Abstract
This study aimed to elucidate the clinicopathological significance of spread through air space (STAS) in non-small cell lung cancer (NSCLC) through a meta-analysis. Using 47 eligible studies, we obtained the estimated rates of STAS in various histological subtypes of NSCLC and compared the clinicopathological characteristics and prognosis between NSCLC with and without STAS. The estimated STAS rate was 0.368 (95% confidence interval [CI], 0.336–0.0.401) in patients with NSCLC. Furthermore, the STAS rates for squamous cell carcinoma and adenocarcinoma were 0.338 (95% CI, 0.273–0.411) and 0.374 (95% CI, 0.340–0.409), respectively. Among the histological subtypes of adenocarcinoma, micropapillary-predominant tumors had the highest rate of STAS (0.719; 95% CI, 0.652–0.778). The STAS rates of solid- and papillary-predominant adenocarcinoma were 0.567 (95% CI, 0.478–0.652) and 0.446 (95% CI, 0.392–0.501), respectively. NSCLCs with STAS showed a higher visceral pleural, venous, and lymphatic invasion than those without STAS. In addition, anaplastic lymphoma kinase mutations and ROS1 rearrangements were significantly more frequent in NSCLCs with STAS than in those without STAS. The presence of STAS was significantly correlated with worse overall and recurrence-free survival (hazard ratio, 2.119; 95% CI, 1.811–2.480 and 2.372; 95% CI, 2.018–2.788, respectively). Taken together, the presence of STAS is useful in predicting the clinicopathological significance and prognosis of patients with NSCLC.
1. Introduction
Lung cancer is one of the most common causes of cancer-related deaths worldwide [1]. In the recent treatment of lung cancer, histological classification, including molecular and biomarker profiles, is important due to the need to decide on systemic therapies [1]. Kadota et al. introduced “spread through airspace (STAS)” in lung tumors [2]. STAS is defined as the spread of lung cancer cells into the air spaces adjacent to the main tumor [2]. STAS should be distinguished from the artificial spreading features. For example, the discontinuity of spread in airspace from the tumor edge is ruled out as an artifact [2]. In addition, in daily practice, the differentiation of spreading tumor cells from normal, benign pneumocytes or bronchial cells can be difficult [2]. They described three morphological patterns (micropapillary structures, solid nests of tumor cells, and discohesive single cells) which are frequently identified in STAS of adenocarcinoma (ADC) [2]. STAS rates according to the histological subtypes of ADC can be different. Non-small cell lung cancer (NSCLC) includes ADC, squamous cell carcinoma (SCC), and large cell carcinoma. Adenocarcinomas contain various histological subtypes, such as lepidic, acinar, papillary, micropapillary, and solid [1]. STAS was significantly correlated with lymphatic and vascular invasions [2]. In addition, STAS was frequently found in lung cancer with papillary, micropapillary, and solid patterns [2]. However, STAS was less frequent in lung cancer with a lepidic pattern than in those without a lepidic pattern [2]. However, although previous studies have reported the prognostic roles of STAS, detailed information on STAS rates according to histological subtypes is unclear [2,3,4,5,6]. Surgical specimens from limited resection can be limited in the evaluation of STAS due to the limitation of the adjacent parenchyma [2,4]. In addition, because STAS does not include the measurement of tumor size, there is no impact of STAS on tumor staging. Therefore, due to the possibility of understaging, further evaluation of the impact of STAS on tumor stage is needed. The clinicopathological implications of the presence of STAS can differ between patients with the same histological subtypes and tumor staging. The correlation between histological subtypes and STAS may be more important. However, detailed information based on histological subtypes is unclear. This study aimed to elucidate the clinicopathological significance of STAS in NSCLC through a meta-analysis. First, the estimated rates of STAS were investigated and evaluated in various histological subtypes and clinicopathological subgroups. In addition, the prognostic implications of STAS were investigated, and a subgroup analysis was performed.
2. Materials and Methods
2.1. Published Studies Search and Selection Criteria
Searching was performed using the PubMed and MEDLINE databases on 30 June 2021. These databases were searched using the following keywords: “lung” and “STAS or spread through air spaces.” The titles and abstracts of all searched articles from databases were screened for exclusion. Review articles were also screened to find additional eligible studies. Articles were included if the study was performed in human NSCLC and if there was information about the clinicopathological characteristics and prognosis of NSCLC with and without STAS. Articles were excluded if they were case reports or non-original articles or if the article was not written in English.
2.2. Data Extraction
The data was extracted from each of the eligible studies by two researchers [2,4,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Extracted data included: the first author’s name, year of publication, study location, number of patients analyzed, and clinicopathological information for patients with and without STAS. Investigated clinicopathological information included histologic subtypes, patients’ age and sex, smoking history, tumor size, tumor location, visceral pleural, venous, and lymphatic invasion, genetic mutations of anaplastic lymphoma kinase (ALK), epithelial growth factor receptor (EGFR), ROS1, and KRAS, and survival rate.
2.3. Statistical Analyses
To perform the meta-analysis, all data were analyzed using the Comprehensive Meta-Analysis software package (Biostat, Englewood, NJ, USA). The incidence rates of STAS were investigated in NSCLCs. In addition, the presence of STAS and various clinicopathological characteristics, including genetic mutations, were investigated and performed in the meta-analysis. The correlations between the presence of STAS and overall and recurrence-free survivals were evaluated. For a quantitative aggregation of survival results, we obtained the hazard ratio (HR) using one of the following methods. In studies not quoting the HR or its confidence interval (CI), these variables were calculated from the presented data using the HR point estimate, log-rank statistic or its p-value, and the O-E statistic (difference between the number of observed and expected events) or its variance. If those data were unavailable, HR was estimated using the total number of events, number of patients at risk in each group, and the log-rank statistic or its p-value. Finally, if the only useful data were in the form of graphical representations of survival distributions, survival rates were extracted at specified times to reconstruct the HR estimate and its variance under the assumption that patients were censored at a constant rate during the time intervals [52]. The published survival curves were read independently by two researchers in order to reduce variability. The HRs were then combined into an overall HR using Peto’s method [53]. Heterogeneity between the studies was checked by the Q and I2 statistics and expressed as p-values. Additionally, sensitivity analysis was conducted to assess the heterogeneity of eligible studies and the impact of each study on the combined effect. In addition, to compare between subgroups with and without STAS, the meta-regression test was performed. Because eligible studies used various populations, the application of the random-effect model rather than the fixed-effect model was more suitable. For the assessment of publication bias, Begg’s funnel plot and Egger’s test were used. If significant publication bias was found, the fail-safe N and trim-fill tests were additionally conducted to confirm the degree of publication bias. The results were considered statistically significant at p < 0.05.
3. Results
3.1. Selection and Characteristics of the Studies
In this study, 47 studies were included among the 201 searched studies. In total, 51 studies were excluded because they were non-original articles. Moreover, 46 articles had insufficient or no information. Overall, 44 articles were studied for other diseases. Two reports were excluded due to duplication of patients. In addition, 11 reports were excluded due to non-English (n = 8) and non-human samples (n = 3). Detailed information for the included and excluded studies is presented in Figure 1 and Table 1.
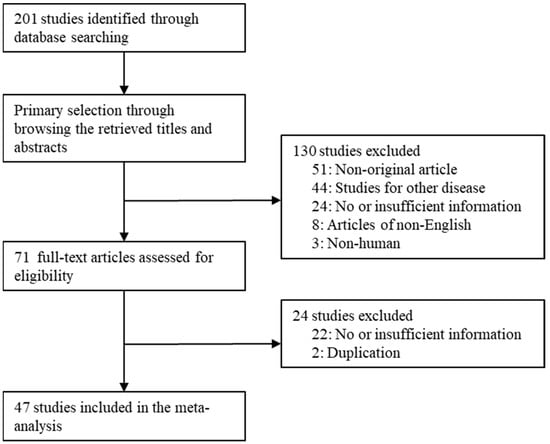
Figure 1.
Flow chart of study search and selection methods.

Table 1.
Main characteristics of eligible studies.
3.2. Estimated Rates of STAS in NSCLC
The estimated rate of STAS was 0.368 (95% CI, 0.336–0.401) in patients with NSCLC (Table 2). STAS was found in 33.8% and 37.4% of the cases of SCC and ADC, respectively. In the subgroup analysis based on histological subtypes of ADC, the STAS rate was the highest in micropapillary-predominant ADC (0.719; 95% CI, 0.652–0.778). The STAS rates were 0.567 (95% CI, 0.478–0.652) and 0.446 (95% CI, 0.392–0.501) in the solid and papillary predominant subgroups, respectively. Additionally, the STAS rates of the lepidic, acinar, mucinous, cribriform, and colloid-predominant subgroups were 0.128 (95% CI, 0.092–0.175), 0.352 (95% CI, 0.312–0.394), 0.278 (95% CI, 0.169–0.42), 0.365 (95% CI, 0.337–0.394), and 0.167 (95% CI, 0.010–0.806), respectively.

Table 2.
Meta-analysis for the rate of spread through air space in non-small cell lung carcinoma.
3.3. Correlation between STAS and Clinicopathological Characteristics in NSCLC
Differences in clinicopathological characteristics between patients with and without STAS were investigated through a meta-analysis. NSCLCs with STAS were significantly more correlated with frequent visceral pleural, venous, and lymphatic invasions than those without STAS (Table 3). In NSCLCs with STAS, the estimated rates of visceral pleural, venous, and lymphatic invasions were 0.322 (95% CI, 0.275–0.373), 0.301 (95% CI, 0.251–0.356), and 0.391 (95% CI, 0.325–0.461), respectively. In addition, STAS is frequently observed in male patients. However, there were no significant differences in age, smoking history, tumor size, and tumor location between patients with and without STAS.

Table 3.
Comparisons of clinicopathological parameters between lung cancers with STAS and non-STAS.
The correlations between genetic alterations and the presence of STAS were investigated in NSCLC. Patients with STAS were significantly more correlated with higher ALK mutations and ROS1 rearrangement than those without STAS (Table 4). The estimated rates of ALK mutation and ROS1 rearrangement in patients with STAS were 0.125 (95% CI, 0.102–0.152) and 0.040 (95% CI, 0.023–0.068), respectively. The estimated rates of ALK mutation and ROS1 rearrangement in patients without STAS were 0.027 (95% CI, 0.011–0.067) and 0.009 (95% CI, 0.004–0.020), respectively. However, there were no significant differences between EGFR mutations and KRAS mutations between patients with and without STAS.

Table 4.
Comparisons of genetic mutation between lung cancers with STAS and non-STAS.
3.4. Prognosis of NSCLC with STAS
Patients with STAS had worse overall and recurrence-free survival (HR, 2.119; 95% CI, 1.811–2.480 and HR, 2.372; 95% CI, 2.018–2.788, respectively) (Figure 2 and Figure 3; Table 5). In the ADC subgroup, patients with STAS were significantly correlated with worse overall and recurrence-free survival (HR, 2.093; 95% CI, 1.756–2.496 and HR, 2.633; 95% CI, 2.145–3.232, respectively). In the SCC subgroup, patients with STAS had worse overall and recurrence-free survival (HR, 4.208; 95% CI, 2.190–8.083 and HR, 1.610; 95% CI, 1.066–2.431, respectively).
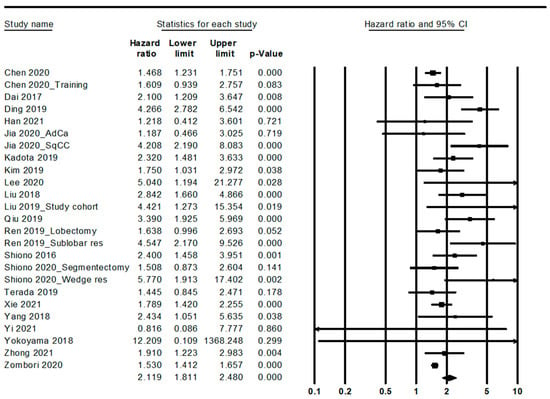
Figure 2.
Forest plots for the overall survival.
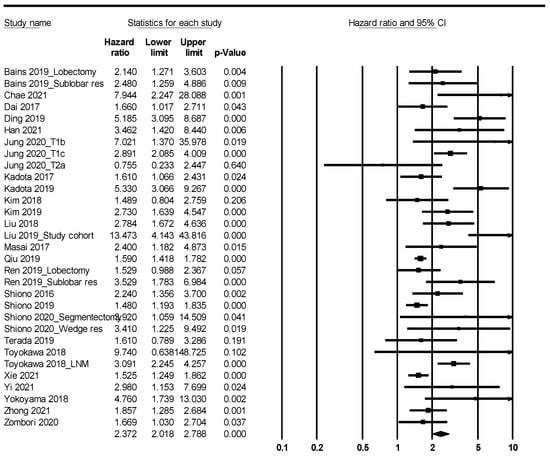
Figure 3.
Forest plots for the recurrence-free survival.

Table 5.
Comparisons of prognosis between lung cancers with STAS and non-STAS.
4. Discussion
Although the concept of STAS was introduced in 2015, it is not included as a diagnostic parameter in daily practice [2]. Because the presence of STAS is correlated with the prognosis and histological subtype of the patient, a detailed evaluation is needed in the pathological examination. However, despite many studies, the diagnostic criteria in daily practice are unclear. Therefore, the meta-analysis may be appropriate to help understand the clinicopathological impacts of STAS. Through this meta-analysis, we obtained the following results: (1) the estimated rate of STAS was 0.368 (95% CI, 0.336–0.401) in NSCLC; (2) STAS was frequently found in the micropapillary and solid predominant subtype; (3) STAS was significantly correlated with visceral pleural, venous, and lymphatic invasion; and (4) patients with STAS had worse overall and recurrence-free survival than those without STAS.
STAS was first defined by Kadota et al. in 2015 [2]. They reported that STAS is the identification of tumor cells that spread in the air spaces of the lung parenchyma adjacent to the edge of the tumor [2]. To evaluate the presence of STAS, the lung parenchyma adjacent to the edge of the tumor must be included in the pathological examination. The identification of STAS can be performed on the histological examination of lung cancer. In addition, the differentiation between tumor cells and other cells within the air space is not easy. Because the evaluation of STAS can be different from that of pathologists, obvious criteria are necessary for daily practice. Kadota et al. introduced the morphological patterns of tumor cells of STAS: (1) micropapillary structures; (2) solid nests of tumor islands; and (3) scattered discohesive single cells [2]. These patterns can easily differ from the lepidic growth patterns. Kadota et al. reported that the presence of STAS was correlated with lepidic, papillary, micropapillary, and solid patterns [2]. In our results, the estimated STAS rate for the lepidic subtype was the lowest among the ADC subtypes (0.128; 95% CI, 0.092–0.175). If the tumor component is a pure lepidic subtype, the actual rate of STAS can be lower than our results. The criterion for a major histopathological subtype of ADC is >5% of the overall tumor. Because the pure histological subtype of ADC is rare, differentiation between the components of STAS and tumors can be difficult. As described above, the morphological patterns of subtypes with low STAS rates, such as lepidic, acinar, and mucinous subtypes, are different from the morphological patterns of STAS. In our results, the estimated rates of STAS ranged from 12.8% to 71.9%, according to the ADC subtypes. The micropapillary subtype showed the highest STAS rate among the ADC subtypes (0.719; 95% CI, 0.652–0.778). In evaluating STAS, artificial spreading features should be distinguished from true STAS. Contamination on sectioning tissue and paraffin block is issued in pathologic examination. Especially in lung resection specimens, the possibility of the displacement of tumor cells may frequently be present along the plane of sectioning by a knife [54,55]. Lee et al. described that three tumor slices were observed under the microscope to avoid confusion with artificially detached cells [25].
STAS has been correlated with aggressive clinical features and a worse prognosis. However, due to the different diagnostic criteria and populations, conclusive information is unclear. Therefore, a meta-analysis is useful for obtaining conclusive information. In this study, STAS was significantly correlated with visceral pleural, venous, and lymphatic invasion. However, there was no significant correlation between STAS and age, smoking history, tumor size, and tumor location. Although previous studies have reported a correlation between STAS and clinicopathological characteristics, the detailed information between studies is different. Kadota et al. reported that STAS was significantly correlated with lymphovascular invasion and histological subtypes [2]. A previous study showed a correlation between STAS and the tumor site and the stage of lymph nodes [7]. In addition, they reported that STAS was significantly higher in micropapillary growth patterns than in other histological patterns [7]. However, there were no statistically significant differences between the presence of STAS and histological subtypes. STAS is more frequently found in the right lower lobe than in the left lower lobe [7]. In this study, no significant differences were observed in STAS rates between upper/middle and lower lobes (p = 0.078 in the meta-regression test).
Kadota et al. reported that STAS was not correlated with visceral pleural invasion [2], unlike in our results. In addition, they divided the patients into limited and lobectomy resection groups. In their study, no significant differences in the visceral pleural invasion were observed according to the presence of STAS. In addition, they suggested that STAS is a risk factor for locoregional recurrence. In patients with limited resection, the evaluation of the presence of STAS is difficult due to the insufficient inclusion of the adjacent parenchyma. In addition, the impact of the fixation method of inflation on the presence of STAS is unclear. Kadota et al. reported different prognostic impacts between the limited and lobectomy groups [2]. STAS was significantly correlated with worse recurrence-free survival in the limited resection group but not in the lobectomy resection group. However, in studies by Bains and Ren, a prognostic impact was found in both the limited and lobectomy resection groups [4,34]. We suggest that upon detecting STAS, close observation or adjuvant therapy is recommended. Cumulative studies for the necessity of further treatments will be needed in patients with STAS. Interestingly, the prognostic roles of STAS were different from those of ADC. The prognostic implications of STAS between stages I and III were not different. There was a significant difference in prognosis between patients with and without STAS in stage I but not in stage III (data not shown). Based on our results, the evaluation of STAS according to the histological subtype can be useful to predict the prognosis of the patient.
We compared the clinicopathological parameters between the STAS and non-STAS subgroups, unlike the previous meta-analysis. In addition, in this study, we showed the results using the estimated rate, but not the odds ratio between the STAS and non-STAS subgroups. Yin et al. reported a correlation between computer tomography and histological STAS in lung ADC [56]. In addition, Eguchi et al. reported the therapeutic effect of surgical treatment in T1 ADC with STAS [57]. Wang et al. demonstrated the prognostic implications of STAS in NSCLC [57]. In the meta-analysis by Liu et al., 12 eligible studies were included [58]. They studied reports from 2015 to 2018. Chen et al. studied using 14 eligible studies [59]. In Wang’s meta-analysis, the number of eligible studies included was eight [58]. Among the eight studies, the reports for ADC and SCC were six and two, respectively [58]. A total of 47 eligible articles were included. In addition, because 33 articles published after 2019 were included, the interest and importance of STAS are gradually increasing. Therefore, our results can be updated and reliable. In addition, we investigated STAS rates in various subtypes of NSCLC and compared clinicopathological characteristics between the STAS and non-STAS subgroups. However, in Wang’s report, information can be obtained based on tumor subtype, unlike our results [58]. In addition, unlike previous meta-analyses, the estimated STAS rates were investigated according to various subgroups.
Unlike previous meta-analyses, our study evaluated the differences in genetic alterations between NSCLC with and without STAS. From our results, detailed information on the clinicopathological characteristics of patients with and without STAS can be useful for the interpretation of patients with STAS. NSCLC with STAS had frequent ALK mutations and ROS1 rearrangement compared to NSCLC without STAS. In lung ADC, EGFR mutations are found more frequently in the micropapillary pattern [60,61]. However, there was no significant correlation between STAS and EGFR mutations or KRAS mutations. Understanding these genetic alterations in STAS may be important for the interpretation of molecular analyses of lung ADC.
There were some limitations to the current meta-analysis. First, the detailed criteria for STAS in NSCLC are unclear. STAS is within air spaces in the lung parenchyma beyond the edge of the main tumor, based on the definition of Kadota’s report [2]. However, the definitive distance was not defined for the edge of the tumor in most eligible studies. Han and Shiono’s reports described the distance as 0.5 mm and 0.25 mm, respectively [13,36]. However, subgroup analysis based on diagnostic criteria could not be performed due to insufficient information. Second, information based on mixed histological patterns could not be obtained from the eligible studies. Third, a detailed evaluation between STAS and distant metastasis could not be performed due to insufficient information. Fourth, a detailed investigation of the morphological patterns of STAS based on the histological subtypes of NSCLC could not be performed. Fourth, evaluating STAS grades may be needed because the extent and amount of STAS can affect a patient’s prognosis. However, it is difficult to assess due to insufficient information from eligible studies. Further evaluation for grading STAS will be needed. Fifth, another limitation concerned the lack of prospective studies for investigating STAS in the included eligible studies. Sixth, the single cell type of STAS was-not prognostic [62]. STAS is composed of three morphologic categories. However, it could not be compared the prognostic differences between morphological categories of STAS.
5. Conclusions
In conclusion, our results showed that STAS is frequently detected as a histological feature, as 36.8% of NSCLC cases. In addition, among adenocarcinomas, STAS is frequently found in the micropapillary and solid predominant subtypes. STAS was significantly correlated with aggressive tumor behavior and a worse prognosis. The recognition of STAS in daily practice is useful to predict the prognosis of the patient.
Author Contributions
Conceptualization, J.-S.P. and N.Y.K.; methodology, J.-S.P.; software, J.-S.P.; validation, J.-S.P.; formal analysis, N.Y.K.; investigation, N.Y.K.; resources, J.-S.P.; data curation, N.Y.K.; writing—original draft preparation, J.-S.P.; writing—review and editing, N.Y.K.; supervision, N.Y.K.; project administration, J.-S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The assistance provided by Soomin Son (Division of Molecular Life and Chemical Sciences, College of Natural Sciences, Ewha Womans University) has been a great help in the revision through searching and classifying the references.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cooper, W.A.; Ladanyi, M.; Van Schil, P.E.Y.; Scagliotti, G.V.; Bubendorf, L.; Bubendorf, L.; Kadota, K.; MacMahon, H.; Matsubara, D.; Russell, P.A.; et al. Tumours of the lung. In WHO Classification of the Lung, Pleura, Thymus and Heart. Thoracic Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021. [Google Scholar]
- Kadota, K.; Nitadori, J.I.; Sima, C.S.; Ujiie, H.; Rizk, N.P.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J. Thorac. Oncol. 2015, 10, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Aly, R.G.; Rekhtman, N.; Li, X.; Takahashi, Y.; Eguchi, T.; Tan, K.S.; Rudin, C.M.; Adusumilli, P.S.; Travis, W.D. Spread Through Air Spaces (STAS) Is Prognostic in Atypical Carcinoid, Large Cell Neuroendocrine Carcinoma, and Small Cell Carcinoma of the Lung. J. Thorac. Oncol. 2019, 14, 1583–1593. [Google Scholar] [CrossRef]
- Bains, S.; Eguchi, T.; Warth, A.; Yeh, Y.C.; Nitadori, J.I.; Woo, K.M.; Chou, T.Y.; Dienemann, H.; Muley, T.; Nakajima, J.; et al. Procedure-Specific Risk Prediction for Recurrence in Patients Undergoing Lobectomy or Sublobar Resection for Small (≤2 cm) Lung Adenocarcinoma: An International Cohort Analysis. J. Thorac. Oncol. 2019, 14, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Onozato, M.L.; Kovach, A.E.; Yeap, B.Y.; Morales-Oyarvide, V.; Klepeis, V.E.; Tammireddy, S.; Heist, R.S.; Mark, E.J.; Dias-Santagata, D.; Iafrate, I.J.; et al. Tumor islands in resected early stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am. J. Surg. Pathol. 2013, 37, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Warth, A.; Muley, T.; Kossakowski, C.A.; Goeppert, B.; Schirmacher, P.; Dienemann, H.; Weichert, W. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am. J. Surg. Pathol. 2015, 39, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Alvarez Moreno, J.C.; Aljamal, A.A.; Bahmad, B.F.; Febres-Aldana, C.A.; Rassaei, N.; Recine, M.; Poppiti, R. Correlation between spread through air spaces (STAS) and other clinicopathological parameters in lung cancer. Pathol. Res. Pract. 2021, 220, 153376. [Google Scholar] [CrossRef]
- Chae, M.; Jeon, J.H.; Chung, J.H.; Lee, S.Y.; Hwang, W.J.; Jung, W.; Hwang, Y.; Cho, S.; Kim, K.; Jheon, S. Prognostic significance of tumor spread through air spaces in patients with stage IA part-solid lung adenocarcinoma after sublobar resection. Lung Cancer 2021, 152, 21–26. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Zhang, F.; Han, R.; Ding, Q.; Xu, X.; Shu, J.; Ye, F.; Shi, L.; Mao, Y.; et al. Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920978147. [Google Scholar] [CrossRef]
- Chen, D.; She, Y.; Wang, T.; Xie, H.; Li, J.; Jiang, G.; Chen, Y.; Zhang, L.; Xie, D.; Chen, C. Radiomics-based prediction for tumour spread through air spaces in stage I lung adenocarcinoma using machine learning. Eur. J. Cardiothorac. Surg. 2020, 58, 51–58. [Google Scholar] [CrossRef]
- Dai, C.; Xie, H.; Su, H.; She, Y.; Zhu, E.; Fan, Z.; Zhou, F.; Ren, Y.; Xie, D.; Zheng, H.; et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J. Thorac. Oncol. 2017, 12, 1052–1060. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Chen, D.; Wang, X.; Wen, J.; Chen, C.; Zhang, Y.; Xu, Z.; Chen, Y. Characterization of lung adenocarcinoma with a cribriform component reveals its association with spread through air spaces and poor outcomes. Lung Cancer 2019, 134, 238–244. [Google Scholar] [CrossRef]
- Han, Y.B.; Kim, H.; Mino-Kenudson, M.; Cho, S.; Kwon, H.J.; Lee, K.R.; Kwon, S.; Lee, J.; Kim, K.; Jheon, S.; et al. Tumor spread through air spaces (STAS): Prognostic significance of grading in non-small cell lung cancer. Mod. Pathol. 2021, 34, 549–561. [Google Scholar] [CrossRef]
- Hara, K.; Mizuguchi, S.; Okada, S.; Izumi, N.; Tsukioka, T.; Komatsu, H.; Ohsawa, M.; Inaba, M.; Shibata, T.; Nishiyama, N. Intensity of SLX predicts distance of tumor spread through alveolar spaces in stage I lung adenocarcinoma. Thorac. Cancer 2019, 10, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.Y.; Hsieh, M.S.; Hsu, H.H.; Tsai, T.M.; Chiang, X.H.; Tsou, K.C.; Liao, H.C.; Lin, M.W.; Chen, J.S. Correlation of tumor spread through air spaces and clinicopathological characteristics in surgically resected lung adenocarcinomas. Lung Cancer 2018, 126, 189–193. [Google Scholar] [CrossRef]
- Ikeda, T.; Kadota, K.; Yoshida, C.; Ishikawa, R.; Go, T.; Haba, R.; Yokomise, H. The epithelial-mesenchymal transition phenotype is associated with the frequency of tumor spread through air spaces (STAS) and a High risk of recurrence after resection of lung carcinoma. Lung Cancer 2021, 153, 49–55. [Google Scholar] [CrossRef]
- Jia, M.; Yu, S.; Yu, J.; Li, Y.; Gao, H.; Sun, P.L. Comprehensive analysis of spread through air spaces in lung adenocarcinoma and squamous cell carcinoma using the 8th edition AJCC/UICC staging system. BMC Cancer 2020, 20, 705. [Google Scholar] [CrossRef]
- Jung, W.; Chung, J.H.; Yum, S.; Kim, K.; Lee, C.T.; Jheon, S.; Cho, S. The differential prognostic impact of spread through air spaces in early-stage lung adenocarcinoma after lobectomy according to the pT descriptor. J. Thorac. Cardiovasc. Surg. 2022, 163, 277–284.e1. [Google Scholar] [CrossRef]
- Kadota, K.; Kushida, Y.; Katsuki, N.; Ishikawa, R.; Ibuki, E.; Motoyama, M.; Nii, K.; Yokomise, H.; Bandoh, S.; Haba, R. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2017, 41, 1077–1086. [Google Scholar] [CrossRef]
- Kadota, K.; Kushida, Y.; Kagawa, S.; Ishikawa, R.; Ibuki, E.; Inoue, K.; Go, T.; Yokomise, H.; Ishii, T.; Kadowaki, N.; et al. Limited Resection Is Associated With a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am. J. Surg. Pathol. 2019, 43, 1033–1041. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, T.J.; Chung, M.J.; Kim, T.S.; Lee, K.S.; Zo, J.I.; Shim, Y.M. Lung Adenocarcinoma: CT Features Associated with Spread through Air Spaces. Radiology 2018, 289, 831–840. [Google Scholar] [CrossRef]
- Kim, M.; Chung, Y.S.; Kim, K.A.; Shim, H.S. Prognostic factors of acinar- or papillary-predominant adenocarcinoma of the lung. Lung Cancer 2019, 137, 129–135. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamura, H.; Omura, A.; Ike, A.; Hiroshima, T.; Maniwa, T.; Honma, K.; Higashiyama, M.; Okami, J. Novel imprint cytological classification is correlated with tumor spread through air spaces in lung adenocarcinoma. Lung Cancer 2020, 148, 62–68. [Google Scholar] [CrossRef]
- Koezuka, S.; Mikami, T.; Tochigi, N.; Sano, A.; Azuma, Y.; Makino, T.; Otsuka, H.; Matsumoto, K.; Shiraga, N.; Iyoda, A. Toward improving prognosis prediction in patients undergoing small lung adenocarcinoma resection: Radiological and pathological assessment of diversity and intratumor heterogeneity. Lung Cancer 2019, 135, 40–46. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, E.K.; Kim, M.; Shim, H.S. Genetic and clinicopathologic characteristics of lung adenocarcinoma with tumor spread through air spaces. Lung Cancer 2018, 123, 121–126. [Google Scholar] [CrossRef]
- Lee, M.A.; Kang, J.; Lee, H.Y.; Kim, W.; Shon, I.; Hwang, N.Y.; Kim, H.K.; Choi, Y.S.; Kim, J.; Zo, J.I.; et al. Spread through air spaces (STAS) in invasive mucinous adenocarcinoma of the lung: Incidence, prognostic impact, and prediction based on clinicoradiologic factors. Thorac. Cancer 2020, 11, 3145–3154. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; Qiu, X.; Duan, S.; Zhang, Y.; Li, F.; Chen, C.; Zhou, Y.; Chen, Y. Relationship between MTA1 and spread through air space and their joint influence on prognosis of patients with stage I-III lung adenocarcinoma. Lung Cancer 2018, 124, 211–218. [Google Scholar] [CrossRef]
- Liu, A.; Hou, F.; Qin, Y.; Song, G.; Xie, B.; Xu, J.; Jiao, W. Predictive value of a prognostic model based on pathologic features in lung invasive adenocarcinoma. Lung Cancer 2019, 131, 14–22. [Google Scholar] [CrossRef]
- Lu, S.; Tan, K.S.; Kadota, K.; Eguchi, T.; Bains, S.; Rekhtman, N.; Adusumilli, P.S.; Travis, W.D. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J. Thorac. Oncol. 2017, 12, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Masai, K.; Sakurai, H.; Sukeda, A.; Suzuki, S.; Asakura, K.; Nakagawa, K.; Asamura, H.; Watanabe, S.I.; Motoi, N.; Hiraoka, N. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J. Thorac. Oncol. 2017, 12, 1788–1797. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, N.; Yoshizawa, A.; Rokutan-Kurata, M.; Noguchi, M.; Teramoto, Y.; Sumiyoshi, S.; Kondo, K.; Sonobe, M.; Hamaji, M.; Menju, T.; et al. Prognostic significance of cribriform adenocarcinoma of the lung: Validation analysis of 1,057 Japanese patients with resected lung adenocarcinoma and a review of the literature. Transl. Lung Cancer Res. 2021, 10, 117–127. [Google Scholar] [CrossRef]
- Qi, L.; Xue, K.; Cai, Y.; Lu, J.; Li, X.; Li, M. Predictors of CT Morphologic Features to Identify Spread Through Air Spaces Preoperatively in Small-Sized Lung Adenocarcinoma. Front. Oncol. 2021, 10, 548430. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, D.; Liu, Y.; Duan, S.; Zhang, F.; Zhang, Y.; Li, F.; Chen, C.; Chen, Y. Relationship between stromal cells and tumor spread through air spaces in lung adenocarcinoma. Thorac. Cancer 2019, 10, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Xie, H.; Dai, C.; She, Y.; Su, H.; Xie, D.; Zheng, H.; Zhang, L.; Jiang, G.; Wu, C.; et al. Prognostic Impact of Tumor Spread Through Air Spaces in Sublobar Resection for 1A Lung Adenocarcinoma Patients. Ann. Surg. Oncol. 2019, 26, 1901–1908. [Google Scholar] [CrossRef]
- Shiono, S.; Yanagawa, N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiono, S.; Endo, M.; Suzuki, K.; Hayasaka, K.; Yanagawa, N. Spread through air spaces in lung cancer patients is a risk factor for pulmonary metastasis after surgery. J. Thorac. Dis. 2019, 11, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Endo, M.; Suzuki, K.; Yanagawa, N. Spread through air spaces affects survival and recurrence of patients with clinical stage IA non-small cell lung cancer after wedge resection. J. Thorac. Dis. 2020, 12, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Jiang, L.; Zhuo, Z.; Luo, J.; Alai, G.; Shen, X.; Lin, Y. Impacts of thoracoscopic surgery and high grade histologic subtypes on spread through air spaces in small stage I lung adenocarcinomas. J. Cancer Res. Clin. Oncol. 2019, 145, 2375–2382. [Google Scholar] [CrossRef]
- Terada, Y.; Takahashi, T.; Morita, S.; Kashiwabara, K.; Nagayama, K.; Nitadori, J.I.; Anraku, M.; Sato, M.; Shinozaki-Ushiku, A.; Nakajima, J. Spread through air spaces is an independent predictor of recurrence in stage III (N2) lung adenocarcinoma. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 442–448. [Google Scholar] [CrossRef]
- Toyokawa, G.; Yamada, Y.; Tagawa, T.; Oda, Y. Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited resection. Thorac. Cancer 2018, 9, 1255–1261. [Google Scholar] [CrossRef]
- Toyokawa, G.; Yamada, Y.; Tagawa, T.; Kinoshita, F.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Takamori, S.; Akamine, T.; Hirai, F.; et al. Significance of Spread Through Air Spaces in Resected Lung Adenocarcinomas With Lymph Node Metastasis. Clin. Lung Cancer 2018, 19, 395–400.e1. [Google Scholar] [CrossRef]
- Vaghjiani, R.G.; Takahashi, Y.; Eguchi, T.; Lu, S.; Kameda, K.; Tano, Z.; Dozier, J.; Tan, K.S.; Jones, D.R.; Travis, W.D.; et al. Tumor Spread Through Air Spaces Is a Predictor of Occult Lymph Node Metastasis in Clinical Stage IA Lung Adenocarcinoma. J. Thorac. Oncol. 2020, 15, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Villalba, J.A.; Shih, A.R.; Sayo, T.M.S.; Kunitoki, K.; Hung, Y.P.; Ly, A.; Kem, M.; Hariri, L.P.; Muniappan, A.; Gaissert, H.A.; et al. Accuracy and Reproducibility of Intraoperative Assessment on Tumor Spread Through Air Spaces in Stage 1 Lung Adenocarcinomas. J. Thorac. Oncol. 2021, 16, 619–629. [Google Scholar] [CrossRef]
- Xie, H.; Su, H.; Zhu, E.; Gu, C.; Zhao, S.; She, Y.; Ren, Y.; Xie, D.; Zheng, H.; Wu, C.; et al. Morphological Subtypes of Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: Prognostic Heterogeneity and Its Underlying Mechanism. Front. Oncol. 2021, 11, 608353. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Y.; Ma, P.; Zheng, B.; Liu, W.; Zhang, Z.; Ding, N.; Liu, L.; Mao, Y.; Lv, N. Spread through air spaces predicts a worse survival in patients with stage I adenocarcinomas >2 cm after radical lobectomy. J. Thorac. Dis. 2018, 10, 5308–5317. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.; Lee, J.H.; Jung, Y.; Chung, J.H.; Lee, Y.; Lee, S. Clinical implication of tumour spread through air spaces in pathological stage I lung adenocarcinoma treated with lobectomy. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Murakami, T.; Tao, H.; Onoda, H.; Hara, A.; Miyazaki, R.; Furukawa, M.; Hayashi, M.; Inokawa, H.; Okabe, K.; et al. Tumor Spread Through Air Spaces Identifies a Distinct Subgroup With Poor Prognosis in Surgically Resected Lung Pleomorphic Carcinoma. Chest 2018, 154, 838–847. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Feng, H.; Xiao, F.; Shao, W.; Liang, C.; Sun, H.; Gu, X.; Liu, D. Predictive value of radiological features on spread through air space in stage cIA lung adenocarcinoma. J. Thorac. Dis. 2020, 12, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, Y.; Deng, J.; Wang, T.; Sun, X.; Chen, D.; Wu, C.; Hou, L.; Xie, H.; She, Y.; et al. Prognostic impact of tumour spread through air space in radiological subsolid and pure solid lung adenocarcinoma. Eur. J. Cardiothorac. Surg. 2021, 59, 624–632. [Google Scholar] [CrossRef]
- Zhuo, Y.; Feng, M.; Yang, S.; Zhou, L.; Ge, D.; Lu, S.; Liu, L.; Shan, F.; Zhang, Z. Radiomics nomograms of tumors and peritumoral regions for the preoperative prediction of spread through air spaces in lung adenocarcinoma. Transl. Oncol. 2020, 13, 100820. [Google Scholar] [CrossRef]
- Zombori, T.; Sejben, A.; Tiszlavicz, L.; Cserni, G.; Pálföldi, R.; Csada, E.; Furák, J. Architectural Grade Combined With Spread Through Air Spaces (STAS) Predicts Recurrence and is Suitable for Stratifying Patients Who Might Be Eligible for Lung Sparing Surgery for Stage I Adenocarcinomas. Pathol. Oncol. Res. 2020, 26, 2451–2458. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Yusuf, S.; Peto, R.; Lewis, J.; Collins, R.; Sleight, P. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog. Cardiovasc. Dis. 1985, 27, 335–371. [Google Scholar] [CrossRef]
- Shih, A.R.; Mino-Kenudson, M. Updates on spread through air spaces (STAS) in lung cancer. Histopathology 2020, 77, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, E.; Blaauwgeers, H.; de Cuba, E.; Yick, C.Y.; Flieder, D.B. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch. Pathol. Lab. Med. 2016, 140, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Wang, H.; Cui, H.; Wang, W.; Yang, G.; Qie, P.; Xun, X.; Han, S.; Liu, H. Meta-analysis of association between CT-based features and tumor spread through air spaces in lung adenocarcinoma. J. Cardiothorac. Surg. 2020, 15, 243. [Google Scholar] [CrossRef]
- Kameda, K.; Eguchi, T.; Lu, S.; Qu, Y.; Tan, K.S.; Kadota, K.; Adusumilli, P.S.; Travis, W.D. Implications of the Eighth Edition of the TNM Proposal: Invasive Versus Total Tumor Size for the T Descriptor in Pathologic Stage I-IIA Lung Adenocarcinoma. J. Thorac. Oncol. 2018, 13, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Hao, J.; Qian, C.; Wang, H. Tumor Spread Through Air Spaces Is a Survival Predictor in Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e584–e591. [Google Scholar] [CrossRef]
- Liu, H.; Yin, Q.; Yang, G.; Qje, P. Prognostic Impact of Tumor Spread Through Air Spaces in Non-small Cell Lung Cancers: A Meta-Analysis Including 3564 Patients. Pathol. Oncol. Res. 2019, 25, 1303–1310. [Google Scholar] [CrossRef]
- Chen, D.; Mao, Y.; Wen, J.; She, Y.; Zhu, E.; Zhu, F.; Zhang, Y.; Fan, M.; Chen, C.; Chen, Y. Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2019, 108, 945–954. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Shi, X.; Hong, W.; Zhao, J.; Shi, L. Correlation of survival and EGFR mutation with predominant histologic subtype according to the new lung adenocarcinoma classification in stage IB patients. World J. Surg. Oncol. 2014, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.R.; Dong, Y.J.; Wu, H.B.; Liu, Z.C.; Zhou, L.J.; Su, D.; Chen, X.J.; Zhang, L.; Zhao, Y.L. Micropapillary: A component more likely to harbour heterogeneous EGFR mutations in lung adenocarcinomas. Sci. Rep. 2016, 6, 23755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).