Poor Performance of Angiotensin II Enzyme-Linked Immuno-Sorbent Assays in Mostly Hypertensive Cohort Routinely Screened for Primary Aldosteronism
Abstract
:1. Introduction
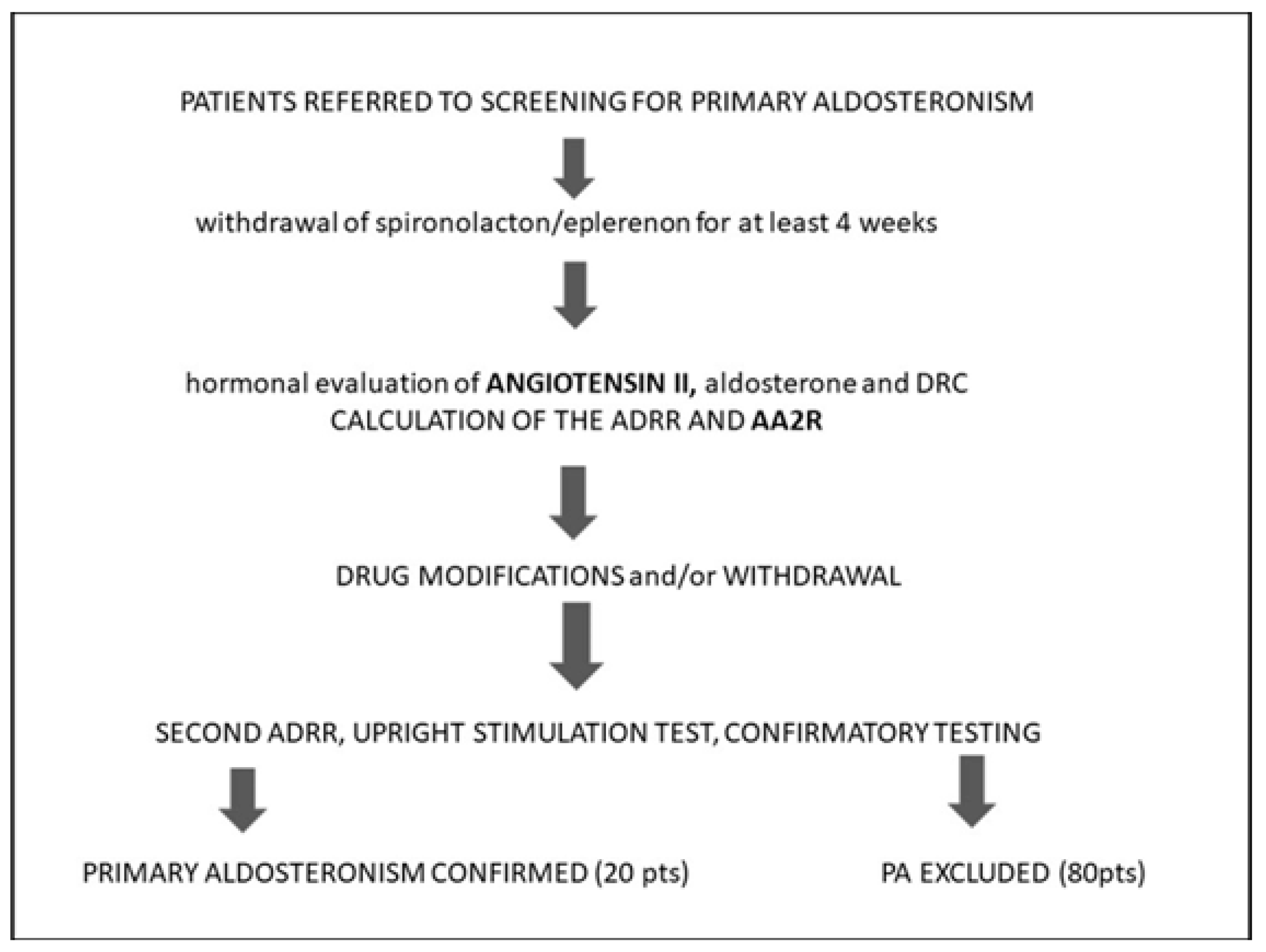
2. Materials and Methods
2.1. Participants
2.2. Hormonal and Biochemical Testing
2.3. Analytical Details
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
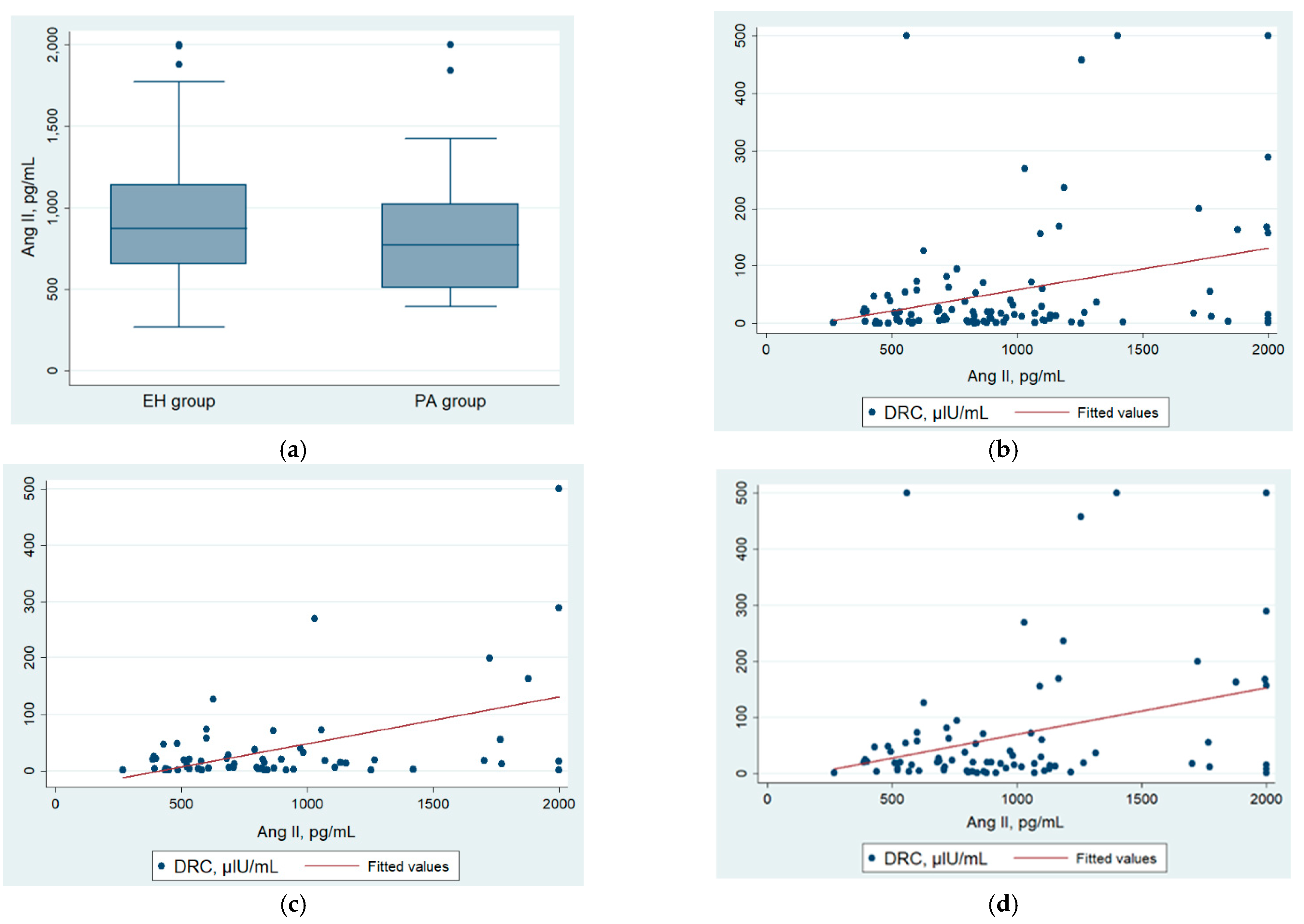
3.2. Angiotensin II, AA2R, and Other Hormonal Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Gronewold, J.; Kropp, R.; Lehmann, N.; Stang, A.; A Mahabadi, A.; Weimar, C.; Dichgans, M.; Moebus, S.; Kröger, K.; Hoffmann, B.; et al. Population impact of different hypertension management guidelines based on the prospective population-based Heinz Nixdorf Recall study. BMJ Open 2021, 11, e039597. [Google Scholar] [CrossRef]
- Young, W.F.; Calhoun, D.A.; Lenders, J.W.; Stowasser, M.; Textor, S.C. Screening for Endocrine Hypertension: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 103–122. [Google Scholar] [CrossRef] [Green Version]
- Mulatero, P.; Monticone, S.; Burrello, J.; Veglio, F.; Williams, T.A.; Funder, J. Guidelines for primary aldosteronism: Uptake by primary care physicians in Europe. J. Hypertens. 2016, 34, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Shen, J.; Fuller, P.; Yang, J. Current pattern of primary aldosteronism diagnosis: Delayed and complicated. Aust. J. Gen. Pr. 2018, 47, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.; Beuschlein, F.; Bidlingmaier, M.; Reincke, M. Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev. Endocr. Metab. Disord. 2011, 12, 43–48. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.J. Hypertension: Renin–Angiotensin–Aldosterone System Alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Vaidya, A.; Mulatero, P.; Baudrand, R.; Adler, G.K. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr. Rev. 2018, 39, 1057–1088. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Vaidya, A.; Carey, R.M. Evolution of the Primary Aldosteronism Syndrome: Updating the Approach. J. Clin. Endocrinol. Metab. 2020, 105, 3771–3783. [Google Scholar] [CrossRef]
- Stowasser, M.; Ahmed, A.; Guo, Z.; Wolley, M.; Ungerer, J.; McWhinney, B.; Poglitsch, M.; Gordon, R. Can Screening and Confirmatory Testing in the Management of Patients with Primary Aldosteronism be Improved? Horm. Metab. Res. 2017, 49, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Yugandhar, V.G.; Clark, M.A. Angiotensin III: A physiological relevant peptide of the renin angiotensin system. Peptides 2013, 46, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Vanderriele, P.-E.; Caroccia, B.; Seccia, T.M.; Piazza, M.; Lenzini, L.; Torresan, F.; Iacobone, M.; Unger, T.; Rossi, G.P. The angiotensin type 2 receptor in the human adrenocortical zona glomerulosa and in aldosterone-producing adenoma: Low expression and no functional role. Clin. Sci. Lond. 2018, 132, 627–640. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Passino, C.; Clerico, A. Evaluation of pathophysiological relationships between renin-angiotensin and ACE-ACE2 systems in cardiovascular disorders: From theory to routine clinical practice in patients with heart failure. Crit. Rev. Clin. Lab. Sci. 2021, 58, 1–16. [Google Scholar] [CrossRef]
- Magness, R.R.; Cox, K.; Rosenfeld, C.R.; Gant, N.F. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am. J. Obstet. Gynecol. 1994, 171, 668–679. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Poglitsch, M.; McWhinney, B.C.; Ungerer, J.P.J.; Ahmed, A.H.; Gordon, R.D.; Wolley, M.; Stowasser, M. Measurement of Equilibrium Angiotensin II in the Diagnosis of Primary Aldosteronism. Clin. Chem. 2020, 66, 483–492. [Google Scholar] [CrossRef]
- Guo, Z.; Poglitsch, M.; Cowley, D.; Domenig, O.; McWhinney, B.C.; Ungerer, J.P.; Wolley, M.; Stowasser, M. Effects of Ramipril on the Aldosterone/Renin Ratio and the Aldosterone/Angiotensin II Ratio in Patients with Primary Aldosteronism. Hypertension 2020, 76, 488–496. [Google Scholar] [CrossRef]
- Vischer, A.; Kuster, G.; Twerenbold, R.; Pfister, O.; Zhou, Q.; Villiger, A.; Poglitsch, M.; Krähenbühl, S.; Mayr, M.; Osswald, S.; et al. Influence of Antihypertensive Treatment on RAAS Peptides in Newly Diagnosed Hypertensive Patients. Cells 2021, 10, 534. [Google Scholar] [CrossRef]
- Van Rooyen, J.; Poglitsch, M.; Huisman, H.; Mels, C.; Kruger, R.; Malan, L.; Botha, S.; Lammertyn, L.; Gafane, L.; Schutte, A. Quantification of systemic renin-angiotensin system peptides of hypertensive black and white African men established from the RAS-Fingerprint®. J. Renin-Angiotensin-Aldosterone Syst. 2016, 17, 1470320316669880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rooyen, J.M.; Poglitsch, M.; Huisman, H.W.; Gafane-Matemane, L.; Breet, Y.; Malan, L. A primary aldosteronism-like phenotype identified with the aldosterone-to-angiotensin II ratio in black men: The SABPA study. Cardiovasc. J. Afr. 2020, 31, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, J.M.; Poglitsch, M.; Mels, C.M.C.; Huisman, H.W.; Gafane-Matemane, L.F.; Le Roux, S.; Lammertyn, L.; Breet, Y.; Uys, L.; Schutte, A.E. Aldosterone and angiotensin II profiles in young black and white women using different hormonal contraceptives: The African-PREDICT study. J. Hum. Hypertens. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pavo, N.; Wurm, R.; Goliasch, G.; Novak, J.F.; Strunk, G.; Gyöngyösi, M.; Poglitsch, M.; Säemann, M.D.; Hülsmann, M. Renin-Angiotensin System Fingerprints of Heart Failure with Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2016, 68, 2912–2914. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Poglitsch, M.; Yogasundaram, H.; Thomas, J.; Rowe, B.H.; Oudit, G.Y. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 805–819. [Google Scholar] [CrossRef]
- Miziołek, B.; Bergler-Czop, B.; Kucharz, E.; Kotyla, P.; Kopeć-Mędrek, M.; Widuchowska, M.; Sieńczyk, M.; Brzezińska-Wcisło, L. Significance of the angiotensin I/angiotensin II /angiotensin-(1-7) axis in the pathogenesis of systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 558–564. [Google Scholar] [CrossRef]
- Osman, I.O.; Melenotte, C.; Brouqui, P.; Million, M.; Lagier, J.-C.; Parola, P.; Stein, A.; La Scola, B.; Meddeb, L.; Mege, J.-L.; et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. 2021, 12, 625732. [Google Scholar] [CrossRef]
- Pucci, F.; Annoni, F.; dos Santos, R.A.S.; Taccone, F.S.; Rooman, M. Quantifying Renin-Angiotensin-System Alterations in COVID-19. Cells 2021, 10, 2755. [Google Scholar] [CrossRef]
- Łebek-Szatańska, A.; Papierska, L.; Glinicki, P.; Zgliczyński, W. Withdrawal of all medications is not necessary for accurate primary aldosteronism: Preliminary results. Pol. Arch. Intern. Med. 2021, 131, 578–581. [Google Scholar] [CrossRef]
- Stowasser, M.; Ahmed, A.H.; Cowley, D.; Wolley, M.; Guo, Z.; McWhinney, B.C.; Ungerer, J.P.; Gordon, R.D. Comparison of Seated with Recumbent Saline Suppression Testing for the Diagnosis of Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2018, 103, 4113–4124. [Google Scholar] [CrossRef] [Green Version]
- Funder, J.W. Primary Aldosteronism. Hypertension 2020, 76, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol. Circ. Physiol. 2016, 310, H137–H152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnihan, K.B.; Chappell, M.C. Measurement of Angiotensin Peptides: HPLC-RIA. Methods Mol. Biol. 2017, 1527, 81–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, A.-L.; Zeng, Z.-P.; Tong, A.-L.; Lu, L.; Chen, S.; Li, M.; Fu, C.-L.; Wang, Y.-H.; Sun, M.-L. Differences of blood plasma renin activity, angiotensin II and aldosterone levels in essential or secondary hypertension. Zhonghua Nei Ke Za Zhi 2012, 51, 294–298. [Google Scholar] [PubMed]
- Burrello, J.; Monticone, S.; Buffolo, F.; Lucchiari, M.; Tetti, M.; Rabbia, F.; Mengozzi, G.; Williams, T.A.; Veglio, F.; Mulatero, P. Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J. Hypertens. 2016, 34, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C.; Pirro, N.T.; South, A.M.; Gwathmey, T.M. Concerns on the Specificity of Commercial ELISAs for the Measurement of Angiotensin (1–7) and Angiotensin II in Human Plasma. Hypertension 2021, 77, e29–e31. [Google Scholar] [CrossRef] [PubMed]
- Pignone, A.; Del Rosso, A.; Brosnihan, K.B.; Perfetto, F.; Livi, R.; Fiori, G.; Guiducci, S.; Cinelli, M.; Rogai, V.; Tempestini, A.; et al. Reduced circulating levels of angiotensin-(1–7) in systemic sclerosis: A new pathway in the dysregulation of endothelial-dependent vascular tone control. Ann. Rheum. Dis. 2007, 66, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Care Res. 2004, 50, 216–226. [Google Scholar] [CrossRef]
- Dai, S.; Ding, M.; Liang, N.; Li, Z.; Li, D.; Guan, L.; Liu, H. Associations of ACE I/D polymorphism with the levels of ACE, kallikrein, angiotensin II and interleukin-6 in STEMI patients. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, D.M.; Nunes-Silva, A.; Rocha, G.C.; Vaz, L.N.; de Faria, M.H.S.; Vieira, E.L.M.; Rocha, N.P.; e Silva, A.C.S. Two protocols of aerobic exercise modulate the counter-regulatory axis of the renin-angiotensin system. Heliyon 2020, 6, e03208. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-W.; Lu, L.-C.; Ching-Chang, C.; Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Lin, Y.-C.; Lin, C.-S. Imbalanced plasma ACE and ACE2 level in the uremic patients with cardiovascular diseases and its change during a single hemodialysis session. Ren. Fail. 2017, 39, 719–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaagib, N.; Sukkar, M.; Kardash, M. The Effects of Salt and Glucose Intake on Angiotensin II and Aldosterone in Obese and Nonobese Patients with Essential Hypertension. Int. J. Hypertens. 2020, 2020, e6017105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, J.; Créminon, C.; Frobert, Y.; Etienne, E.; Ezan, E.; Volland, H.; Pradelles, P. Two different approaches for developing immunometric assays of haptens. Clin. Chem. 1996, 42, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
| Variable | Study Population (n = 100) | Females (n = 64) | Males (n = 36) | p |
|---|---|---|---|---|
| Age (years) | 56 (40–63) | 58.5 (40–63) | 53 (43–62) | 0.78 |
| BMI (kg/m2) | 28.42 (±4.4) | 28.04 (±5) | 29.25 (±3.3) | 0.20 |
| Hypertension (n, %) | 95 (95) | 60 (93.7) | 35 (97.2) | 0.58 |
| Normotension (n, %) | 5 (5) | 4 (6.25) | 1 (2.8) | 0.58 |
| SBP (mmHg) | 144.95 (±21.6) | 144.22 (±23.5) | 146.25 (±17.9) | 0.65 |
| DBP (mmHg) | 89.75 (±12.6) | 88.98 (±12.3) | 91.11 (±13.2) | 0.42 |
| Hypokalemia (n, %) | 40 (40) | 24 (37.5) | 16 (44.4) | 0.24 |
| spontaneous | 24 (24) | 12 (18.8) | 12 (33.3) | |
| diuretic-induced | 16 (16) | 12 (18.8) | 4 (11.1) | |
| Serum sodium (mmol/L) | 141.43 (±2) | 141.49 (±2.2) | 141.31 (±1.8) | 0.67 |
| Serum potassium (mmol/L) | 4.2 (±0.4) | 4.23 (±0.4) | 4.14 (±0.4) | 0.53 |
| Serum creatinine (mg/dL) | 0.76 (0.69–0.9) | 0.71 (0.66–0.8) | 0.91 (0.76–1) | <0.01 |
| eGFR (mg/mL/1.73 m2) | 89.23 (79.8–90) | 88.39 (80.8–90) | 90 (77.8–90) | 0.99 |
| Angiotensin II (pg/mL) | 852 (600.3–1131.4) | 834.4 (543.9–1149.2) | 877.9 (713.3–1102.2) | 0.33 |
| Aldosterone (ng/dL) | 12.2 (8.7–18.8) | 11.3 (7–16) | 14.15 (10.6–20.1) | 0.02 |
| DRC (μIU/mL) | 16.13 (3.9–51) | 15.07 (3.5–26.5) | 27.85 (8–72.2) | 0.049 |
| Variable | Primary Aldosteronism (n = 20) | Essential Hypertension (n = 80) | p |
|---|---|---|---|
| Age (years) | 57.5 (46.5–61) | 55.5 (40–63) | 0.93 |
| Sex (n, %F) | 11 (55) | 53 (66.2) | 0.35 |
| BMI (kg/m2) | 28.42 (±5.3) | 28.42 (±4.6) | 0.997 |
| Hypokalemia (n, %) | 15 (75) | 25 (31.2) | <0.001 |
| Spontaneous hypokalemia (n, %) | 12 (60) | 12 (15) | <0.001 |
| Diuretic-induced hypokalemia (n, %) | 3 (15) | 13 (16.2) | 0.24 |
| Adrenal lesions (n, %) | 15 (75) | 50 (62.5) | 0.43 |
| Serum sodium (mmol/L) | 142.24 (±1.9) | 141.22 (±2) | 0.04 |
| Serum potassium (mmol/L) | 3.84 (±0.1) | 4.29 (±0.04) | <0.001 |
| Serum creatinine (mg/dL) | 0.77 (0.7–0.9) | 0.76 (0.69-0.9) | 0.46 |
| eGFR (mg/mL/1.73 m2) | 85.95 (78.8–90) | 90 (80.3–90) | 0.64 |
| Variable | Study Population (n = 95) | Primary Aldosteronism (n = 19) | Essential Hypertension (n = 76) | p |
|---|---|---|---|---|
| Duration of HTN (years) | 7 (2–15) | 10 (5–20) | 7 (2–13) | 0.07 |
| Age of HTN onset (years) | 43 (35–55) | 42 (38–48) | 44.5 (33–55) | 0.46 |
| Onset of HTN < 40 years of age (n, %) | 37 (38.9) | 7 (36.8) | 30 (39.5) | 0.83 |
| Fixed (vs. paroxysmal) HTN (n, %) | 82 (86.3) | 17 (89.5) | 65 (85.5) | 0.66 |
| Uncontrolled HTN (n, %) | 66 (69.5) | 17 (89.5) | 49 (64.5) | 0.049 |
| Resistant HTN (n, %) | 21 (22.1) | 3 (15.8) | 18 (23.7) | 0.55 |
| Mean SBP, mmHg | 146.53 (±20.9) | 148.95 (±17.2) | 145.13 (±22.1) | 0.48 |
| Mean DBP, mmHg | 90.53 (±12.3) | 91.05 (±13.7) | 90.39 (±12) | 0.84 |
| No treatment (n, %) | 11 (11.6) | 2 (10.5) | 9 (11.8) | 1.0 |
| No RAAS-interfering drugs—amlodipine only (n, %) | 13 (13.7) 7 (7.4) | 4 (21.1) 2 (10.5) | 9 (15.8) 5 (6.6) | 0.36 |
| Number of drugs | 2 (1–4) | 3 (1–4) | 2 (2–3) | 0.59 |
| Number of drugs in the time of screening | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.83 |
| Modifications of treatment—MRA withdrawal (n, %) | 34 (35.8) 17 (17.9) | 11 (57.9) 5 (26.3) | 23 (30.3) 12 (15.8) | 0.02 0.32 |
| Hormonal Test Results | Study Population (n = 100) | PA (n = 20) | EH Group (n = 80) | p |
|---|---|---|---|---|
| Screening test | ||||
| DRC (μIU/mL) | 16.13 (3.89–50.98) | 2.87 (0.66–5.76) | 20.85 (8.97–61.63) | <0.001 |
| Serum aldosterone (ng/dL) | 12.2 (8.67–18.75) | 18.75 (13.25–24.3) | 11.3 (7.27–16) | <0.001 |
| ADRR I (ng/dL/ µIU/mL) | 0.64 (0.22–2.62) | 5.08 (3.33–20.74) | 0.45 (0.2–1.12) | <0.001 |
| Plasma angiotensin II (pg/mL) | 852 (600–1131) | 773.5 (509.4–1025) | 873.2 (653.2–1142.5) | 0.23 |
| AA2R (ng/dL/pg/mL) | 0.015 (0.008–0.023) | 0.024 (0.016–0.032) | 0.012 (0.007–0.02) | <0.001 |
| After patients’ preparation | ||||
| DRC (µIU/mL) | 18.33 (4.84–53.98) | 2.53 (0.88–3.91) | 24.37 (12.77–71.5) | <0.001 |
| Serum aldosterone (ng/dL) | 13.95 (8.83–20.25) | 17 (14–25.15) | 12.15 (8.31–18.75) | 0.003 |
| ADRR II (ng/dL/ µIU/mL) | 0.69 (0.22–2.57) | 6.03 (3.98–19) | 0.48 (0.19–0.98) | <0.001 |
| Confirmatory testing | ||||
| Number of participants who underwent SSST (n, %) | 27 (27) | 20 (100) | 7 (8.7) | |
| Post -SSST aldosterone level (ng/dL) | 11.6 (5.9–14.8) | 14.35 (9.38–17.7) | 4.19 (3.4–5.61) | <0.001 |
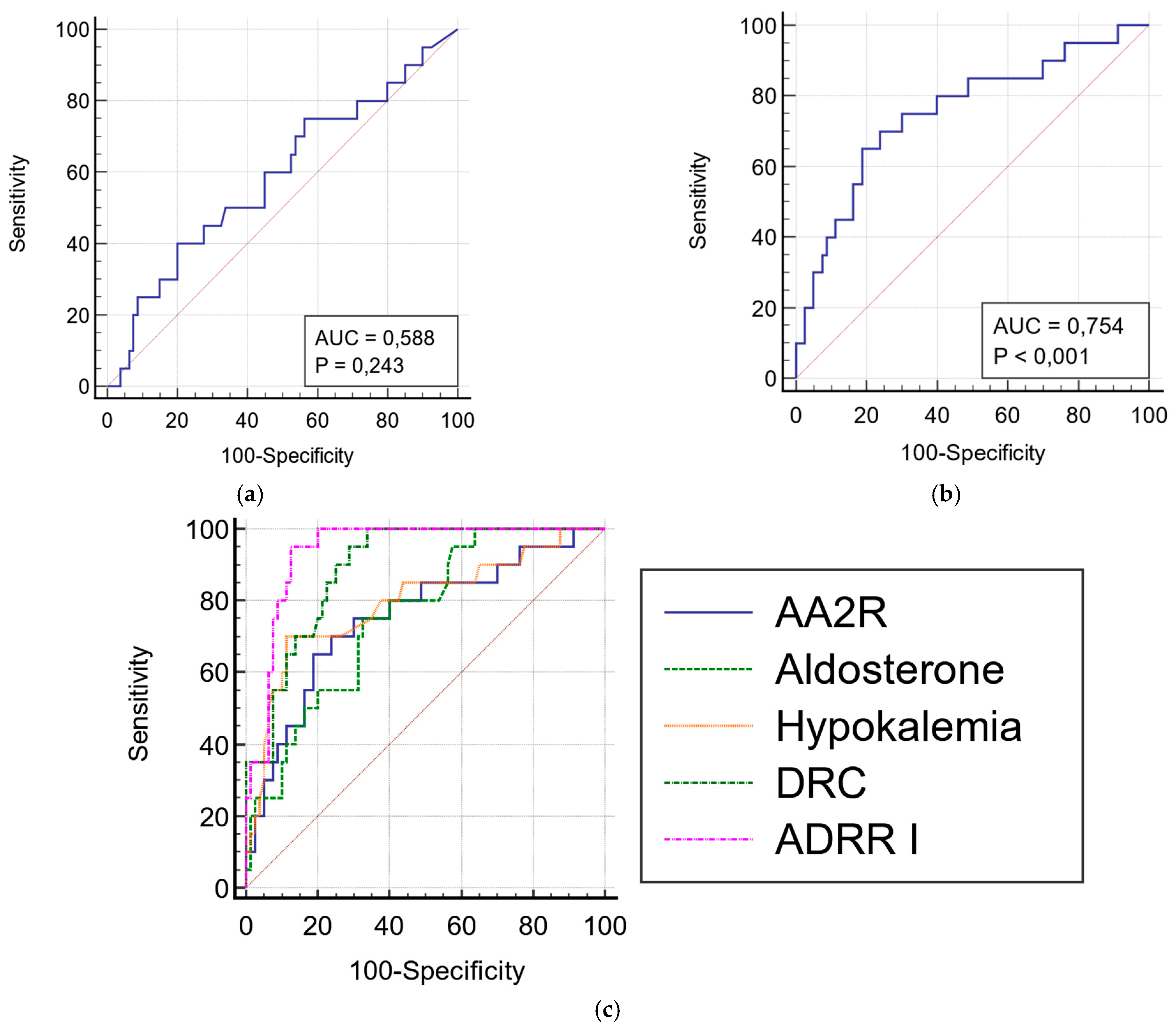
| Variable | Associated Criterion | Sensitivity, % | Specificity, % | AUROC | 95% CI * |
|---|---|---|---|---|---|
| AA2R | 0.02 ng/dL/pg/mL | 70 | 76.2 | 0.754 | 0.657–0.834 |
| Aldosterone | 14 ng/dL | 75 | 67.5 | 0.757 | 0.661–0.837 |
| Hypokalemia | 3.9 mmol/L | 88.7 | 70 | 0.794 | 0.702–0.868 |
| DRC | 10.5 µIU/mL | 95 | 71.2 | 0.892 | 0.814–0.945 |
| ADRR I | 2 ng/dL/µIU/mL | 95 | 87.5 | 0.939 | 0.873–0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łebek-Szatańska, A.; Papierska, L.; Glinicki, P.; Zgliczyński, W. Poor Performance of Angiotensin II Enzyme-Linked Immuno-Sorbent Assays in Mostly Hypertensive Cohort Routinely Screened for Primary Aldosteronism. Diagnostics 2022, 12, 1124. https://doi.org/10.3390/diagnostics12051124
Łebek-Szatańska A, Papierska L, Glinicki P, Zgliczyński W. Poor Performance of Angiotensin II Enzyme-Linked Immuno-Sorbent Assays in Mostly Hypertensive Cohort Routinely Screened for Primary Aldosteronism. Diagnostics. 2022; 12(5):1124. https://doi.org/10.3390/diagnostics12051124
Chicago/Turabian StyleŁebek-Szatańska, Agnieszka, Lucyna Papierska, Piotr Glinicki, and Wojciech Zgliczyński. 2022. "Poor Performance of Angiotensin II Enzyme-Linked Immuno-Sorbent Assays in Mostly Hypertensive Cohort Routinely Screened for Primary Aldosteronism" Diagnostics 12, no. 5: 1124. https://doi.org/10.3390/diagnostics12051124
APA StyleŁebek-Szatańska, A., Papierska, L., Glinicki, P., & Zgliczyński, W. (2022). Poor Performance of Angiotensin II Enzyme-Linked Immuno-Sorbent Assays in Mostly Hypertensive Cohort Routinely Screened for Primary Aldosteronism. Diagnostics, 12(5), 1124. https://doi.org/10.3390/diagnostics12051124






