruíz de Arcos, María | Hospital Universitario Virgen Macarena, Sevilla, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ruíz Martínez, Javier | Hospital Universitario Donostia, San Sebastián, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Alonso, Pilar | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez-Carpintero, Macarena | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Sánchez Díez, Gema | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Rodríguez, Antonio | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Santacruz, Pilar | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Santos García, Diego | CHUAC, Complejo Hospitalario Universitario de A Coruña | Coordinator of the Project | Coordination of the COPPADIS-2015 |
| Segundo Rodríguez, José Clemente | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Seijo, Manuel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Sierra, María | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Solano, Berta | Institut d’Assistència Sanitària (IAS)-Instituí Cátala de la Salud. Girona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Suárez Castro, Ester | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Tartari, Juan Pablo | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Valero, Caridad | Hospital Arnau de Vilanova, Valencia, Spain | Site investigator | Evaluation of participants and/or data management |
| Vargas, Laura | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Vela, Lydia | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Villanueva, Clara | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Vives, Bárbara | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator | Evaluation of participants and/or data management |
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Schapira, A.; Chaudhuri, K.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Lloret, S.P.; Weintraub, D.; Sampaio, C.; the Collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for non-motor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Mattay, V.; Tessitore, A.; Callicott, J.; Bertolino, A.; Goldberg, T.E.; Chase, T.N.; Hyde, T.M.; Weinberger, D.R. Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann. Neurol. 2002, 51, 156–164. [Google Scholar] [CrossRef]
- Barone, P.; Scarzella, L.; Marconi, R.; Antonini, A.; Morgante, L.; Bracco, F.; Zappia, M.; Musch, B.; the Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease. A national multicenter parallel group randomized study. J. Neurol. 2006, 253, 601–607. [Google Scholar] [CrossRef]
- Fernández-Pajarín, G.; Sesar, Á.; Jiménez Martín, I.; Ares, B.; Castro, A. Continuous subcutaneous apomorphine infusion in the early phase of advanced Parkinson’s disease: A prospective study of 22 patients. Clin. Park. Relat. Disord. 2021, 6, 100129. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Kies, B.; Rudzinska, M.; Fine, J.; Nikl, J.; Honczarenko, K.; Dioszeghy, P.; Hill, D.; Anderson, T.; Myllyla, V.; et al. Recover Study Group. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov. Disord. 2011, 26, 90–99. [Google Scholar] [CrossRef]
- Martinez-Castrillo, J.C.; Vela, L.; del Val, J.; Alonso-Canovas, A. Non-motor disorders and their correlation with dopamine: Can they be treated by currently available methods? Neurologist 2011, 17 (Suppl. 1), S9–S17. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Schmitt, E.; Martinez-Martin, P.; Krack, P. The hidden sister of motor fluctuations in Parkinson’s disease: A review on non-motor fluctuations. Mov. Disord. 2016, 31, 1080–1094. [Google Scholar] [CrossRef]
- Witjas, T.; Kaphan, E.; Azulay, J.P.; Blin, O.; Ceccaldi, M.; Pouget, J.; Poncet, M.; Chérif, A.A. Non-motor fluctuations in Parkinson’s disease: Frequent and disabling. Neurology 2002, 59, 408–413. [Google Scholar] [CrossRef]
- Honig, H.; Antonini, A.; Martinez-Martin, P.; Forgacs, I.; Faye, G.C.; Fox, T.; Fox, K.; Mancini, F.; Canesi, M.; Odin, P.; et al. Intrajejunal levodopa infusion in Parkinson’s disease: A pilot multicenter study of effects on non-motor symptoms and quality of life. Mov. Disord. 2009, 24, 1468–1474. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Reddy, P.; Katzenschlager, R.; Antonini, A.; Todorova, A.; Odin, P.; Henriksen, T.; Martin, A.; Calandrella, D.; Rizos, A.; et al. EuroInf: A multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov. Disord. 2015, 30, 510–516. [Google Scholar] [CrossRef]
- Santos García, D.; Labandeira Guerra, C.; Yáñez Baña, R.; Cimas Hernando, M.I.; Cabo López, I.; Paz Gonález, J.M.; Alonso Losada, M.G.; González Palmás, M.J.; Martínez Miró, C. Safinamide Improves Non-Motor Symptoms Burden in Parkinson’s Disease: An Open-Label Prospective Study. Brain Sci. 2021, 11, 316. [Google Scholar] [CrossRef]
- Santos García, D.; Fernández-Pajarín, G.; Oropesa Ruíz, J.M.; Escamilla Sevilla, F.; Rahim López, R.R.A.; Muñoz Enríquez, J.G. Opicapone Improves Global Non-Motor Symptoms Burden in Parkinson´s Disease: An Open-label Prospective Study. Brain Sci. 2022, 12, 383. [Google Scholar] [CrossRef]
- Santos-García, D.; de Deus Fonticoba, T.; Suárez Castro, E.; Díaz, A.A.; McAfee, D.; Catalán, M.J.; Alonso-Frech, F.; Villanueva, C.; Jesús, S.; Mir, P.; et al. Non-motor symptom burden is strongly correlated to motor complications in patients with Parkinson’s disease. Eur. J. Neurol. 2020, 27, 1210–1223. [Google Scholar] [CrossRef]
- Santos García, D.; De Deus Fonticoba, T.; Paz González, J.M.; Bartolomé, C.C.; Aymerich, L.V.; Enríquez, J.G.M.; Suárez, E.; Jesús, S.; Aguilar, M.; Pastor, P.; et al. Staging Parkinson’s Disease Combining Motor and Non-motor Symptoms Correlates with Disability and Quality of Life. Parkinsons Dis. 2021, 2021, 8871549. [Google Scholar]
- Santos García, D.; de Deus Fonticoba, T.; Cores, C.; Muñoz, G.; Paz González, J.M.; Martínez Miró, C.; Suárez, E.; Jesús, S.; Aguilar, M.; Pastor, P.; et al. Predictors of clinically significant quality of life impairment in Parkinson’s disease. NPJ Parkinsons Dis. 2021, 7, 118. [Google Scholar] [CrossRef]
- Malaty, I.A.; Martinez-Martin, P.; Chaudhuri, K.R.; Odin, P.; Skorvanek, M.; Jimenez-Shahed, J.; Soileau, M.J.; Lindvall, S.; Domingos, J.; Jones, S.; et al. Does the 5-2-1 criteria identify patients with advanced Parkinson’s disease? Real-world screening accuracy and burden of 5-2-1-positive patients in 7 countries. BMC Neurol. 2022, 22, 35. [Google Scholar] [CrossRef]
- Santos García, D.; Jesús, S.; Aguilar, M.; Planellas, L.L.; Caldentey, J.G.; Caballol, N.; Legarda, I.; Hernández Vara, J.; Cabo, I.; Manzanares, L.L.; et al. COPPADIS-2015 (COhort of Patients with Parkinson’s DIsease in Spain, 2015): An ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur. J. Neurol. 2019, 26, 1399–1407. [Google Scholar] [CrossRef]
- Santos-García, D.; Mir, P.; Cubo, E.; Vela, L.; Rodríguez-Oroz, M.C.; Martí, M.J.; Arbelo, J.M.; Infante, J.; Kulisevsky, J.; Martínez-Martín, P.; et al. COPPADIS-2015 (COhort of Patients with Parkinson’s DIsease in Spain, 2015), a global--clinical evaluations, serum biomarkers, genetic studies and neuroimaging--prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 2016, 16, 26. [Google Scholar]
- Fahn, S.; Elton, R.L.; Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Calne, D.B., Goldstein, M., Eds.; Macmillan Health Care Information: Florham Park, NJ, USA, 1987; Volume 2, pp. 153–164. [Google Scholar]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; MacPhee, G.; MacMahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Kurtis, M.M.; Chaudhuri, K.R.; NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 2011, 26, 399–406. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson´s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson´s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Da Rocha, N.S.; Power, M.J.; Bushnell, D.M.; Fleck, M.P. The EUROHIS-QOL 8-item index: Comparative psychometric properties to its parent WHOQOL-BREF. Value Health 2012, 15, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of non-motor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Abe, K.; Bhattacharyya, K.B.; Bloem, B.R.; Carod-Artal, F.J.; Prakash, R.; Esselink, R.A.J.; Falup-Pecurariu, C.; Gallardo, M.; et al. International study on the psychometric attributes of the non-motor symptoms scale in Parkinson disease. Neurology 2009, 73, 1584–1591. [Google Scholar] [CrossRef]
- Hauser, R.A.; McDermott, M.P.; Messing, S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch. Neurol. 2006, 63, 1756–1760. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef]
- Barrachina-Fernández, M.; Maitín, A.M.; Sánchez-Ávila, C.; Romero, J.P. Wearable Technology to Detect Motor Fluctuations in Parkinson’s Disease Patients: Current State and Challenges. Sensors 2021, 21, 4188. [Google Scholar] [CrossRef]
- Stocchi, F.; Antonini, A.; Barone, P.; Tinazzi, M.; Zappia, M.; Onofrj, M.; Ruggieri, S.; Morgante, L.; Bonuccelli, U.; Lopiano, L.; et al. Early DEtection of wEaring off in Parkinson disease: The DEEP study. Parkinsonism Relat. Disord. 2014, 20, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Fox, S.H.; Katzenschlager, R.; Lim, S.Y.; Barton, B.; De Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C.; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Antonini, A.; Barone, P.; Marconi, R.; Morgante, L.; Zappulla, S.; Pontieri, F.E.; Ramat, S.; Ceravolo, M.G.; Meco, G.; Cicarelli, G.; et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. J. Neurol. 2012, 259, 2621–2631. [Google Scholar] [CrossRef]
- Todorova, A.; Jenner, P.; Ray Chaudhuri, K. Non-motor parkinson’s: Integral to motor parkinson’s, yet often neglected. Pract. Neurol. 2014, 14, 310–322. [Google Scholar] [CrossRef]
- Fernandes, M.; Pierantozzi, M.; Stefani, A.; Cattaneo, C.; Bonizzoni, E.A.; Cerroni, R.; Mercuri, N.B.; Liguori, C. Frequency of Non-motor Symptoms in Parkinson’s Patients With Motor Fluctuations. Front. Neurol. 2021, 12, 678373. [Google Scholar] [CrossRef]
- Watanabe, H.; Saiki, H.; Chiu, S.W.; Yamaguchi, T.; Kashihara, K.; Tsuboi, Y.; Nomoto, M.; Hattori, N.; Maeda, T.; Shimo, Y.; et al. Real-World Non-motor Changes I Patients with Parkinson’s Disease and Motor Fluctuations: J-FIRST. Mov. Disord. Clin. Pract. 2020, 7, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Santos-García, D.; de Deus, T.; Cores, C.; Canfield, H.; Paz González, J.M.; Martínez Miró, C.; Valdés Aymerich, L.; Suárez, E.; Jesús, S.; Aguilar, M.; et al. Predictors of Global Non-Motor Symptoms Burden Progression in Parkinson’s Disease. Results from the COPPADIS Cohort at 2-Year Follow-Up. J. Pers. Med. 2021, 11, 626. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schrag, A.; Weintraub, D.; Rizos, A.; Rodriguez-Blazquez, C.; Mamikonyan, E.; Martinez-Martin, P. The movement disorder society non-motor rating scale: Initial validation study. Mov. Disord. 2020, 35, 116–133. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, R.S.; Weng, Y.H.; Huang, Y.-Z.; Chen, C.C.; Hung, J.; Lin, Y.-Y. The severity progression of non-motor symptoms in Parkinson’s disease: A 6-year longitudinal study in Taiwanese patients. Sci. Rep. 2021, 11, 14781. [Google Scholar] [CrossRef]
- Ou, R.; Yang, J.; Cao, B.; Wei, Q.; Chen, K.; Chen, X.; Zhao, B.; Wu, Y.; Song, W.; Shang, H. Progression of non-motor symptoms in Parkinson’s disease among different age populations: A two-year follow-up study. J. Neurol. Sci. 2016, 360, 72–77. [Google Scholar] [CrossRef]
- Cilia, R.; Akpalu, A.; Sarfo, F.S.; Cham, M.; Amboni, M.; Cereda, E.; Fabbri, M.; Adjei, P.; Akassi, J.; Bonetti, A.; et al. The modern pre-levodopa era of Parkinson’s disease: Insights into motor complications from sub-Saharan Africa. Brain 2014, 137 Pt 10, 2731–2742. [Google Scholar] [CrossRef] [Green Version]
- Simuni, T.; Caspell-Garcia, C.; Coffey, C.S.; Weintraub, D.; Mollenhauer, B.; Lasch, S.; Tanner, C.M.; Jennings, D.; Kieburtz, K.; Chahine, L.M.; et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: The PPMI cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Bugalho, P.; Ladeira, F.; Barbosa, R.; Marto, J.P.; Borbinha, C.; Da Conceição, L.; Salavisa, M.; Saraiva, M.; Meira, B.; Fernandes, M. Progression in Parkinson’s Disease: Variation in Motor and Non-motor Symptoms Severity and Predictors of Decline in Cognition, Motor Function, Disability, and Health-Related Quality of Life as Assessed by Two Different Methods. Mov. Disord. Clin. Pract. 2021, 8, 885–895. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).