Noninvasive Evaluation of Myocardial Work in Patients with Chronic Kidney Disease Using Left Ventricular Pressure-Strain Loop Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Study Design and Population
2.3. Clinical Features
2.4. Echocardiographic Analysis
2.5. Left Ventricular Global Longitudinal Strain and Myocardial Motion Synchronization
2.6. Quantitative Analysis of Myocardial Work Done by the Left Ventricle
- GWI (mmHg%): the total work done in the LV PSL analysis calculated from mitral valve closure to mitral valve opening (except in diastole).
- Global constructive work (GCW, mmHg%): contributes to LV ejection work, including systolic myocardial shortening and isovolumic diastolic myocardial elongation.
- Global waste work (GWW, mmHg%): not conducted for LV ejection work, including systolic myocardial elongation and isovolumic diastolic period shortening and increasing.
- Global work efficiency (GWE, %): (GCW/(GCW + GWW) × 100%.
2.7. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Characteristics
3.2. Transthoracic Echocardiographic Parameters
3.3. Left Ventricular Global Longitudinal Strain and Myocardial Asynchrony
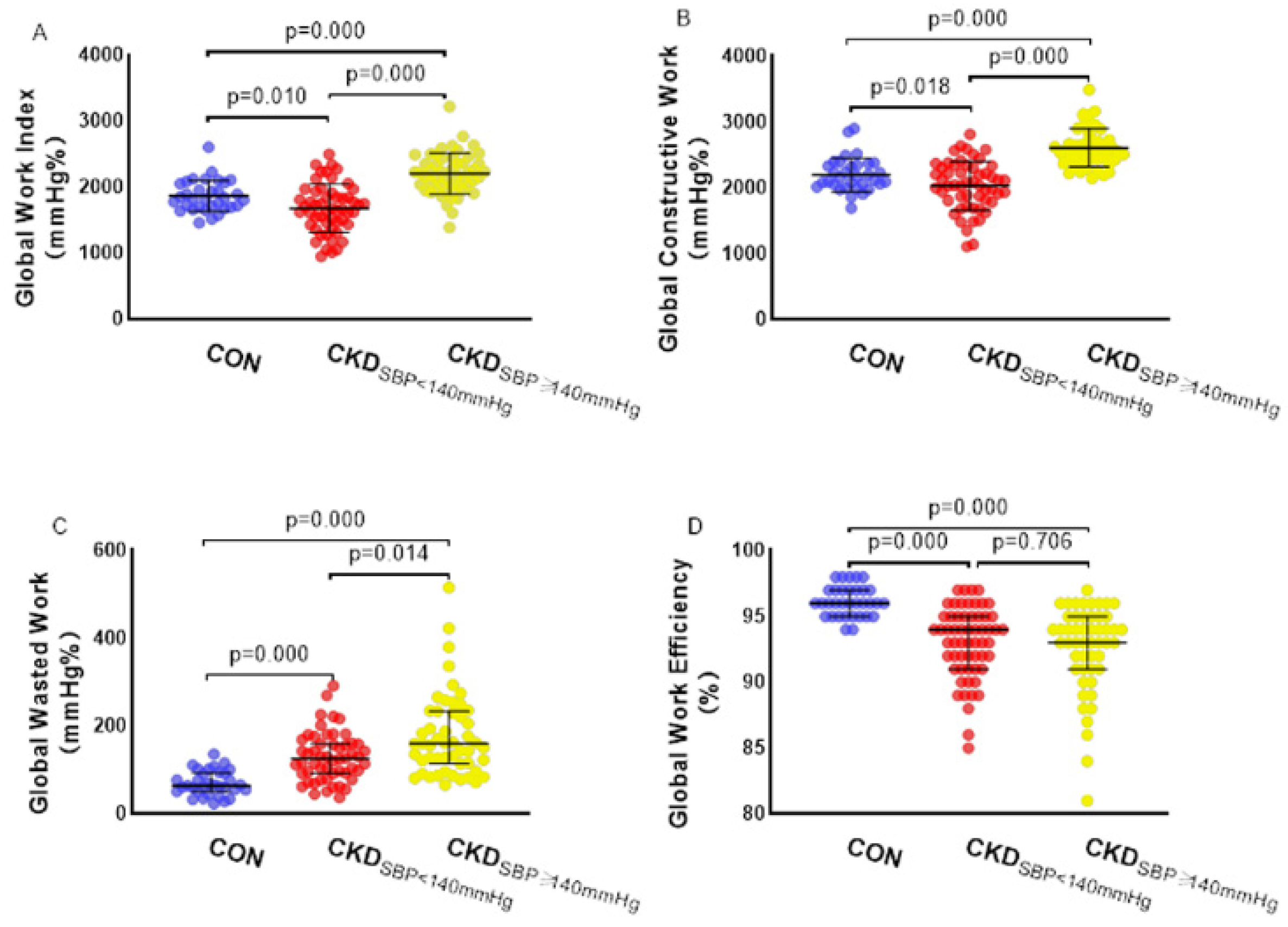
3.4. Myocardial Work Analysis
3.5. Independent Correlation Analysis of Various Parameters of Myocardial Work in Patients with Chronic Kidney Disease
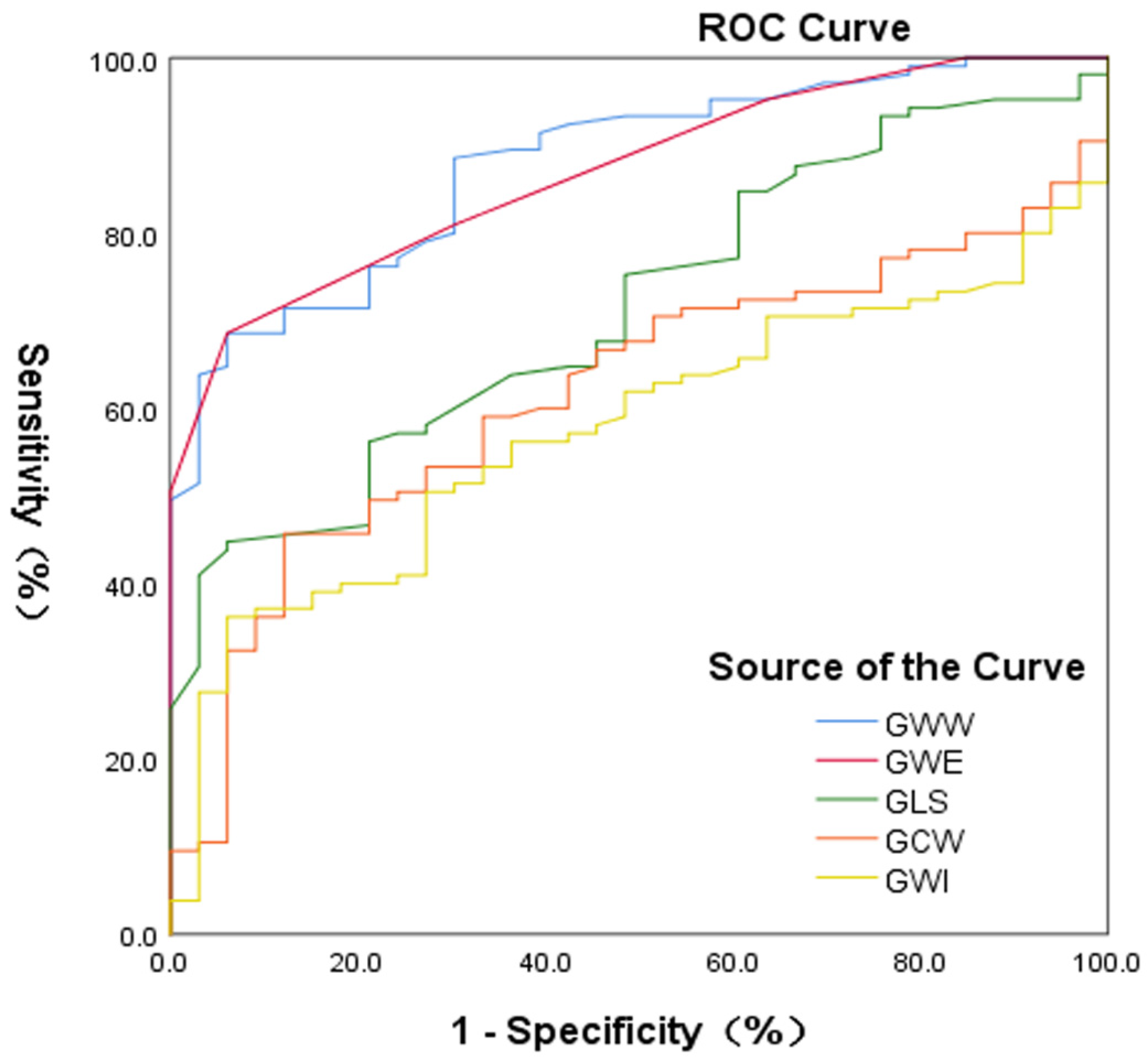
3.6. Accuracy of Identification of Myocardial Injury between Patients with Chronic Kidney Disease and the Controls
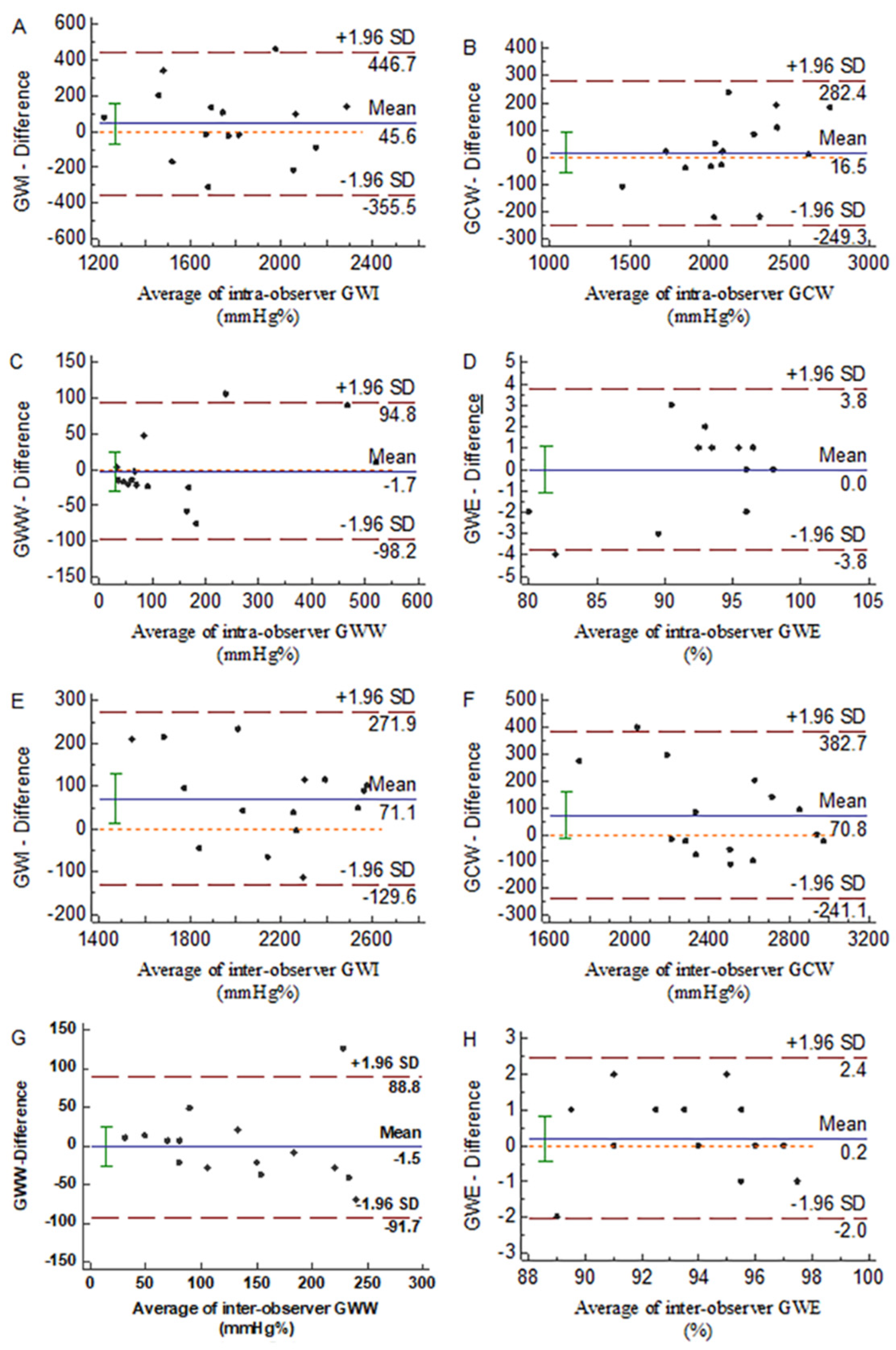
3.7. Repeatability of the Parameters of Myocardial Work
4. Discussion
4.1. Main Findings
4.2. Influence of Left Ventricular Hypertrophy on Myocardial Work
4.3. Influence of the Systolic Blood Pressure on Myocardial Work
4.4. A New Index for Detecting Myocardial Injury in Patients with Chronic Kidney Disease
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Essig, M.; Escoubet, B.; De Zuttere, D.; Blanchet, F.; Arnoult, F.; Dupuis, E.; Michel, C.; Mignon, F.; Mentre, F.; Clerici, C.; et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol. Dial. Transplant. 2008, 23, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saran, A.M.; Dubose, T.D. Cardiovascular disease in chronic kidney disease. Ther. Adv. Cardiovasc. Dis. 2008, 2, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Li, S.; Ma, J.Z.; Herzog, C. Cardiovascular disease in end-stage renal disease patients. Am. J. Kidney Dis. 2001, 38, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Pecoits-Filho, R.; Barberato, S.H. Left ventricular mass in chronic kidney disease and ESRD. Clin. J. Am. Soc. Nephrol. 2009, 4, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Guyton, A.C.; Coleman, T.G. Quantitative analysis of the pathophysiology of hypertension. J. Am. Soc. Nephrol. 1999, 10, 2248–2258. [Google Scholar]
- Locatelli, F.; Bommer, J.; London, G.M.; Martín-Malo, A.; Wanner, C.; Yaqoob, M.; Zoccali, C. Cardiovascular disease determinants in chronic renal failure: Clinical approach and treatment. Nephrol. Dial. Transplant. 2001, 16, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Amann, K.; Breitbach, M.; Ritz, E.; Mall, G. Myocyte/Capillary in the Heart of Uremic Patients. J. Am. Soc. Nephrol. 1998, 9, 1018–1022. [Google Scholar] [CrossRef]
- Mignot, A.; Donal, E.; Zaroui, A.; Reant, P.; Salem, A.; Hamon, C.; Monzy, S.; Roudaut, R.; Habib, G.; Lafitte, S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: A multicenter study. J. Am. Soc. Echocardiogr. 2010, 23, 1019–1024. [Google Scholar] [CrossRef]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of myocardial work from pressure–strain loops analysis: An experimental evaluation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.K.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Boe, E.; Russell, K.; Eek, C.; Eriksen, M.; Remme, E.W.; Smiseth, O.A.; Skulstad, H. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-STsegment elevation-acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society ofHypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESCTask Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, T.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Singer, J.; Thompson, C.R.; Ross, H.; Lewis, M. Prevalent left ventricular hypertrophy in the predialysis population: Identifying opportunities for intervention. Am. J. Kidney Dis. 1996, 27, 347–354. [Google Scholar] [CrossRef]
- Chang, J.M.; Chen, S.C.; Huang, J.C.; Su, H.M.; Chen, H.C. Anemia and left ventricular hypertrophy with renal function decline and cardiovascular events in chronic kidney disease. Am. J. Med. Sci. 2014, 347, 183–189. [Google Scholar] [CrossRef]
- Shi, F.; Feng, S.; Zhu, J.; Wu, Y.; Chen, J. Left Ventricular Strain and Dyssynchrony in Young and Middle-Aged Peritoneal Dialysis Patients and Healthy Controls: A Case-Matched Study. CardioRenal Med. 2018, 8, 271–284. [Google Scholar] [CrossRef]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressurestrain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.Y.; Seeberger, A.; Lind, B.; Nowak, J.; Do Nascimento, M.M.; Lindholm, B.; Brodin, L.Å. A single session of haemodialysis improves left ventricular synchronicity in patients with end-stage renal disease: A pilot tissue synchronization imaging study. Nephrol. Dial. Transplant. 2008, 23, 3622–3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, T.; Dohi, K.; Onishi, K.; Sugiura, E.; Fujimoto, N.; Ichikawa, K.; Ishikawa, E.; Nakamura, M.; Nomura, S.; Takeuchi, H.; et al. Role of haemodialytic therapy on left ventricular mechanical dyssynchrony in patients with end-stage renal disease quantified by speckle-tracking strain imaging. Nephrol. Dial. Transplant. 2011, 26, 1655–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlaug, B.A.; Lam, C.S.P.; Roger, V.L.; Rodeheffer, R.J.; Redfield, M.M. Contractility and Ventricular Systolic Stiffening in Hypertensive Heart Disease. J. Am. Coll. Cardiol. 2009, 54, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, S.; Gendelman, G.; Ayzenberg, O.; Smirin, N.; Lysyansky, P.; Edri, O.; Deutsch, L.; Caspi, A.; Friedman, Z. Differential effects of coronary artery stenosis on myocardial function: The value of myocardial strain analysis for the detection of coronary artery disease. J. Am. Soc. Echocardiogr. 2011, 24, 748–757. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Skulstad, H.; Edvardsen, T.; Urheim, S.; Rabben, S.I.; Stugaard, M.; Lyseggen, E.; Ihlen, H.; Smiseth, O.A. Postsystolic shortening in ischemic myocardium: Active contraction or passive recoil? Circulation 2002, 106, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Galli, E.; Lancellotti, P.; Sengupta, P.P.; Donal, E. LV mechanics in mitral and aortic valve diseases: Value of functional assessment beyond ejection fraction. JACC Cardiovasc. Imaging 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [Green Version]
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients With Normal Left Ventricular Function and Wall Motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957. [Google Scholar] [CrossRef]
- Galli, E.; Vitel, E.; Schnell, F.; Le Rolle, V.; Hubert, A.; Lederlin, M.; Donal, E. Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography 2019, 36, 74–82. [Google Scholar] [CrossRef] [Green Version]

| CON (n = 33) | CKDN-LVH (n = 59) | CKDLVH (n = 46) | p | |
|---|---|---|---|---|
| Age (years) | 48.1 ± 8.9 | 53.2 ± 16.1 | 54.4 ± 15.3 | 0.134 |
| Male gender, n (%) | 18 (54.5%) | 39 (66.1%) | 22 (47.8%) | 0.161 |
| SBP (mmHg) | 119.8 ± 9.4 | 130.8 ± 17.5 * | 150.9 ± 17.2 *† | 0.000 |
| DBP (mmHg) | 78.6 ± 7.8 | 81.4 ± 10.5 | 87.7 ± 13.1 *† | 0.001 |
| BMI (kg/m2) | 23.7 ± 2.1 | 22.9 ± 3.2 | 23.5 ± 3.5 | 0.459 |
| BSA (m2) | 1.7 ± 0.18 | 1.69 ± 0.17 | 1.65 ± 0.17 | 0.441 |
| HR (bpm) | 66.2 ± 8.8 | 73.6 ± 10.4 * | 73.2 ± 10.5 * | 0.002 |
| eGFR (mL/min/1.73 m2) | 104.5 ± 14.9 | 35.1 ± 27.8 * | 15.4 ± 17.5 *† | 0.000 |
| SCR (umol/L) | 69.5 ± 15.9 | 356.7 ± 340.2 * | 596.7 ± 359.5 *† | 0.000 |
| BUN (mmol/L) | 4.68 ± 1.14 | 14.67 ± 9.45 * | 20.43 ± 11.91 *† | 0.000 |
| CON (n = 33) | CKDN-LVH (n = 59) | CKDLVH (n = 46) | p | |
|---|---|---|---|---|
| IVSd (mm) | 9.24 ± 1.25 | 9.51 ± 1.33 | 11.7 ± 1.94 *† | 0.000 |
| LVIDd (mm) | 46.55 ± 4.15 | 46.1 ± 4.02 | 50.26 ± 5.14 *† | 0.000 |
| PWTd (mm) | 7.94 ± 1.12 | 9.01 ± 1.43 | 11.07 ± 1.82 *† | 0.000 |
| LVEDV (mL) | 101.36 ± 21.61 | 98.97 ± 19.51 | 121.3 ± 28.52 *† | 0.000 |
| LVEDVI (mL/m2) | 59.76 ± 10.35 | 58.6 ± 9.54 | 73.69 ± 15.43 *† | 0.000 |
| LVEF (%) | 68.52 ± 6.85 | 69.37 ± 6.06 | 66 ± 7.26 † | 0.036 |
| FS (%) | 38.58 ± 5.43 | 39.08 ± 4.88 | 36.87 ± 5.68 | 0.098 |
| LVMI (g/m2) | 78.7 ± 15.37 | 85.62 ± 15.56 | 133.99 ± 25.1 *† | 0.000 |
| CON (n = 33) | CKDN-LVH (n = 59) | CKDLVH (n = 46) | p | |
|---|---|---|---|---|
| GLS (%) | 20.05 ± 1.77 | 18.87 ± 2.16 * | 17.82 ± 2.48 *† | 0.000 |
| PSD (%) | 39.31 ± 7.94 | 50.7 ± 10.96 * | 61.23 ± 13.85 *† | 0.000 |
| GWI (mmHg%) | 1865.1 ± 235.3 | 1822.3 ± 427.9 | 2081.7 ± 393.5 *† | 0.002 |
| GCW (mmHg%) | 2201.5 ± 256.2 | 2196.7 ± 445.3 | 2462.9 ± 391.4 *† | 0.001 |
| GWW (mmHg%) | 64 (51, 92) | 129 (92.5, 158.5) * | 162 (98, 235) *† | 0.000 |
| GWE (%) | 96 (95, 97) | 94 (92, 95) * | 93 (90, 95) * | 0.000 |
| GLS (%) | PSD (%) | GWI (mmHg%) | GCW (mmHg%) | GWW (mmHg%) | GWE (%) | |
|---|---|---|---|---|---|---|
| CON (n = 33) | 20 ± 1.8 | 39.3 ± 7.9 | 1865.1 ± 235.3 | 2201.5 ± 256.2 | 64 (51, 92) | 96 (95, 97) |
| CKD (SBP < 140 mmHg, n = 54) | 18.5 ± 2.5 * | 50.7 ± 10.6 * | 1679.6 ± 366.2 * | 2033.5 ± 372.7 * | 126 (92, 159) * | 93.5 (91, 95) * |
| CKD (SBP ≥ 140 mmHg, n = 51) | 18.3 ± 2.2 * | 60.2 ± 14.3 *† | 2207.5 ± 313.3 *† | 2609.7 ± 288.3 *† | 160 (115, 232) *† | 93 (91, 95) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, L.; Zhong, X.; Peng, G.; Sheng, Y.; Li, J.; Liu, Q.; Shi, B.; Huang, Y.; Xu, J.; et al. Noninvasive Evaluation of Myocardial Work in Patients with Chronic Kidney Disease Using Left Ventricular Pressure-Strain Loop Analysis. Diagnostics 2022, 12, 856. https://doi.org/10.3390/diagnostics12040856
Liu X, Chen L, Zhong X, Peng G, Sheng Y, Li J, Liu Q, Shi B, Huang Y, Xu J, et al. Noninvasive Evaluation of Myocardial Work in Patients with Chronic Kidney Disease Using Left Ventricular Pressure-Strain Loop Analysis. Diagnostics. 2022; 12(4):856. https://doi.org/10.3390/diagnostics12040856
Chicago/Turabian StyleLiu, Xiaohua, Lixin Chen, Xiaofang Zhong, Guijuan Peng, Yuanyuan Sheng, Jian Li, Qian Liu, Bobo Shi, Yuxiang Huang, Jinfeng Xu, and et al. 2022. "Noninvasive Evaluation of Myocardial Work in Patients with Chronic Kidney Disease Using Left Ventricular Pressure-Strain Loop Analysis" Diagnostics 12, no. 4: 856. https://doi.org/10.3390/diagnostics12040856
APA StyleLiu, X., Chen, L., Zhong, X., Peng, G., Sheng, Y., Li, J., Liu, Q., Shi, B., Huang, Y., Xu, J., & Liu, Y. (2022). Noninvasive Evaluation of Myocardial Work in Patients with Chronic Kidney Disease Using Left Ventricular Pressure-Strain Loop Analysis. Diagnostics, 12(4), 856. https://doi.org/10.3390/diagnostics12040856






