Radiothermometric Study of the Effect of Amino Acid Mutation on the Characteristics of the Enzymatic System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Agents
2.2. Proteins
2.3. Analytical Measurements
2.4. Methods of Monitoring Microwave Radiation of CYP102 A1 Solution
2.4.1. Catalytic Reaction in CYP102 A1 System
2.4.2. Measuring Microwave Radiation of CYP102 A1 Solution
2.5. AFM Visualization
2.5.1. Sample Preparation
2.5.2. Atomic force Microscopy Measurements
2.6. AFM Data Processing
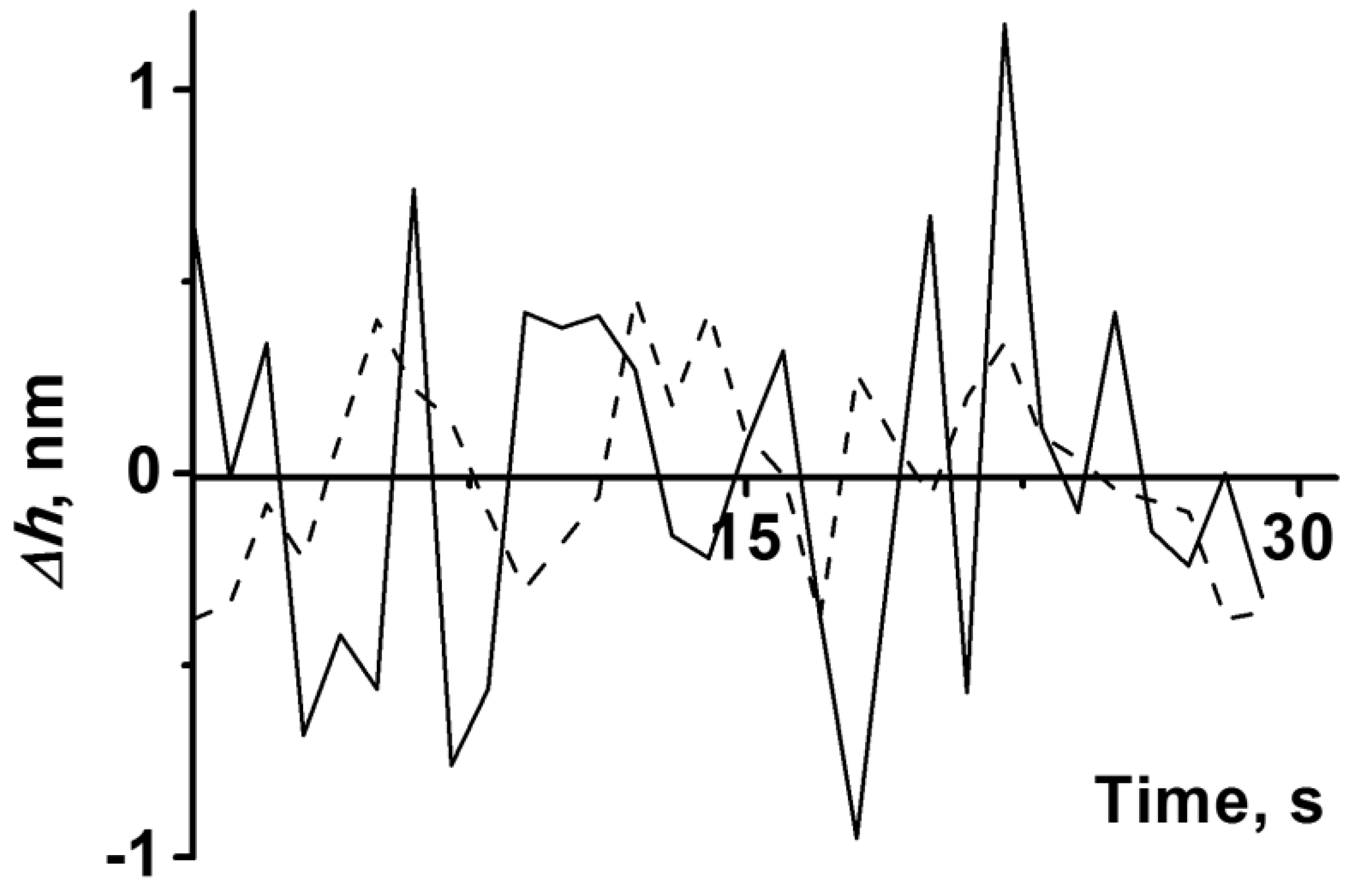
2.7. Analysis of CYP102A1 Molecules’ Activity Using the AFM Method
3. Results
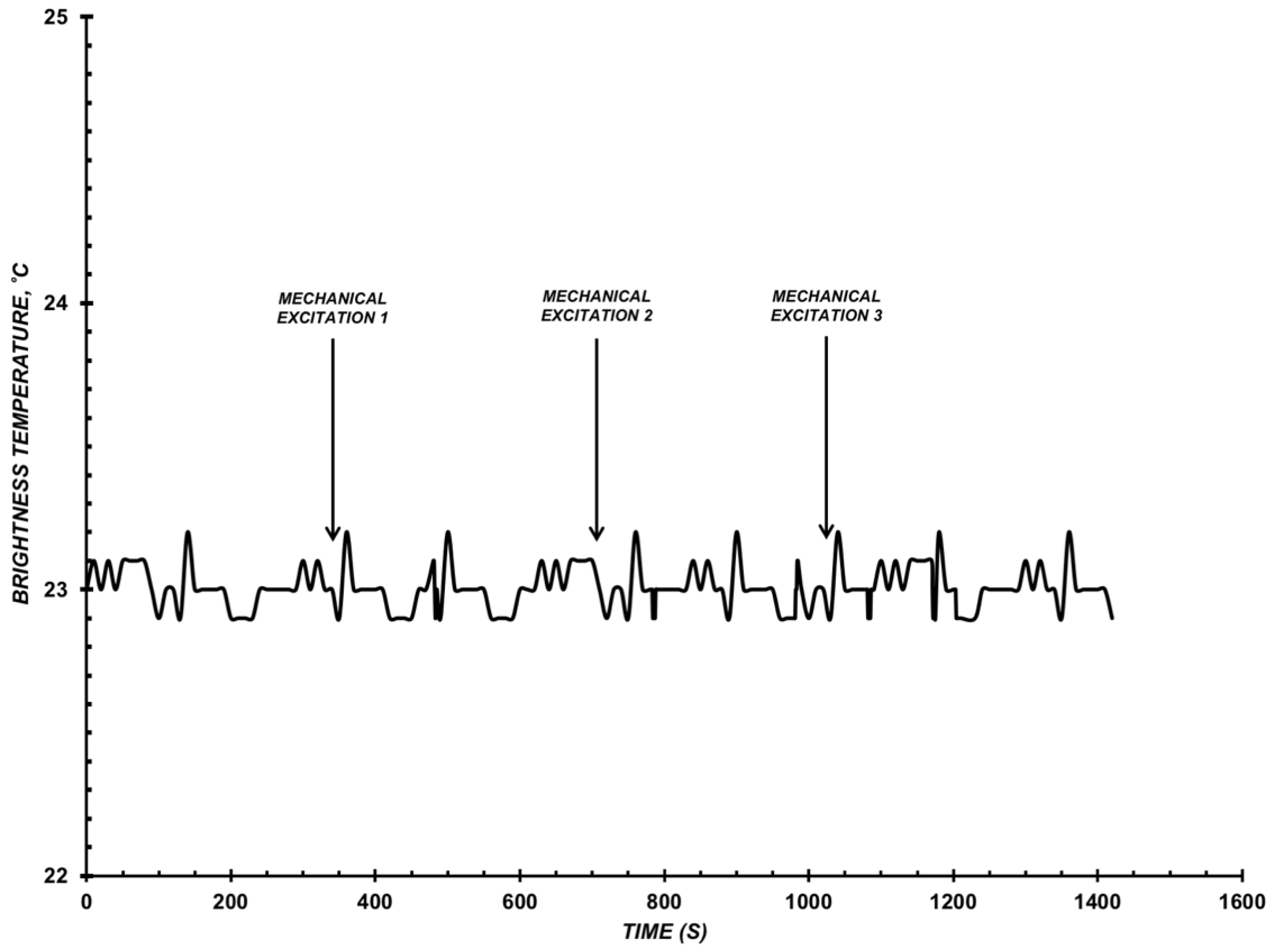
3.1. Results of Microwave Radiation Check-Measurements
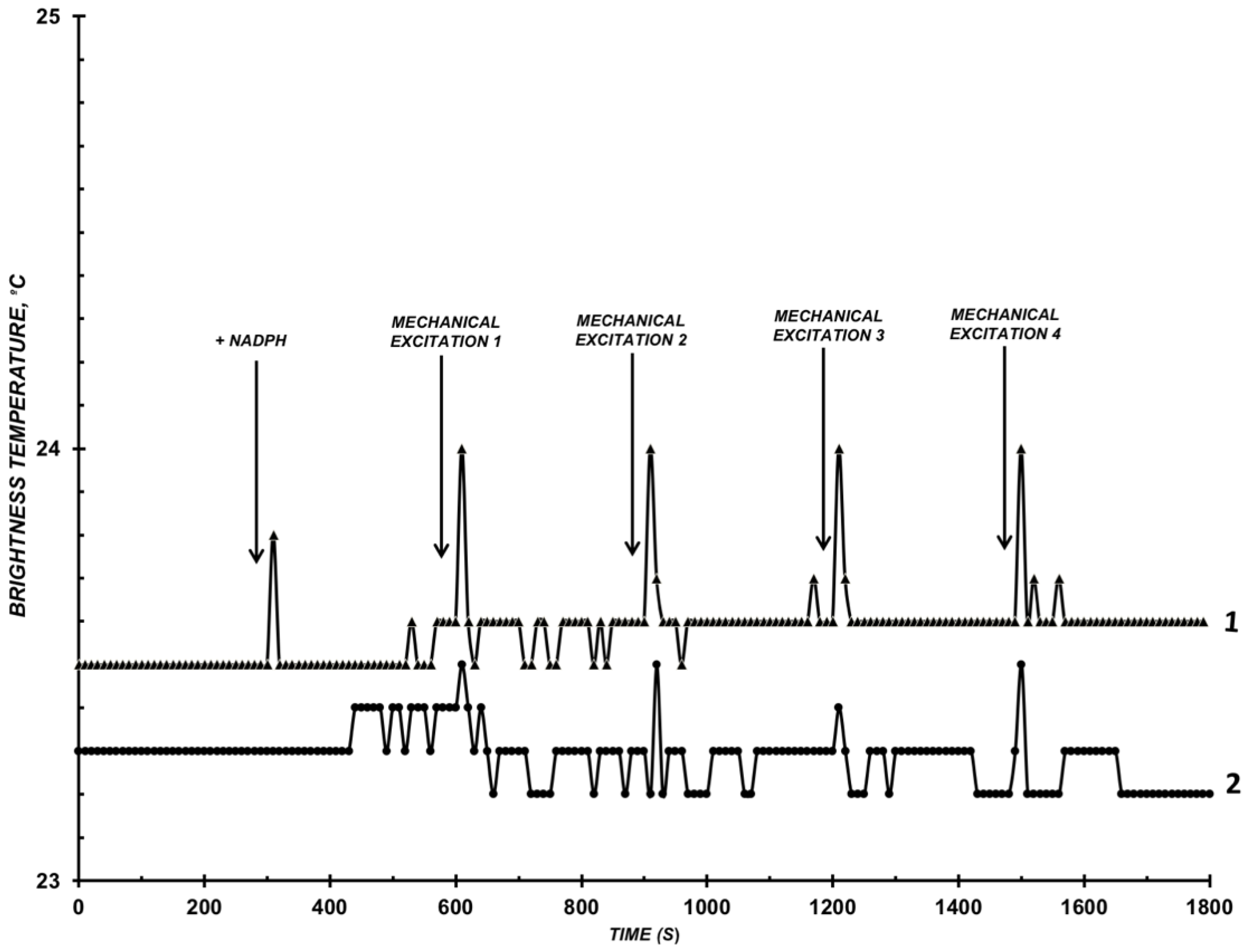
3.2. Results of Detecting Microwave Radiation of CYP102 A1 WT and A264K Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semiglazov, V.F. Diverse Biology of Breast Cancer: Search for Adequate Treatment. Malig. Tumours 2016, 3, 5–10. [Google Scholar] [CrossRef][Green Version]
- Vesnin, S.G. Using RTM Method in Regions Where Mammography Screening Is Implemented; Association for Microwave Radiothermometry. Available online: http://www.resltd.ru/rus/literature/507/Use_reg.pdf (accessed on 7 April 2022).
- Burdina, L.M.; Pinhosevich, E.G.; Khailenko, V.A. Radiometry in the Algorithm of Complex Examination of Mammary Glands. Mod. Oncol. 2001, 6, 8–10. [Google Scholar]
- Caferova, S.; Uysal, F.; Balcı, P.; Saydam, S.; Canda, T. Efficacy and Safety of Breast Radiothermometry in the Differential Diagnosis of Breast Lesions. Współczesna Onkol. 2014, 3, 197–203. [Google Scholar] [CrossRef]
- Rozhkova, N.I.; Smirnova, N.A.; Nazarov, A.A. Interventional Radiology, Nuclear Medicine and the Latest Non-Invasive Technologies in the Diagnosis and Treatment of Breast Diseases. In Proceedings of the Materials of the IV All-Russian Scientific and Practical Conference, Novokuznetsk, Russia, 12–15 April 2016; pp. 118–120. [Google Scholar]
- Gautherie, M.; Gros, C.M. Breast Thermography and Cancer Risk Prediction. Cancer 1980, 45, 51–56. [Google Scholar] [CrossRef]
- Vesnin, S.G.; Kaplan, M.A.; Avakyan, R.S. Modern Microwave Radiometry of the Mammary Glands. Tumours Female Reprod. Syst. 2008, 3, 28–33. [Google Scholar]
- Burdina, L.M.; Pinkhosevich, E.G.; Haylenko, V.A.; Burdina, I.I.; Vesnin, S.G.; Tikhomirova, N.N. Comparative Analysis of the Results of Examination of Patients with Breast Cancer According to the Data of X-ray Mammographic and Radiothermometric Examinations. Mod. Oncol. 2006, 6, 8–10. [Google Scholar]
- Ivanov, Y.D.; Malsagova, K.A.; Izotov, A.A.; Pleshakova, T.O.; Tatur, V.Y.; Vesnin, S.G.; Ivanova, N.D.; Usanov, S.A.; Archakov, A.I. Detection of Microwave Radiation of Cytochrome CYP102 A1 Solution during the Enzyme Reaction. Biochem. Biophys. Rep. 2016, 5, 285–289. [Google Scholar] [CrossRef][Green Version]
- Zinoviev, S.V.; Ivanov, A.V. Functional Microwave Thermography of Primary Malignant Tumors: An Experimental Study. Med. Vis. 2017, 4, 57–58. [Google Scholar]
- Ivanov, Y.D.; Malsagova, K.A.; Pleshakova, T.O.; Vesnin, S.G.; Tatur, V.Y.; Yarygin, K.N. Monitoring of Brightness Temperature of Suspension of Follicular Thyroid Carcinoma Cells in SHF Range by Radiothermometry. Pathol. Fiziol. Exp. Ther. 2016, 60, 174–177. [Google Scholar]
- Ivanov, Y.D.; Kozlov, A.F.; Malsagova, K.A.; Pleshakova, T.O.; Vesnin, S.G.; Tatur, V.Y.; Ivanova, N.D.; Ziborov, V.S. Monitoring of Microwave Emission of HRP System during the Enzyme Functioning. Biochem. Biophys. Rep. 2016, 7, 20–25. [Google Scholar] [CrossRef]
- Wang, Q.; Chaerkady, R.; Wu, J.; Hwang, H.J.; Papadopoulos, N.; Kopelovich, L.; Maitra, A.; Matthaei, H.; Eshleman, J.R.; Hruban, R.H.; et al. Mutant Proteins as Cancer-Specific Biomarkers. Proc. Natl. Acad. Sci. USA 2011, 108, 2444–2449. [Google Scholar] [CrossRef]
- Suzuki, M.; Aki, A.; Mizuki, T.; Maekawa, T.; Usami, R.; Morimoto, H. Encouragement of Enzyme Reaction Utilizing Heat Generation from Ferromagnetic Particles Subjected to an AC Magnetic Field. PLoS ONE 2015, 10, e0127673. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, W.; Zhang, Y.; Zhang, Y.; Chen, Y.; He, Y.; Fan, H. Remote and Real Time Control of an FVIO-Enzyme Hybrid Nanocatalyst Using Magnetic Stimulation. Nanoscale 2019, 11, 18081–18089. [Google Scholar] [CrossRef]
- Andreeva, Y.I.; Drozdov, A.S.; Avnir, D.; Vinogradov, V.V. Enzymatic Nanocomposites with Radio Frequency Field-Modulated Activity. ACS Biomater. Sci. Eng. 2018, 4, 3962–3967. [Google Scholar] [CrossRef]
- Collins, C.B.; Riskowski, R.A.; Ackerson, C.J. Radiofrequency Remote Control of Thermolysin Activity. Sci. Rep. 2021, 11, 6070. [Google Scholar] [CrossRef]
- Collins, C.B.; Ackerson, C.J. Remote enzyme activation using gold coated magnetite as antennae for radio frequency fields. In Proceedings of the Proceedings SPIE 10507, Colloidal Nanoparticles for Biomedical Applications XIII, San Francisco, CA, USA, 23 February 2018. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Armenia, I.; Serantes, D.; Veintemillas-Verdaguer, S.; Zeballos, N.; López-Gallego, F.; Grazu, V. Selective Magnetic Nanoheating: Combining Iron Oxide Nanoparticles for Multi-Hot-Spot Induction and Sequential Regulation. Nano Lett. 2021, 21, 7213–7220. [Google Scholar] [CrossRef]
- Didenko, N.P.; Zelentsov, V.I.; Cha, V.A. Study of the Multi-Resonance Interaction of Electromagnetic Oscillations with a Hemoglobin Molecule Using Mössbauer Spectrometry. Work. Res. Inst. Nucl. Phys. 1983, 10, 77–81. [Google Scholar]
- Didenko, N.P.; Gurevich, M.E.; Perelmuter, V.M.; Cha, V.A. Influence of Electromagnetic Radiation on the Affinity of Hemagglutinating Immunoglobulins; Federal State Budgetary Institution of Science All-Russian Institute of Scientific and Technical Information of the Russian Academy of Sciences: Moscow, Russia, 1984; p. 10. [Google Scholar]
- Narhi, L.O.; Fulco, A.J. Identification and Characterization of Two Functional Domains in Cytochrome P-450BM-3, a Catalytically Self-Sufficient Monooxygenase Induced by Barbiturates in Bacillus Megaterium. J. Biol. Chem. 1987, 262, 6683–6690. [Google Scholar] [CrossRef]
- Sevrioukova, I.F.; Li, H.; Zhang, H.; Peterson, J.A.; Poulos, T.L. Structure of a Cytochrome P450-Redox Partner Electron-Transfer Complex. Proc. Natl. Acad. Sci. USA 1999, 96, 1863–1868. [Google Scholar] [CrossRef]
- Iggo, R.; Bartek, J.; Lane, D.; Gatter, K.; Harris, A.L.; Bartek, J. Increased Expression of Mutant Forms of P53 Oncogene in Primary Lung Cancer. Lancet 1990, 335, 675–679. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-Function Mutant P53 in Cancer Progression and Therapy. J. Mol. Cell Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 Pharmacogenetics and Cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef]
- Miura, Y.; Fulco, A.J. ω-1, ω-2 and ω-3 Hydroxylation of Long-Chain Fatty Acids, Amides and Alcohols by a Soluble Enzyme System from Bacillus Megatyerium. Biochim. Biophys. Acta BBA-Lipids Lipid Metab. 1975, 388, 305–317. [Google Scholar] [CrossRef]
- Neeli, R.; Girvan, H.M.; Lawrence, A.; Warren, M.J.; Leys, D.; Scrutton, N.S.; Munro, A.W. The Dimeric Form of Flavocytochrome P450 BM3 Is Catalytically Functional as a Fatty Acid Hydroxylase. FEBS Lett. 2005, 579, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Girvan, H.M.; Toogood, H.S.; Littleford, R.E.; Seward, H.E.; Smith, W.E.; Ekanem, I.S.; Leys, D.; Cheesman, M.R.; Munro, A.W. Novel Haem Co-Ordination Variants of Flavocytochrome P450BM3. Biochem. J. 2009, 417, 65–76. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Pleshakova, T.O.; Frantsuzov, P.A.; Krokhin, N.V.; Ziborov, V.S.; Archakov, A.I. Atomic Force Microscopy Visualization and Measurement of the Activity and Physicochemical Properties of Single Monomeric and Oligomeric Enzymes. Biophysics 2011, 56, 892–896. [Google Scholar] [CrossRef]
- Archakov, A.I.; Bachmanova, G.I. Cytochrome P450 and Active Oxygen; Taylor & Francis: New York, NY, USA, 1990; p. 275. [Google Scholar]
- Vaisblat, A.V. Radiothermography as a Diagnostic Method in Medicine; National Medical Research Center for Children’s Health: Moscow, Russia, 2003. [Google Scholar]
- Radmacher, M.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Direct Observation of Enzyme Activity with the Atomic Force Microscope. Science 1994, 265, 1577–1579. [Google Scholar] [CrossRef]
- Pershin, S.M. Conversion of Ortho-Para-H2O Isomers in Water and a Jump in Erythrocyte Fluidity through a Microcapillary at a Temperature of 36.6 ± 0.3 °C. Phys. Wave Phenom. 2009, 17, 241–250. [Google Scholar] [CrossRef]
- de Montellano, P.R.O. (Ed.) Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer: Boston, MA, USA, 1995. [Google Scholar] [CrossRef]
- Pershin, S.M.; Bunkin, A.F. Temperature Evolution of the Relative Concentration of the H2O Ortho/Para Spin Isomers in Water Studied by Four-Photon Laser Spectroscopy. Laser Phys. 2009, 19, 1410–1414. [Google Scholar] [CrossRef]
- Pershin, S.M. Signal Exchange between Bio-Objects on the Principle of Carrier Modulation: Coherent Radiation of Cosmic OH (1.6–1.7 GHz) and H2O (22.3 GHz) Masers. Available online: https://www.phys.msu.ru/rus/about/sovphys/ISSUES-2010/03(80)-2010/9883/ (accessed on 7 April 2022).
- Ivanov, Y.D.; Malsagova, K.A.; Vesnin, S.G.; Tatur, V.Y.; Ivanova, N.D.; Ziborov, V.S. The Registration of a Biomaser-Like Effect in an Enzyme System with an RTM Sensor. J. Sens. 2019, 2019, 7608512. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Malsagova, K.A.; Bukharina, N.S.; Vesnin, S.G.; Usanov, S.A.; Tatur, V.Y.; Lukyanitsa, A.A.; Ivanova, N.D.; Konev, V.A.; Ziborov, V.S. Radiothermometric Study of the Effect of Amino Acid Mutation on the Characteristics of the Enzymatic System. Diagnostics 2022, 12, 943. https://doi.org/10.3390/diagnostics12040943
Ivanov YD, Malsagova KA, Bukharina NS, Vesnin SG, Usanov SA, Tatur VY, Lukyanitsa AA, Ivanova ND, Konev VA, Ziborov VS. Radiothermometric Study of the Effect of Amino Acid Mutation on the Characteristics of the Enzymatic System. Diagnostics. 2022; 12(4):943. https://doi.org/10.3390/diagnostics12040943
Chicago/Turabian StyleIvanov, Yuri D., Kristina A. Malsagova, Natalia S. Bukharina, Sergey G. Vesnin, Sergey A. Usanov, Vadim Yu. Tatur, Andrei A. Lukyanitsa, Nina D. Ivanova, Vladimir A. Konev, and Vadim S. Ziborov. 2022. "Radiothermometric Study of the Effect of Amino Acid Mutation on the Characteristics of the Enzymatic System" Diagnostics 12, no. 4: 943. https://doi.org/10.3390/diagnostics12040943
APA StyleIvanov, Y. D., Malsagova, K. A., Bukharina, N. S., Vesnin, S. G., Usanov, S. A., Tatur, V. Y., Lukyanitsa, A. A., Ivanova, N. D., Konev, V. A., & Ziborov, V. S. (2022). Radiothermometric Study of the Effect of Amino Acid Mutation on the Characteristics of the Enzymatic System. Diagnostics, 12(4), 943. https://doi.org/10.3390/diagnostics12040943






