Lower Macromolecular Content in Tendons of Female Patients with Osteoporosis versus Patients with Osteopenia Detected by Ultrashort Echo Time (UTE) MRI
Abstract
1. Introduction
2. Material and Methods
2.1. Participants’ Recruitment
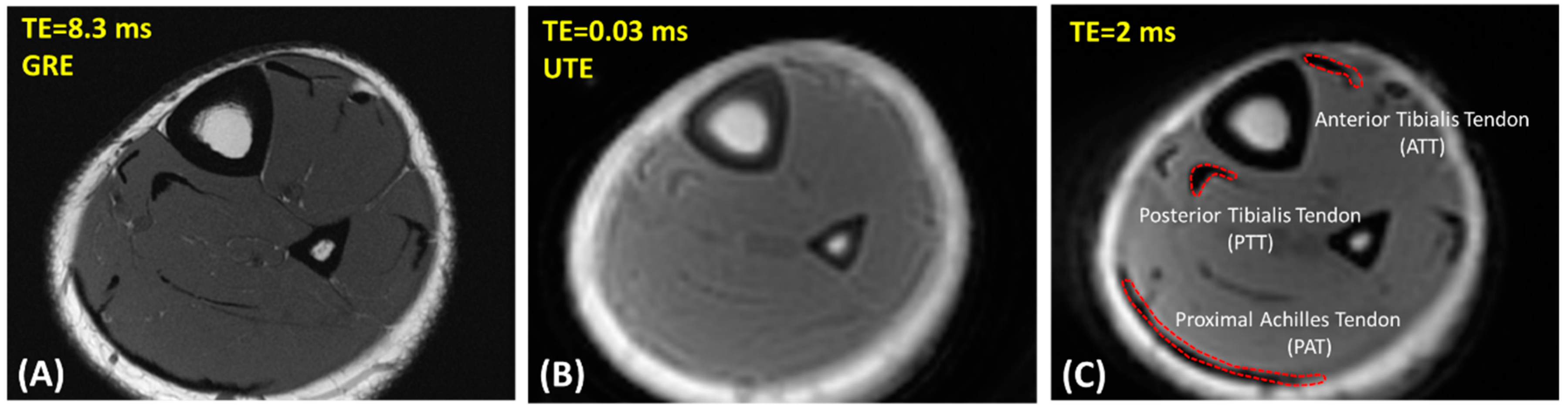
2.2. UTE-MRI Scanning
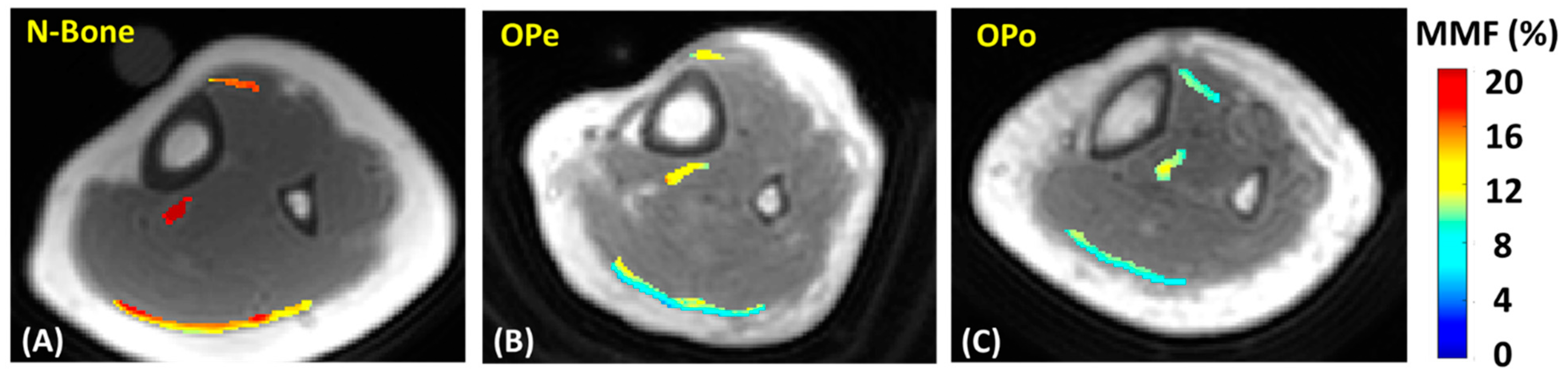
2.3. Data Analysis
2.4. Statistical Analysis
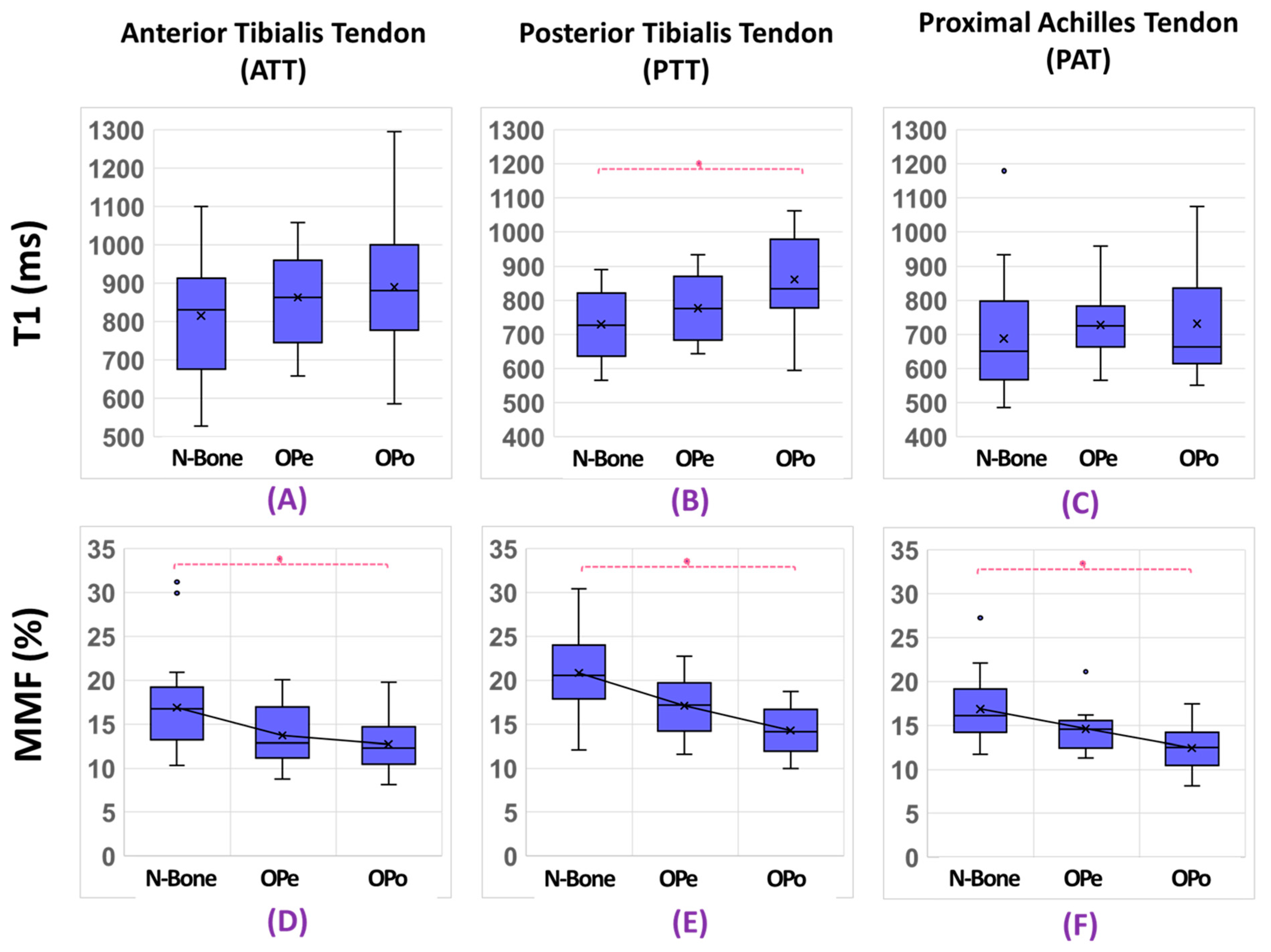
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, M.H.; Dennison, E.M.; Aihie Sayer, A.; Fielding, R.; Cooper, C. Osteoporosis and sarcopenia in older age. Bone 2015, 80, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Cruz-Jentoft, A.J.; Maggi, S. Sarcopenia and fragility fractures. Eur. J. Phys. Rehabil. Med. 2013, 49, 111–117. [Google Scholar] [PubMed]
- Boesen, A.P.; Dideriksen, K.; Couppé, C.; Magnusson, S.P.; Schjerling, P.; Boesen, M.; Aagaard, P.; Kjaer, M.; Langberg, H. Effect of growth hormone on aging connective tissue in muscle and tendon: Gene expression, morphology, and function following immobilization and rehabilitation. J. Appl. Physiol. 2014, 116, 192–203. [Google Scholar] [CrossRef]
- Chen, X.; Giambini, H.; Ben-Abraham, E.; An, K.N.; Nassr, A.; Zhao, C. Effect of bone mineral density on rotator cuff tear: An osteoporotic rabbit model. PLoS ONE 2015, 10, e0139384. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Mokbel, N.; DiGirolamo, D.J. Therapies for musculoskeletal disease: Can we treat two birds with one stone? Curr. Osteoporos. Rep. 2014, 12, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Urban, R.J. Growth hormone and testosterone: Anabolic effects on muscle. Horm. Res. Paediatr. 2011, 76, 81–83. [Google Scholar] [CrossRef]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef]
- Pierre-Jerome, C.; Moncayo, V.; Terk, M.R. MRI of the achilles tendon: A Comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010, 51, 438–454. [Google Scholar] [CrossRef]
- Weinreb, J.H.; Sheth, C.; Apostolakos, J.; McCarthy, M.-B.B.; Barden, B.; Cote, M.P.; Mazzocca, A.D. Tendon structure, disease, and imaging. Muscle Ligaments Tendons J. 2014, 4, 66–73. [Google Scholar] [CrossRef]
- Hodgson, R.; O’Connor, P.J.; Grainger, A.J. Tendon and ligament imaging. Br. J. Radiol. 2012, 85, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chiang, A.J.T.; Chung, C.B.; Statum, S.; Znamirowski, R.; Takahashi, A.; Bydder, G.M. Orientational analysis of the achilles tendon and enthesis using an ultrashort echo time spectroscopic imaging sequence. Magn. Reson. Imaging 2010, 28, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Juras, V.; Zbyn, S.; Pressl, C.; Valkovic, L.; Szomolanyi, P.; Frollo, I.; Trattnig, S. Regional variations of T2* in healthy and pathologic achilles tendon in vivo at 7 Tesla: Preliminary results. Magn. Reson. Med. 2012, 68, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Du, J.; Chung, C.B. UTE imaging in the musculoskeletal system. J. Magn. Reson. Imaging 2015, 41, 870–883. [Google Scholar] [CrossRef]
- Syha, R.; Springer, F.; Grözinger, G.; Würslin, C.; Ipach, I.; Ketelsen, D.; Schabel, C.; Gebhard, H.; Hein, T.; Martirosian, P.; et al. Short-term exercise-induced changes in hydration state of healthy achilles tendons can be visualized by effects of off-resonant radiofrequency saturation in a three-dimensional ultrashort echo time MRI sequence applied at 3 tesla. J. Magn. Reson. Imaging 2014, 40, 1400–1407. [Google Scholar] [CrossRef]
- Du, J.; Carl, M.; Bydder, M.; Takahashi, A.; Chung, C.B.; Bydder, G.M. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J. Magn. Reson. 2010, 207, 304–311. [Google Scholar] [CrossRef]
- Koff, M.F.; Pownder, S.L.; Shah, P.H.; Yang, L.W.; Potter, H.G. Ultrashort echo imaging of cyclically loaded rabbit patellar tendon. J. Biomech. 2014, 47, 3428–3432. [Google Scholar] [CrossRef]
- Juras, V.; Apprich, S.; Szomolanyi, P.; Bieri, O.; Deligianni, X.; Trattnig, S. Bi-Exponential T2*analysis of healthy and diseased achilles tendons: An in vivo preliminary magnetic resonance study and correlation with clinical score. Eur. Radiol. 2013, 23, 2814–2822. [Google Scholar] [CrossRef]
- Chang, E.Y.; Du, J.; Bae, W.C.; Statum, S.; Chung, C.B. Effects of achilles tendon immersion in saline and perfluorochemicals on T2 and T2*. J. Magn. Reson. Imaging 2014, 40, 496–500. [Google Scholar] [CrossRef]
- Chang, E.Y.; Bae, W.C.; Statum, S.; Du, J.; Chung, C.B. Effects of repetitive freeze-thawing cycles on T2 and T2* of the achilles tendon. Eur. J. Radiol. 2014, 83, 349–353. [Google Scholar] [CrossRef]
- Du, J.; Carl, M.; Diaz, E.; Takahashi, A.; Han, E.; Szeverenyi, N.M.; Chung, C.B.; Bydder, G.M. Ultrashort TE T1rho (UTE T1rho) imaging of the achilles tendon and meniscus. Magn. Reson. Med. 2010, 64, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C. Nuclear magnetic resonance study of collagen hydration. J. Chem. Phys. 1962, 36, 3297–3305. [Google Scholar] [CrossRef]
- Krasnosselskaia, L.V.; Fullerton, G.D.; Dodd, S.J.; Cameron, I.L. Water in tendon: Orientational analysis of the free induction decay. Magn. Reson. Med. 2005, 54, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Szeverenyi, N.M.; Statum, S.; Chung, C.B. Rotator Cuff tendon ultrastructure assessment with reduced-orientation dipolar anisotropy fiber imaging. Am. J. Roentgenol. 2014, 202, 376–378. [Google Scholar] [CrossRef]
- Ma, Y.; Shao, H.; Du, J.; Chang, E.Y. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: Magic angle independent biomarkers of tissue properties. NMR Biomed. 2016, 29, 1546–1552. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, X.; Ma, Y.; Wong, J.H.; Xie, Y.; Du, J.; Chang, E.Y. Rotator cuff tendon assessment using magic-angle insensitive 3D ultrashort echo time cones magnetization transfer (UTE-Cones-MT) imaging and modeling with histological correlation. J. Magn. Reson. Imaging 2018, 48, 160–168. [Google Scholar] [CrossRef]
- Shao, H.; Pauli, C.; Li, S.; Ma, Y.; Tadros, A.S.; Kavanaugh, A.; Chang, E.Y.; Tang, G.; Du, J. Magic angle effect plays a major role in both T1rho and T2 relaxation in articular cartilage. Osteoarthr. Cartil. 2017, 25, 2022–2030. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, E.Y.; Carl, M.; Du, J. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke cones sequence. Magn. Reson. Med. 2017, 79, 692–700. [Google Scholar] [CrossRef]
- Jerban, S.; Ma, Y.; Li, L.; Jang, H.; Wan, L.; Guo, T.; Searleman, A.; Chang, E.Y.; Du, J. Volumetric mapping of bound and pore water as well as collagen protons in cortical bone using 3D ultrashort echo time cones MR imaging techniques. Bone 2019, 127, 120–128. [Google Scholar] [CrossRef]
- Jerban, S.; Ma, Y.; Wan, L.; Searleman, A.C.; Jang, H.; Sah, R.L.; Chang, E.Y.; Du, J. Collagen proton fraction from ultrashort echo time magnetization transfer (UTE-MT) MRI modelling correlates significantly with cortical bone porosity measured with micro-computed tomography (ΜCT). NMR Biomed. 2019, 32, e4045. [Google Scholar] [CrossRef]
- Jerban, S.; Ma, Y.; Wong, J.H.; Nazaran, A.; Searleman, A.; Wan, L.; Williams, J.; Du, J.; Chang, E.Y. Ultrashort echo time magnetic resonance imaging (UTE-MRI) of cortical bone correlates well with histomorphometric assessment of bone microstructure. Bone 2019, 123, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Jerban, S.; Ma, Y.; Nazaran, A.; Dorthe, E.W.; Cory, E.; Carl, M.; D’Lima, D.; Sah, R.L.; Chang, E.Y.; Du, J.; et al. Detecting stress injury (fatigue fracture) in fibular cortical bone using quantitative ultrashort echo time-magnetization transfer (UTE-MT): An ex vivo study. NMR Biomed. 2018, 31, e3994. [Google Scholar] [CrossRef] [PubMed]
- Jerban, S.; Ma, Y.; Dorthe, E.W.; Kakos, L.; Le, N.; Alenezi, S.; Sah, R.L.; Chang, E.Y.; D’Lima, D.; Du, J. Assessing cortical bone mechanical properties using collagen proton fraction from ultrashort echo time magnetization transfer (UTE-MT) MRI modeling. Bone Rep. 2019, 8, 100220. [Google Scholar] [CrossRef] [PubMed]
- Jerban, S.; Hananouchi, T.; Ma, Y.; Namiranian, B.; Dorthe, E.W.; Wong, J.H.; Shojaeiadib, N.; Wu, M.; Du, J.; D’Lima, D.; et al. Correlation between the elastic modulus of anterior cruciate ligament (ACL) and quantitative ultrashort echo time (UTE) magnetic resonance imaging. J. Orthop. Res. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Namiranian, B.; Jerban, S.; Ma, Y.; Dorthe, E.W.; Masoud-Afsahi, A.; Wong, J.H.; Zhao, W.; Wei, Z.; Chen, Y.; D’Lima, D.; et al. Assessment of mechanical properties of articular cartilage with quantitative three-dimensional ultrashort echo time (UTE) cones magnetic resonance imaging. J. Biomech. 2020, 113, 110085. [Google Scholar] [CrossRef]
- de Aro, A.A.; de Campos Vidal, B.; Pimentel, E.R. Biochemical and anisotropical properties of tendons. Micron 2012, 43, 205–214. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of common tendinopathies: Update and implications for clinical management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef]
- Jerban, S.; Ma, Y.; Namiranian, B.; Ashir, A.; Shirazian, H.; Zhao, W.; Wu, M.; Cai, Z.; Le, N.; Du, J.; et al. Age-related decrease in collagen proton fraction in tibial tendons estimated by magnetization transfer modeling of ultrashort echo time magnetic resonance imaging (UTE-MRI). Sci. Rep. 2019, 9, 17974. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Carl, M.; Zhu, Y.; Szeverenyi, N.M.; Bydder, G.M.; Chang, E.Y.; Du, J. Accurate T1 mapping of short T2 tissues using a three-dimensional ultrashort echo time cones actual flip angle imaging-variable repetition time (3D UTE-Cones AFI-VTR) method. Magn. Reson. Med. 2018, 80, 598–608. [Google Scholar] [CrossRef]
- Gurney, P.T.; Hargreaves, B.A.; Nishimura, D.G. Design and analysis of a practical 3D cones trajectory. Magn. Reson. Med. 2006, 55, 575–582. [Google Scholar] [CrossRef]
- Carl, M.; Bydder, G.M.; Du, J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn. Reson. Med. 2016, 76, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Zhu, Y.; Lu, X.; Carl, M.; Chang, E.Y.; Du, J. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn. Reson. Med. 2017, 79, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tadros, A.; Du, J.; Chang, E.Y. Quantitative two-dimensional ultrashort echo time magnetization transfer (2D UTE-MT) imaging of cortical bone. Magn. Reson. Med. 2018, 79, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Paavola, M.; Józsa, L. Aging and degeneration of tendons. In Tendon Injuries; Spinger: Berlin/Heidelberg, Germany, 2005; pp. 25–31. [Google Scholar]
- McCrum, C.; Leow, P.; Epro, G.; König, M.; Meijer, K.; Karamanidis, K. Alterations in leg extensor muscle-tendon unit biomechanical properties with ageing and mechanical loading. Front. Physiol. 2018, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.C.; Dickinson, J.M.; Haus, J.M.; Lee, G.A.; Hollon, C.J.; Aagaard, P.; Magnusson, S.P.; Trappe, T.A. Influence of aging on the in vivo properties of human patellar tendon. J. Appl. Physiol. 2008, 105, 1907–1915. [Google Scholar] [CrossRef]
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1353–1362. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Beyer, N.; Abrahamsen, H.; Aagaard, P.; Neergaard, K.; Kjaer, M. Increased cross-sectional area and reduced tensile stress of the achilles tendon in elderly compared with young women. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 123–127. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef]
- Adams, G.R. Insulin-like growth factor in muscle growth and its potential abuse by athletes. West. J. Med. 2001, 175, 7–9. [Google Scholar] [CrossRef][Green Version]
- Frizziero, A.; Vittadini, F.; Gasparre, G.; Masiero, S. Impact of oestrogen deficiency and aging on tendon: Concise review. Muscle Ligaments Tendons J. 2014, 4, 324–328. [Google Scholar] [CrossRef]
- Hodgson, R.J.; Evans, R.; Wright, P.; Grainger, A.J.; O’connor, P.J.; Helliwell, P.; Mcgonagle, D.; Emery, P.; Robson, M.D. Quantitative magnetization transfer ultrashort echo time imaging of the achilles tendon. Magn. Reson. Med. 2011, 65, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.K.; Arruda, E.M.; Brooks, S.V. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J. Appl. Physiol. 2011, 111, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhao, W.; Ma, Y.; Jerban, S.; Searleman, A.C.; Carl, M.; Chang, E.Y.; Tang, G.; Du, J. Fast quantitative three-dimensional ultrashort echo time (UTE) magnetic resonance imaging of cortical bone using extended cones sampling. Magn. Reson. Med. 2019, 82, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Pease, L.I.; Clegg, P.D.; Proctor, C.J.; Shanley, D.J.; Cockell, S.J.; Peffers, M.J. Cross platform analysis of transcriptomic data identifies ageing has distinct and opposite effects on tendon in males and females. Sci. Rep. 2017, 7, 14443. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.C.; Kharaz, Y.A.; Sugg, K.B.; Gumucio, J.P.; Comerford, E.; Mendias, C.L. Sex differences in tendon structure and function. J. Orthop. Res. 2017, 35, 2117–2126. [Google Scholar] [CrossRef]
- Kohls-Gatzoulis, J.; Woods, B.; Angel, J.C.; Singh, D. The prevalence of symptomatic posterior tibialis tendon dysfunction in women over the age of 40 in England. Foot Ankle Surg. 2009, 15, 75–81. [Google Scholar] [CrossRef]
- Carmody, D.; Bubra, P.; Keighley, G.; Rateesh, S. Posterior tibial tendon dysfunction: An overlooked cause of foot deformity. J. Fam. Med. Prim. Care 2015, 4, 26–29. [Google Scholar] [CrossRef]
- Kohls-Gatzoulis, J.; Angel, J.; Singh, D. Tibialis posterior dysfunction as a cause of flatfeet in elderly patients. Foot 2004, 14, 207–209. [Google Scholar] [CrossRef]
| T1 (ms) | MMF (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| ATT | PTT | PAT | Mean | ATT | PTT | PAT | Mean | |
| N-Bone | 829 ± 145 | 733 ± 91 | 698 ± 156 | 758 ± 86 | 16.8 ± 4.7 | 21.0 ± 4.2 | 16.8 ± 3.5 | 18.2 ± 3.6 |
| OPe | 832 ± 144 | 766 ± 101 | 702 ± 111 | 757 ± 99 | 14.3 ± 3.6 | 17.2 ± 3.1 | 15.0 ± 2.9 | 15.8 ± 3.6 |
| OPo | 890 ± 180 | 861 ± 127 | 732 ± 164 | 825 ± 145 | 12.7 ± 2.7 | 14.3 ± 2.6 | 12.4 ± 2.5 | 13.2 ± 2.5 |
| ICC | 0.98 ± 0.01 | 0.97 ± 0.02 | 0.96 ± 0.02 | 0.98 ± 0.01 | 0.98 ± 0.01 | 0.99 ± 0.01 | 0.97 ± 0.01 | 0.99 ± 0.01 |
| T1 Difference (%) | MMF Difference (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| ATT | PTT | PAT | Mean | ATT | PTT | PAT | Mean | |
| N-Bone Vs. OPe | 0.3 (p = 1.00) | 4.5 (p = 0.70) | 0.7 (p = 0.79) | −0.1 (p = 0.98) | −18.3 (p = 0.03) | −14.6 (p = 0.17) | −10.7 (p = 0.28) | −12.9 (p = 0.17) |
| N-Bone Vs. OPo | 7.3 (p = 0.47) | 17.6 (p < 0.01) | 4.9 (p = 0.67) | 8.7 (p = 0.31) | −32.1 (p < 0.01) | −24.2 (p < 0.01) | −26.1 (p < 0.01) | −27.5 (p < 0.01) |
| OPe Vs. OPo | 6.9 (p = 0.65) | 12.5 (p = 0.06) | 4.2 (p = 1.00) | 8.9 (p = 0.32) | −16.9 (p = 0.07) | −11.3 (p = 0.30) | −17.3 (p = 0.04) | −16.8 (p = 0.02) |
| T1 Difference (%) | MMF Difference (%) | |||||
|---|---|---|---|---|---|---|
| ATT-PTT | ATT-PAT | PTT-PAT | ATT-PTT | ATT-PAT | PTT-PAT | |
| N-Bone | −11.67 (p = 0.05) | −15.90 (p < 0.01) | −4.78 (p = 0.34) | 25.43 (p < 0.01) | 0.45 (p = 0.93) | −19.92 (p < 0.01) |
| OPe | −7.95 (p = 0.4) | −15.61 (p = 0.02) | −8.32 (p = 0.38) | 19.94 (p = 0.04) | 5.04 (p = 0.92) | −12.42 (p = 0.10) |
| OPo | −3.16 (p = 0.95) | −17.75 (p < 0.01) | −15.06 (p < 0.01) | 12.38 (p = 0.06) | −2.10 (p = 0.95) | −12.88 (p = 0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerban, S.; Ma, Y.; Afsahi, A.M.; Lombardi, A.; Wei, Z.; Shen, M.; Wu, M.; Le, N.; Chang, D.G.; Chung, C.B.; et al. Lower Macromolecular Content in Tendons of Female Patients with Osteoporosis versus Patients with Osteopenia Detected by Ultrashort Echo Time (UTE) MRI. Diagnostics 2022, 12, 1061. https://doi.org/10.3390/diagnostics12051061
Jerban S, Ma Y, Afsahi AM, Lombardi A, Wei Z, Shen M, Wu M, Le N, Chang DG, Chung CB, et al. Lower Macromolecular Content in Tendons of Female Patients with Osteoporosis versus Patients with Osteopenia Detected by Ultrashort Echo Time (UTE) MRI. Diagnostics. 2022; 12(5):1061. https://doi.org/10.3390/diagnostics12051061
Chicago/Turabian StyleJerban, Saeed, Yajun Ma, Amir Masoud Afsahi, Alecio Lombardi, Zhao Wei, Meghan Shen, Mei Wu, Nicole Le, Douglas G. Chang, Christine B. Chung, and et al. 2022. "Lower Macromolecular Content in Tendons of Female Patients with Osteoporosis versus Patients with Osteopenia Detected by Ultrashort Echo Time (UTE) MRI" Diagnostics 12, no. 5: 1061. https://doi.org/10.3390/diagnostics12051061
APA StyleJerban, S., Ma, Y., Afsahi, A. M., Lombardi, A., Wei, Z., Shen, M., Wu, M., Le, N., Chang, D. G., Chung, C. B., Du, J., & Chang, E. Y. (2022). Lower Macromolecular Content in Tendons of Female Patients with Osteoporosis versus Patients with Osteopenia Detected by Ultrashort Echo Time (UTE) MRI. Diagnostics, 12(5), 1061. https://doi.org/10.3390/diagnostics12051061







