Experience of Low-Pass Whole-Genome Sequencing-Based Copy Number Variant Analysis: A Survey of Chinese Tertiary Hospitals
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Participants
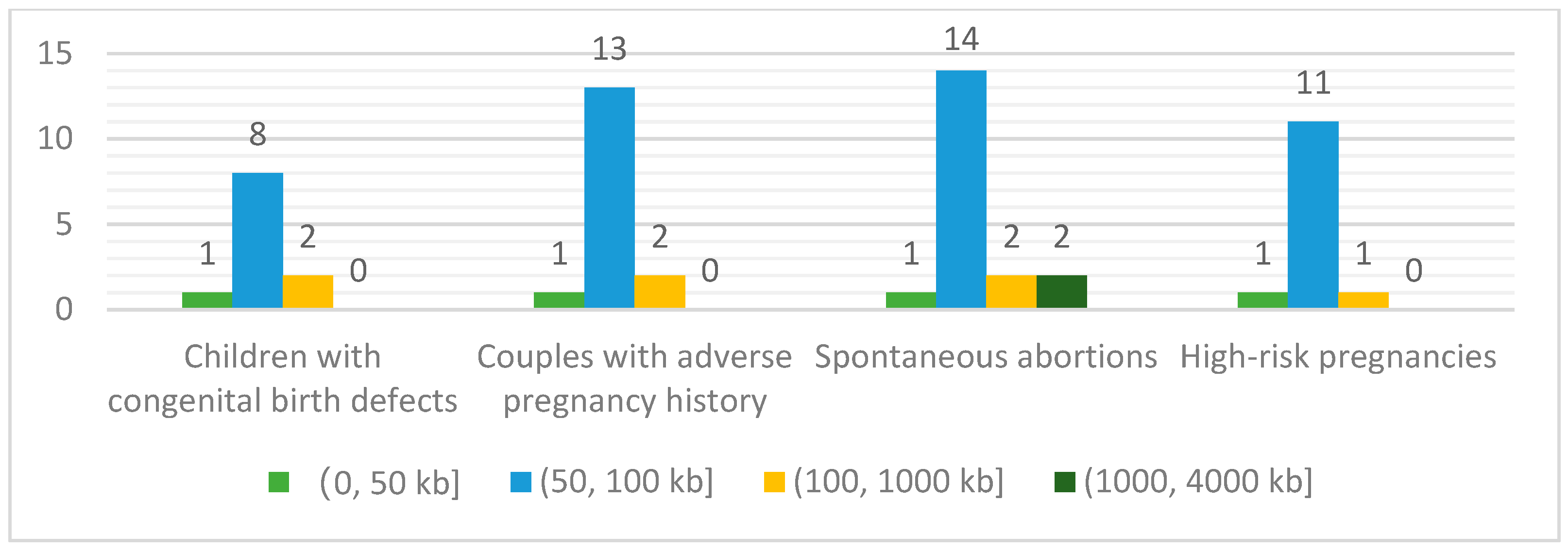
3.2. Compositions of Patients’ Referrals and Annual Sample Volumes of Low-Pass WGS-Based CNV Analysis
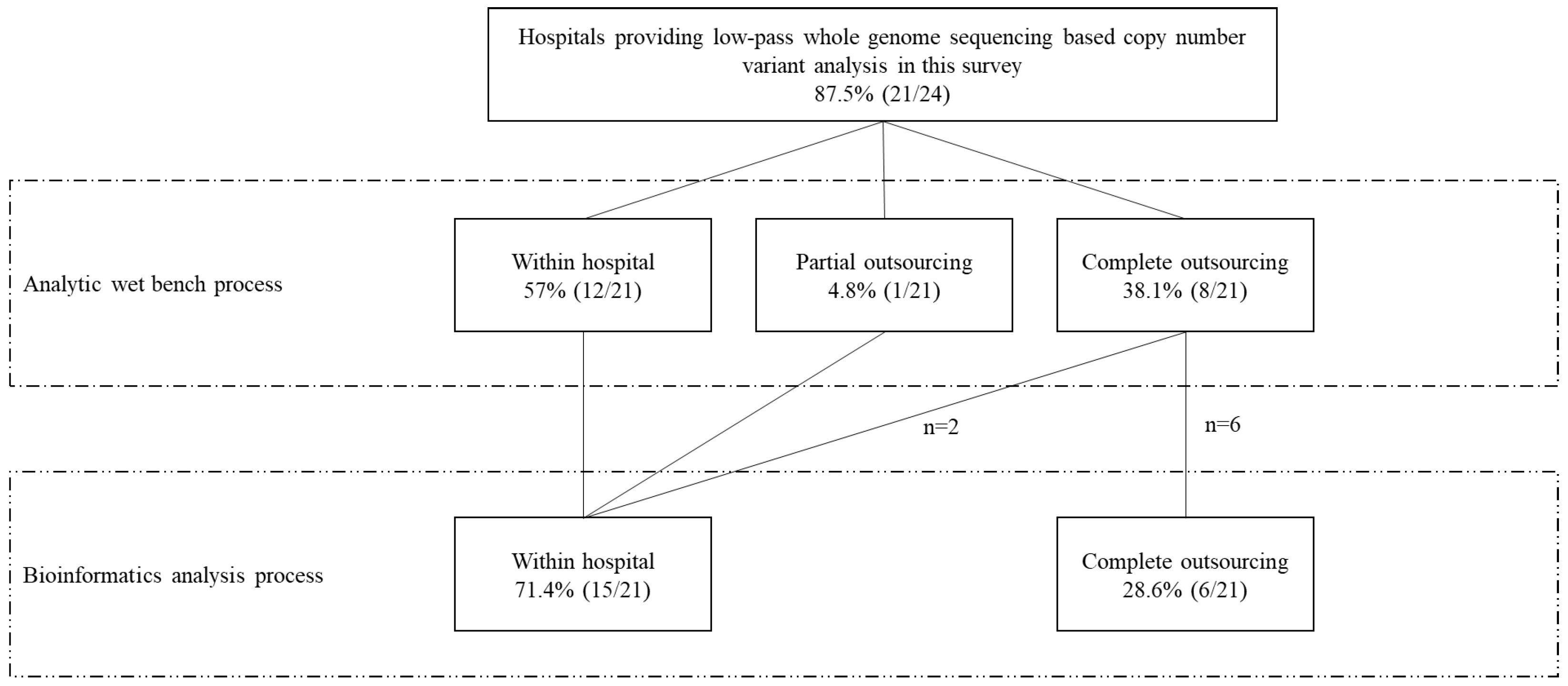
3.3. The Hospital’s Capacity to Conduct Low-Pass WGS-Based CNV Analysis Internally
3.4. Sequencing Platforms and Parameters Utilized for Low-Pass WGS-Based CNV Analysis
3.5. Sequencing Parameters Utilized for Low-Pass WGS-Based CNV Analysis
3.6. The Compliance of CNV Reporting Nomenclature with Internationally-Recognized Criteria
4. Discussion
4.1. Low-Pass WGS-Based CNV Analysis Is in Great Demand in China
4.2. Various Patterns of Low-Pass WGS-Based CNV Analysis Bring Challenges to Quality Assessment
4.3. Challenges Regarding the Establishment of Standard Criteria for Low-Pass WGS-Based CNV Analysis
4.4. Challenges Regarding Implementation of Existing Criteria for CNV Nomenclature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1
- Prenatal Genetic Diagnosis Centre, Department of Obstetrics & Gynecology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China
- 2
- Department of Genetics Medicine, First People’s Hospital of Yunnan Province, Kunming 650021, China
- 3
- Reproductive Medicine Centre, Shengjing Hospital of China Medical University, Shenyang 110055, China
- 4
- Reproductive Medicine Centre, Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
- 5
- Obstetrics Centre, Peking Union Medical College Hospital, Beijing 100006, China
- 6
- Genetics Centre, Hunan Provincial Maternal and Child Health Care Hospital, Changsha 410008, China
- 7
- Genetics Centre, Reproductive & Genetic Hospital of CITIC-Xiangya, Changsha 410008, China
- 8
- Reproductive Medicine Centre, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu 610032, China
- 9
- Centre of Reproductive Medicine, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou 350004, China
- 10
- Laboratory of the Obstetrics and Gynecology Institute, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou 510150, China
- 11
- Reproductive Medicine Centre, The Affiliated Hospital of Guizhou Medical University, Guiyang 550031, China
- 12
- Reproductive Genetics Centre, Women’s Hospital School of Medicine Zhejiang University, Hangzhou 310006, China
- 13
- Reproductive Medicine Center, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, China
- 14
- Department of Reproductive Genetics, Hospital for Reproductive Medicine Affiliated to Shandong University, Jinan 250021, China
- 15
- Prenatal Diagnosis Center, Jiangxi Maternal and Child Health Hospital, Nanchang 330006, China
- 16
- Pediatrics Research Institute, The Children’s Hospital of Fudan University, Shanghai 201102, China
- 17
- Prenatal Diagnosis Center, Shanghai JIAI Genetics & IVF Institute, Obstetrics & Gynecology Hospital of Fudan University, Shanghai 200082, China
- 18
- Shanghai Key Laboratory of Maternal Fetal Medicine, Department of Fetal Medicine & Prenatal Diagnosis Center, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai 200125, China
- 19
- Reproductive Medicine Centre, The Second Hospital of Hebei Medical University, Shijiazhuang 050004, China
- 20
- Centre of Reproduction and Genetics, Suzhou Municipal Hospital, Suzhou 215005, China
- 21
- Reproductive Medicine Center, Children’s Hospital of Shanxi (Women Health Center of Shanxi), Taiyuan 030083, China
- 22
- Prenatal Diagnosis Center, Xinjiang Jiaying Hospital, Urumqi 830041, China
- 23
- Reproductive Medicine Centre, Renmin Hospital of Wuhan University, Wuhan 430064, China
- 24
- Obstetrics and Gynecology Centre, Tangdu Hospital, Xi’an 710024, China
References
- MacDonald, J.R.; Ziman, R.; Yuen, R.K.C.; Feuk, L.; Scherer, S.W. The Database of Genomic Variants: A curated collection of structural variation in the human genome. Nucleic Acids Res. 2014, 42, 986–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A copy number variation map of the human genome. Nat. Rev. Genet. 2015, 16, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Weischenfeldt, J.; Symmons, O.; Spitz, F.; Korbel, J.O. Phenotypic impact of genomic structural variation: Insights from and for human disease. Nat. Rev. Genet. 2013, 14, 125–138. [Google Scholar] [CrossRef]
- Manning, M.; Hudgins, L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet. Med. 2010, 12, 742–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.L.; Tzika, A.; Thygesen, H.; Berri, S.; Wood, H.M.; Hewitt, S.; Pendlebury, M.; Coates, A.; Willoughby, L.; Watson, C.M.; et al. Diagnosis of copy number variation by Illumina next generation sequencing is comparable in performance to oligonucleotide array comparative genomic hybridisation. Genomics 2013, 102, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Chau, M.H.K.; Wang, H.; Lai, Y.; Zhang, Y.; Xu, F.; Tang, Y.; Wang, Y.; Chen, Z.; Leung, T.Y.; Chung, J.P.W.; et al. Low-pass genome sequencing: A validated method in clinical cytogenetics. Hum. Genet. 2020, 139, 1403–1415. [Google Scholar] [CrossRef]
- Xie, C.; Tammi, M.T. CNV-seq, a new method to detect copy number variation using high-throughput sequencing. BMC Bioinform. 2009, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, Q.; Wang, Q.; Jia, P.; Zhao, Z. Computational tools for copy number variation (CNV) detection using next-generation sequencing data: Features and perspectives. BMC Bioinform. 2013, 14, S1. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Z.; Zhang, R.; Chau, M.H.K.; Yang, Z.; Tsang, K.Y.C.; Wong, H.K.; Gui, B.; Meng, Z.; Xiao, K.; et al. Low-pass genome sequencing versus chromosomal microarray analysis: Implementation in prenatal diagnosis. Genet. Med. 2020, 22, 500–510. [Google Scholar] [CrossRef]
- Clinical Genetics Group of Medical Genetics Branch Chinese Medical Association; Professional Committee for Prenatal Diagnosis of Genetic Diseases Medical Genetics Branch of Chinese Medical Association; Group of Genetic Disease Prevention and Control Birth Defect Prevention and Control Committee of Chinese Society of Preventive Medicine. Expert consensus on the application of low-depth whole genome sequencing in prenatal diagnosis. Chin. J. Med. Genet. 2019, 36, 293–296. [Google Scholar] [CrossRef]
- Liang, D.; Peng, Y.; Lv, W.; Deng, L.; Zhang, Y.; Li, H.; Yang, P.; Zhang, J.; Song, Z.; Xu, G.; et al. Copy number variation sequencing for comprehensive diagnosis of chromosome disease syndromes. J. Mol. Diagn. 2014, 16, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, J.; Hu, P.; Chen, H.; Xu, J.; Tian, Q.; Meng, L.; Ye, Y.; Wang, J.; Zhang, M.; et al. Low-pass whole-genome sequencing in clinical cytogenetics: A validated approach. Genet. Med. 2016, 18, 940–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, M.H.K.; Cao, Y.; Kwok, Y.K.Y.; Chan, S.; Chan, Y.M.; Wang, H.; Yang, Z.; Wong, H.K.; Leung, T.Y.; Choy, K.W. Characteristics and mode of inheritance of pathogenic copy number variants in prenatal diagnosis. Am. J. Obstet. Gynecol. 2019, 221, 493.e1–493.e11. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P. Next-Generation Proficiency Testing. J. Mol. Diagn. 2016, 18, 469–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yohe, S.; Thyagarajan, B. Review of clinical next-generation sequencing. Arch. Pathol. Lab. Med. 2017, 141, 1544–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Shen, Y.; Gu, W.; Wang, W.; Wang, Y.; Qi, M.; Shen, J.; Qiu, Z.; Yu, S.; Zhou, Z.; et al. Discussion on the standard of clinical genetic testing report and the consensus of gene testing industry. Chin. J. Med. Genet. 2018, 35, 1–8. [Google Scholar] [CrossRef]
- Riggs, E.R.; Andersen, E.F.; Cherry, A.M.; Kantarci, S.; Kearney, H.; Patel, A.; Raca, G.; Ritter, D.I.; South, S.T.; Thorland, E.C.; et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med. 2020, 22, 245–257. [Google Scholar] [CrossRef] [Green Version]
- McGowan-Jordan, J.; Simons, A.; Schmid, M. ISCN: An International System for Human Cytogenomic Nomenclature (2016); Karger: Basel, Switzerland; Paris, France, 2016; ISBN 9783318058574. [Google Scholar]
- National Stocktaking Report on Birth Defect Prevention. 2012. Available online: http://www.gov.cn/gzdt/att/att/site1/20120912/1c6f6506c7f811bacf9301 (accessed on 31 March 2022).
- Zheng, D.; Li, C.; Wu, T.; Tang, K. Factors associated with spontaneous abortion: A cross-sectional study of Chinese populations. Reprod. Health 2017, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, Y.; Li, C.; Zhang, W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil. Steril. 2014, 101, 385–391.e2. [Google Scholar] [CrossRef]
- Shan, D.; Qiu, P.Y.; Wu, Y.X.; Chen, Q.; Li, A.L.; Ramadoss, S.; Wang, R.R.; Hu, Y.Y. Pregnancy Outcomes in Women of Advanced Maternal Age: A Retrospective Cohort Study from China. Sci. Rep. 2018, 8, 12239. [Google Scholar] [CrossRef]
- Guha, S.; Thomas-Wilson, A.; Rahman, A.; Abhyankar, A.; Poisner, H.; Wilson, A.; Griffin, E.; Chung, W.; Jobanputra, V. OP035: Rapid Whole Genome Sequencing (rWGS) in the cardiac NICU. Genet. Med. 2022, 24, S362–S363. [Google Scholar] [CrossRef]
- Saunders, C.J.; Miller, N.A.; Soden, S.E.; Dinwiddie, D.L.; Noll, A.; Alnadi, N.A.; Andraws, N.; Patterson, M.L.A.; Krivohlavek, L.A.; Fellis, J.; et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci. Transl. Med. 2012, 4, 154ra135. [Google Scholar] [CrossRef] [Green Version]
- Schrijver, I.; Aziz, N.; Jennings, L.J.; Richards, C.S.; Voelkerding, K.V.; Weck, K.E. Methods-based proficiency testing in molecular genetic pathology. J. Mol. Diagnostics 2014, 16, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.G.; Jones, G.R.D.; Horowitz, G.L.; Weykamp, C. Proficiency testing/external quality assessment: Current challenges and future directions. Clin. Chem. 2011, 57, 1670–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremond, J.; Plebani, M. IVD industry role for quality and accreditation in medical laboratories. Clin. Chim. Acta 2001, 309, 167–171. [Google Scholar] [CrossRef]
- McGowan-Jordan, J.; Hastings, R.J.; Moore, S.; GmbH, S.K. ISCN 2020: An international System for Human Cytogenomic Nomenclature (2020); Karger: Basel, Switzerland, 2020; ISBN 9783318067064. [Google Scholar]


| Participant Code | Hospital Code | Hospital Location | Low-Pass WGS-Based CNV Analysis | Department of Participant | Low-Pass WGS-Related Services | |||
|---|---|---|---|---|---|---|---|---|
| General Molecular Genetic Testing | Assisted Reproduction | Prenatal Diagnosis | Pediatrics | |||||
| P1 | Hospital 1 | Hefei, Anhui | Y | Reproductive Medicine Center | Y | Y | N | N |
| P2 | Hospital 2 | Beijing, Beijing | Y | Obstetrics Centre | Y | N | Y | N |
| P3 | Hospital 3 | Shanghai, Shanghai | Y | Pediatrics Research Institute | N | N | N | Y |
| P4 | Hospital 4 | Shanghai, Shanghai | Y | Prenatal Diagnosis Center | Y | N | Y | N |
| P5 | Hospital 5 | Guangzhou, Guangdong | Y | Laboratory of the Obstetrics and Gynecology Institute | Y | N | Y | N |
| P6 | Hospital 6 | Guiyang, Guizhou | Y | Reproductive Medicine Centre | N | Y | N | N |
| P7 | Hospital 7 | Shijiazhuang, Hebei | Y | Reproductive Medicine Centre | N | Y | N | N |
| P8 | Hospital 8 | Changsha, Hunan | Y | Genetics Centre | Y | N | N | N |
| P9 | Hospital 9 | Nanchang, Jiangxi | Y | Prenatal Diagnosis Center | Y | N | Y | N |
| P10 | Hospital 10 | Xi’an, Shaanxi | Y | Obstetrics and Gynecology Centre | N | Y | N | N |
| P11 | Hospital 11 | Jinan, Shandong | Y | Department of Reproductive Genetics | Y | N | N | N |
| P12 | Hospital 12 | Taiyuan, Shanxi | Y | Reproductive Medicine Center | N | Y | N | N |
| P13 | Hospital 13 | Shanghai, Shanghai | N | Shanghai Key Laboratory of Maternal Fetal Medicine, Department of Fetal Medicine & Prenatal Diagnosis Center | Y | N | Y | N |
| P14 | Hospital 14 | Chengdu, Sichuan | N | Reproductive Medicine Centre | N | Y | N | N |
| P15 | Hospital 15 | Suzhou, Jiangsu | Y | Centre of Reproduction and Genetics | Y | Y | Y | N |
| P16 | Hospital 16 | Shatin, Hong Kong SAR | Y | Prenatal Genetic Diagnosis Centre, Department of Obstetrics & Gynecology | Y | N | N | N |
| P17 | Hospital 17 | Urumqi, Xinjiang | Y | Prenatal Diagnosis Center | Y | Y | Y | N |
| P18 | Hospital 18 | Kunming, Yunnan | Y | Department of Genetics Medicine | Y | N | Y | N |
| P19 | Hospital 19 | Hangzhou, Zhejiang | Y | Reproductive Genetics Centre | Y | N | N | N |
| P20 | Hospital 20 | Changsha, Hunan | Y | Genetics Centre | Y | N | N | N |
| P21 | Hospital 21 | Shenyang, Liaoning | Y | Reproductive Medicine Centre | N | Y | N | N |
| P22 | Hospital 22 | Fuzhou, Fujian | Y | Centre of Reproductive Medicine | N | Y | N | N |
| P23 | Hospital 23 | Wuhan, Hubei | N | Reproductive Medicine Centre | Y | Y | Y | N |
| P24 | Hospital 24 | Zhengzhou, Henan | Y | Reproductive Medicine Centre | N | Y | N | N |
| - | - | - | 21/24 (87.5%) | - | 15/24 (62.5%) | 12/24 (50.0%) | 9/24 (37.5%) | 1/24 (4.2%) |
| Manufacturer | Platform | No. of Hospital |
|---|---|---|
| MGI | MGISEQ-2000 | 3 |
| BGISEQ-500 | 1 | |
| Illumina | NovaSeq 6000 | 2 |
| NextSeq 500 | 2 | |
| Annoroad NextSeq 550AR | 1 | |
| Berry Genomics NextSeq CN500 | 4 | |
| MiSeq/MiSeqDx | 2 | |
| HiSeq 2000 | 1 | |
| Thermo Fisher Scientific | Ion Proton | 6 |
| Hospital Code | Manufacture | Platform | DiGeorge Syndrome (DGS) seq[GRCh37]del(22)(q11.21) chr22:g.19009792_21452445del | Charcot-Marie-Tooth Disease Type 1 (CMT1) seq[GRCh37]dup(17)(p12) chr17:g.14097915_15470903dup | Score Points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +a | +b | −c | −d | −e | −f | −g | −h | −i | −ji | Sum | |||||
| Hospital 1 | Thermo Fisher | Ion proton | seq[GCRh37]del(22)(q11.2)#chr22:g.19009792-21452445del | seq[GCRh37]dup(17)(p12)#chr17:g.14097915-15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| Hospital 2 | Illumina | NovaSeq 6000 | seq[GRCh37] 22q11.2(19009792_21452445)X1 | seq[GRCh37] 17p12(14097915_15470903)X3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 7 |
| Hospital 3 | Illumina | NovaSeq | There may be 22q11 microdeletion syndrome | There may be duplication on chromosome 17p12 | 0 | 0 | - | - | - | - | - | - | - | - | 0 |
| Hospital 4 | Illumina | Berry Genomics NextSeq CN500, HiSeq 2000 | seq[hg19]del(22)(q11.2) chr22:g.19009792_21452445del | seq[hg19]dup(17)(p12) chr17:g.14097915_15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| Hospital 5 | Thermo Fisher | Ion proton | del(22)(q11.2).seq[GRCh37](19009792-21452445)×1 | dup(17)(p12).seq[GRCh37](14097915-15470903)×3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 6 | Thermo Fisher | Ion proton | del(22)(q11.2).seq[GRCh37/hg19](19009792-21452445)×1 | dup(17)(p12).seq[GRCh37/hg19](14097915-15470903)×3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 7 | Thermo Fisher | Ion proton | del(22)(q11.2).seq[GRCh37/hg19](19009792-21452445)X1 | dup(17)(p12).seq[GRCh37/hg19](14097915-15470903)X3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 8 | Illumina | Berry Genomics NextSeq CN500 | seq[hg19]del(22)(q11.2)#chr22:g.19009792_21452445del | seq[hg19]dup(17)(p12)#chr17:g.14097915_15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| Hospital 9 | BGI | MGISEQ-2000 | seq[GRCh37] del(22)(q11.2)chr22:g.19009792_21452445 del | seq[GRCh37] dup(17)(p12)chr17:g.14097915_15470903 dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| Hospital 10 | Illumina | MiSeqDx | del(22)(q11.2).(19009792-21452445)X1 | dup(17)(p12).(14097915-15470903)X3 | 5 | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 6 |
| Hospital 11 | Illumina | NextSeq 500 | seq[hg19] 22q11.2(19009792-21452445 )x1 CNV type: heterozygous deletion length: 2.3 Mb classification: pathogenic | seq[hg19] 17p12(14097915-15470903)x3 CNV type: duplication length: 1.3Mb classification:pathogenic | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 12 | BGI | MGISEQ-2000 | 46,XN,del(22q11.2).seq[GRCh37/hg19](19009792-21452445)x1 | 46,XN,dup(17p12).seq[GRCh37/hg19](14097915-15470903)x3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 15 | Illumina | MiSeq | seq[GRCh37]del(22)(q11.2)(19009792-21452445) | seq[GRCh37]dup(17)(p12)(14097915-15470903) | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 16 | BGI | MGISEQ-2000 | seq[GRCh37] del(22)(q11.21) mat/pat/dn chr22:g.19009792_21452445del | seq[GRCh37] dup(17)(p12) mat/pat/dn chr17:g.14097915_15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Hospital 17 | Thermo Fisher | Ion proton | del(22)(q11.2).seq[GRCh37/hg19](19009792-21452445)X1 | dup(17)(p12).seq[GRCh37/hg19](14097915-15470903)X3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 18 | Illumina | NextSeq 550AR | DiGeorge syndrome #band: 22q11.2 #Genomic coordinate (GRCh37) 22:g.19009792-21452445 #type: heterozygous deletion | CMT syndrome type 1 #band: 17p12 #Genomic coordinate (GRCh37) 17:g.1409795-15470903 #type: duplication | 5 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 |
| Hospital 20 | Thermo Fisher | Ion proton | seq[hg19] 22q11.21(18620001_21820000)X1 | seq[hg19] 17p12(14097915_15470903)X3 | 5 | 5 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 7 |
| Hospital 21 | Illumina | NextSeq 500 | seq[hg19]del(22)(q11.2) chr22:g.19009792_21452445del | seq[hg19]dup(17)(p12) chr17:g.14097915_15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| Hospital 22 | Illumina | Berry Genomics NextSeq CN500 | DGS seq(hg19)del(22)(q11.2) chr22:g.19009792_21452445dup | CMT seq(hg19)dup(17)(p12) chr17:g.14097915_15470903dup | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 |
| 19 hospitals | 3 manufacturers | 11 Platforms | 12 types of nomenclature | 12 types of nomenclature | 1# | 1# | 2& | 1& | 10& | 0& | 0& | 2& | 0& | 17& | 7.79 (95% CI, 6.78–8.80) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhu, B.; Tan, J.; Guan, Y.; The Chinese Genomic Structural Variants Consortium; Morton, C.C.; Lu, G. Experience of Low-Pass Whole-Genome Sequencing-Based Copy Number Variant Analysis: A Survey of Chinese Tertiary Hospitals. Diagnostics 2022, 12, 1098. https://doi.org/10.3390/diagnostics12051098
Zheng Y, Zhu B, Tan J, Guan Y, The Chinese Genomic Structural Variants Consortium, Morton CC, Lu G. Experience of Low-Pass Whole-Genome Sequencing-Based Copy Number Variant Analysis: A Survey of Chinese Tertiary Hospitals. Diagnostics. 2022; 12(5):1098. https://doi.org/10.3390/diagnostics12051098
Chicago/Turabian StyleZheng, Yu, Baosheng Zhu, Jichun Tan, Yichun Guan, The Chinese Genomic Structural Variants Consortium, Cynthia C. Morton, and Guangxiu Lu. 2022. "Experience of Low-Pass Whole-Genome Sequencing-Based Copy Number Variant Analysis: A Survey of Chinese Tertiary Hospitals" Diagnostics 12, no. 5: 1098. https://doi.org/10.3390/diagnostics12051098
APA StyleZheng, Y., Zhu, B., Tan, J., Guan, Y., The Chinese Genomic Structural Variants Consortium, Morton, C. C., & Lu, G. (2022). Experience of Low-Pass Whole-Genome Sequencing-Based Copy Number Variant Analysis: A Survey of Chinese Tertiary Hospitals. Diagnostics, 12(5), 1098. https://doi.org/10.3390/diagnostics12051098






