Compliance with Cardiovascular Prevention Guidelines in Type 2 Diabetes Individuals in a Middle-Income Region: A Cross-Sectional Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
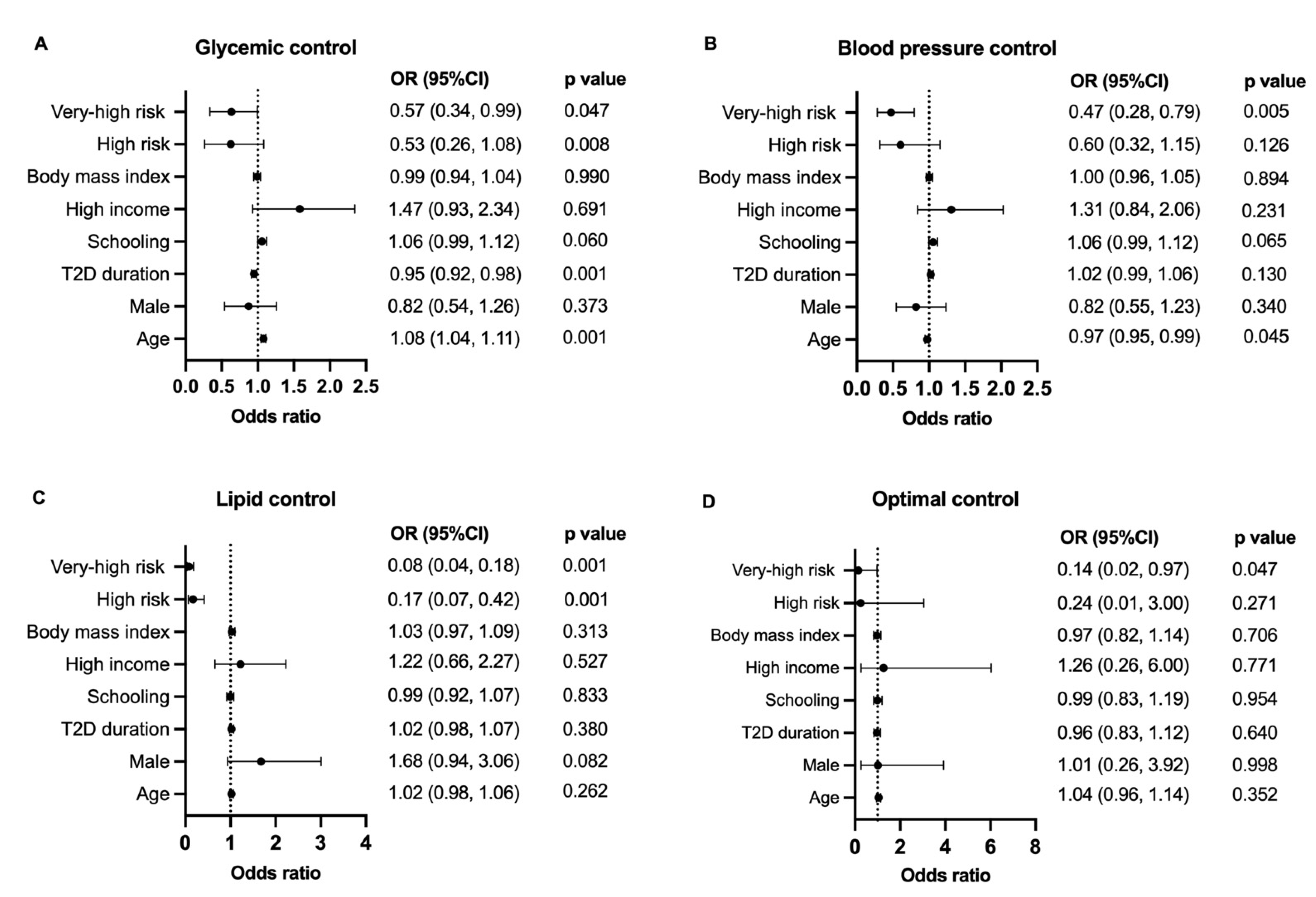
2.2. Definition of Cardiovascular Risk and Goals
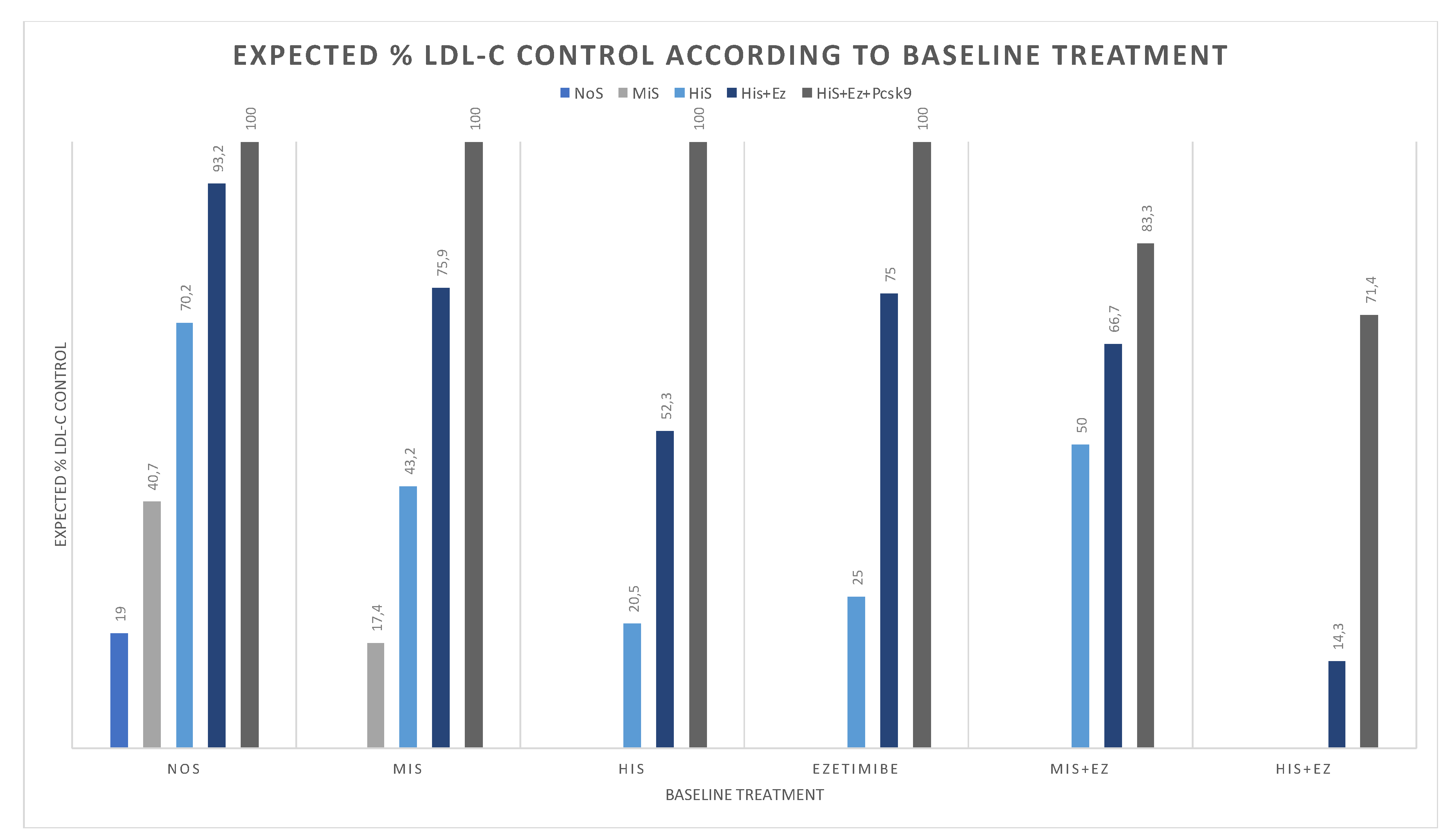
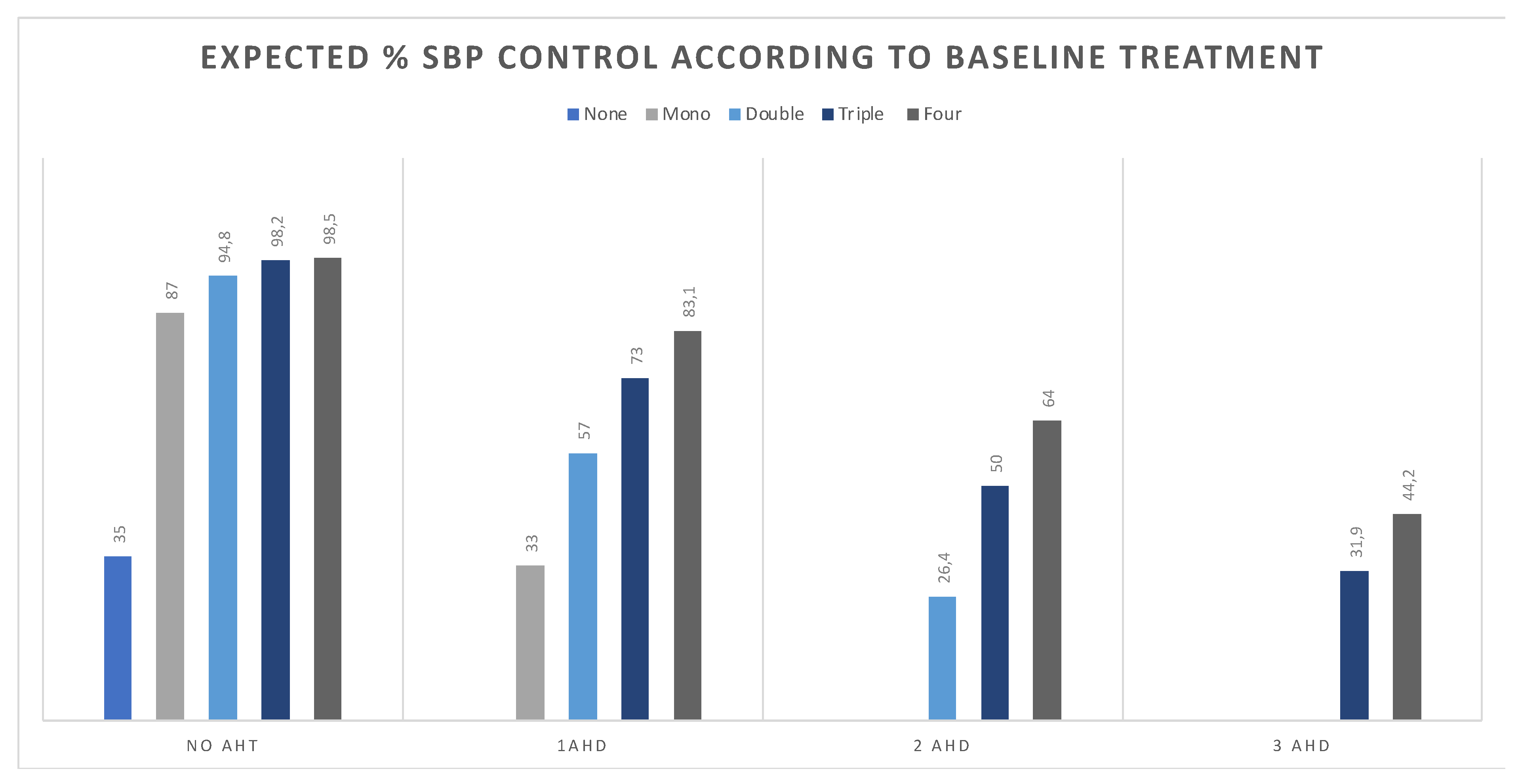
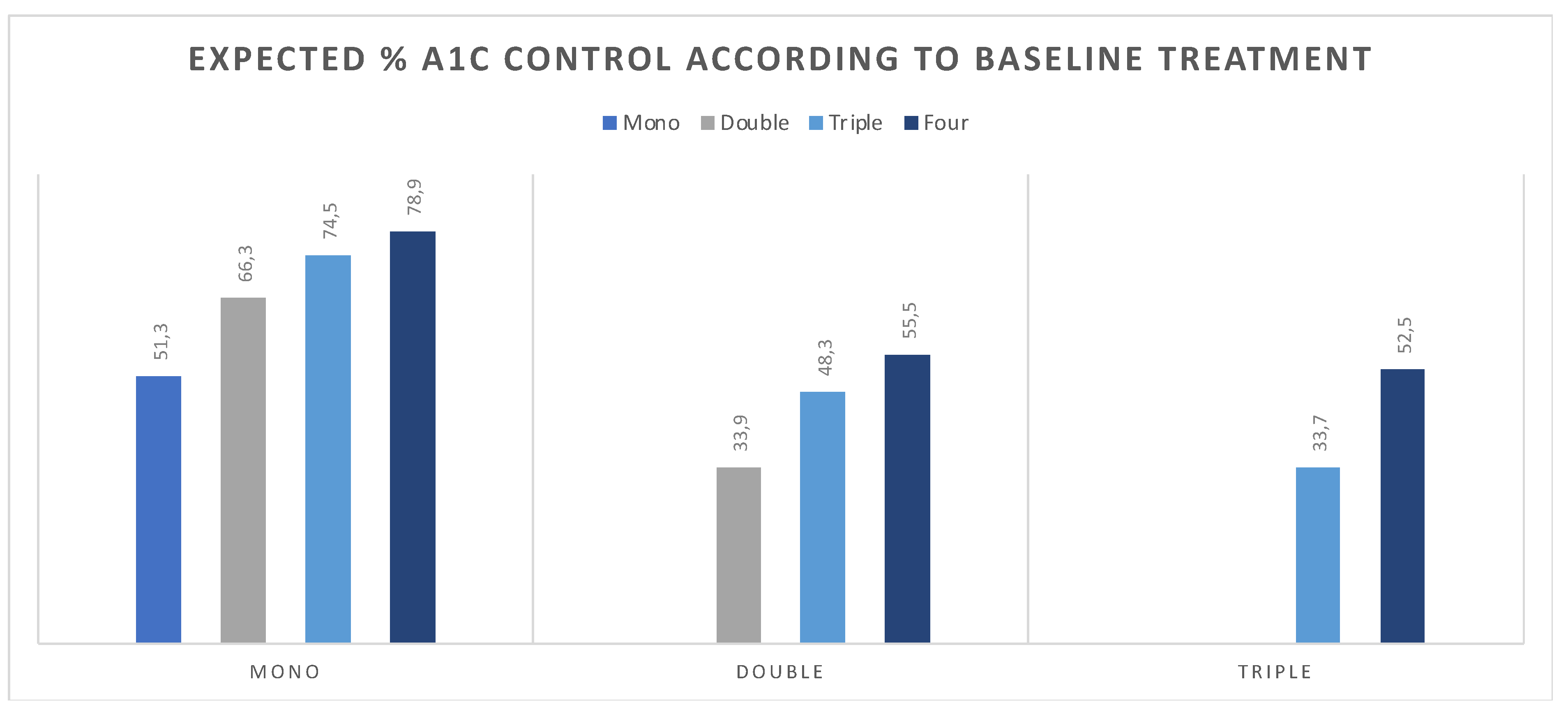
2.3. Estimate Change in Attainment Rate According to Intervention
2.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- IDF Diabetes Atlas 2021. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 12 October 2021).
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. Available online: https://www.ncbi.nlm.nih.gov/pubmed/9742976 (accessed on 12 October 2021). [CrossRef]
- Dhindsa, D.S.; Sandesara, P.B.; Shapiro, M.D.; Wong, N.D. The Evolving Understanding and Approach to Residual Cardiovascular Risk Management. Front. Cardiovasc. Med. 2020, 7, 88. [Google Scholar] [CrossRef]
- Luo, D.; Cheng, Y.; Zhang, H.; Ba, M.; Chen, P.; Li, H.; Chen, K.; Sha, W.; Zhang, C.; Chen, H. Association between high blood pressure and long term cardiovascular events in young adults: Systematic review and meta-analysis. BMJ 2020, 370, m3222. [Google Scholar] [CrossRef]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: Findings from the China National Stroke Screening and prevention project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef]
- Wang, C.C.; Gurevich, I.; Draznin, B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes 2003, 52, 2562–2569. [Google Scholar] [CrossRef] [Green Version]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Shen, X.; An, Y.; Gong, Q.; Li, H.; Zhang, B.; Shuai, Y.; Chen, Y.; Hu, Y.; et al. Higher blood pressure predicts diabetes and enhances long-term risk of cardiovascular disease events in individuals with impaired glucose tolerance: Twenty-three-year follow-up of the Daqing diabetes prevention study. J. Diabetes 2019, 11, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Adler, A.I.; Stratton, I.M.; Neil, H.A.; Yudkin, J.S.; Matthews, D.R.; Cull, C.A.; Wright, A.D.; Turner, R.C.; Holman, R.R. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ 2000, 321, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1) as a Cardiovascular Risk Predictor: Mechanistic Insight and Potential Clinical Use. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, J.; Barter, P.; Carmena, R.; Deedwania, P.; Fruchart, J.C.; Haffner, S.; Hsia, J.; Breazna, A.; LaRosa, J.; Grundy, S.; et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: The Treating to New Targets (TNT) study. Diabetes Care 2006, 29, 1220–1226. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists, C.; Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- Khunti, K.; Ceriello, A.; Cos, X.; De Block, C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2018, 137, 137–148. [Google Scholar] [CrossRef]
- Grintsova, O.; Maier, W.; Mielck, A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (SES) and regional deprivation: A systematic literature review. Int. J. Equity Health 2014, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Mosenzon, O.; Alguwaihes, A.; Leon, J.L.A.; Bayram, F.; Darmon, P.; Davis, T.M.E.; Dieuzeide, G.; Eriksen, K.T.; Hong, T.; Kaltoft, M.S.; et al. CAPTURE: A multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc. Diabetol. 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandao, A.A.; Feitosa, A.D.D.M.; Nadruz, W. Brazilian Guidelines of Hypertension–2020. Arq. Bras. Cardiol. 2020, 116, 516–658. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.; Wolf, V.; Bonilha, I.; Luchiari, B.; Lima, M.; Oliveira, A.; Vitte, S.; Machado, G.; Cunha, J.; Borges, C.; et al. Rationale and design of the Brazilian diabetes study: A prospective cohort of type 2 diabetes. Curr. Med. Res. Opin. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Xavier, H.T.; Izar, M.C.; Faria Neto, J.R.; Assad, M.H.; Rocha, V.Z.; Sposito, A.C.; Fonseca, F.A.; dos Santos, J.E.; Santos, R.D.; Bertolami, M.C.; et al. V Brazilian Guidelines on Dyslipidemias and Prevention of Atherosclerosis. Arq. Bras. Cardiol. 2013, 101, 1–20. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Byyny, R.L. Losartan potassium lowers blood pressure measured by ambulatory blood pressure monitoring. J. Hypertens. Suppl. 1995, 13, S29–S33. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Simpson, R.L.; Toh, J.; Arcuri, K.E.; Goldberg, A.I.; Sweet, C.S. Controlled trial of losartan given concomitantly with different doses of hydrochlorothiazide in hypertensive patients. Blood Press. 1996, 5, 32–40. [Google Scholar] [CrossRef]
- Rakugi, H.; Tsuchihashi, T.; Shimada, K.; Numaguchi, H.; Nishida, C.; Yamaguchi, H.; Shirakawa, M.; Azuma, K.; Fujita, K.P. Efficacy and safety of fixed-dose losartan/hydrochlorothiazide/amlodipine combination versus losartan/hydrochlorothiazide combination in Japanese patients with essential hypertension. Clin. Exp. Hypertens. 2015, 37, 260–266. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Cardiovascular Disease and Risk Management. Diabetes Care 2020, 43, S111–S134. [Google Scholar] [CrossRef] [Green Version]
- Garber, A.J.; Donovan, D.S.; Dandona, P.; Bruce, S.; Park, J.S. Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 3598–3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, C.; Ranetti, A.E.; Li, D.; Ekholm, E.; Cook, W.; Hirshberg, B.; Chen, H.; Hansen, L.; Iqbal, N. Randomized, Double-Blind, Phase 3 Trial of Triple Therapy With Dapagliflozin Add-on to Saxagliptin Plus Metformin in Type 2 Diabetes. Diabetes Care 2015, 38, 2009–2017. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Kim, S.S.; Kim, J.H.; Kim, M.K.; Kim, T.N.; Lee, S.H.; Lee, C.W.; Park, J.Y.; Kim, E.S.; Lee, K.J.; et al. Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study. Diabetes Metab. J. 2020, 44, 67–77. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Cohen, S.S.; Killian, J.M.; Monda, K.L.; Weston, S.A.; Okerson, T. Lipid-Lowering Prescription Patterns in Patients With Diabetes Mellitus or Cardiovascular Disease. Am. J. Cardiol. 2019, 124, 995–1001. [Google Scholar] [CrossRef]
- De Luca, L.; Arca, M.; Temporelli, P.L.; Meessen, J.; Riccio, C.; Bonomo, P.; Colavita, A.R.; Gabrielli, D.; Gulizia, M.M.; Colivicchi, F.; et al. Current lipid lowering treatment and attainment of LDL targets recommended by ESC/EAS guidelines in very high-risk patients with established atherosclerotic cardiovascular disease: Insights from the START registry. Int. J. Cardiol. 2020, 316, 229–235. [Google Scholar] [CrossRef]
- Virani, S.S.; Woodard, L.D.; Chitwood, S.S.; Landrum, C.R.; Urech, T.H.; Wang, D.; Murawsky, J.; Ballantyne, C.M.; Petersen, L.A. Frequency and correlates of treatment intensification for elevated cholesterol levels in patients with cardiovascular disease. Am. Heart J. 2011, 162, 725–732.e721. [Google Scholar] [CrossRef]
- Chen, G.; McAlister, F.A.; Walker, R.L.; Hemmelgarn, B.R.; Campbell, N.R. Cardiovascular outcomes in framingham participants with diabetes: The importance of blood pressure. Hypertension 2011, 57, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Wang, D.; Coresh, J.; Selvin, E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999–2018. N. Engl. J. Med. 2021, 384, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, G.; Yuan, Z.; Chen, L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2012, 7, e42551. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, M.A.; Charpentier, G.; Doggen, K.; Kuss, O.; Lindblad, U.; Kellner, C.; Nolan, J.; Pazderska, A.; Rutten, G.; Trento, M.; et al. Quality of care of people with type 2 diabetes in eight European countries: Findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care 2013, 36, 2628–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Funck, K.L.; Knudsen, J.S.; Hansen, T.K.; Thomsen, R.W.; Grove, E.L. Real-world use of cardioprotective glucose-lowering drugs in patients with type 2 diabetes and cardiovascular disease: A Danish nationwide cohort study, 2012 to 2019. Diabetes Obes. Metab. 2021, 23, 520–529. [Google Scholar] [CrossRef]

| Overall | MR | HR | VHR | p-Value | |
|---|---|---|---|---|---|
| n | 1030 | 314 | 155 | 561 | |
| Age. years | 57.8 ± 8 | 54 ± 8.7 | 58 ± 8.4 | 59 ± 6.8 | 0.001 a,b |
| Male. % | 59.3 | 58.9 | 58.1 | 59.9 | 0.905 |
| Married. % | 72.2 | 72.9 | 73.5 | 71.5 | 0.936 |
| Schooling. years | 11 ± 4.2 | 11 ± 4.2 | 11 ± 3.7 | 10 ± 4.3 | 0.006 c,b |
| Family income, USD | 640 (760) | 800 (800) | 800 (600) | 600 (740) | 0.001 |
| Caucasian, % | 68.7 | 67.2 | 72.3 | 68.6 | 0.510 |
| T2D duration, years | 9.7 ± 7.3 | 4.7 ± 3.3 | 14 ± 4.5 | 10 ± 7.9 | 0.001 a,b,c |
| Hypertension. % | 81.4 | 61.1 | 73.5 | 94.8 | 0.001 |
| Dyslipidemia. % | 74.2 | 68.5 | 67.1 | 79.3 | 0.001 |
| Prior CVD, % | 17.4 | 0 | 0 | 31.9 | 0.001 |
| Smoker. % | 6.7 | 3.5 | 1.9 | 9.8 | 0.001 |
| Obese. % | 45.4 | 25.6 | 14.1 | 64.8 | 0.001 |
| SBP, mm Hg | 141 ± 20.4 | 135 ± 19.1 | 139 ± 19.4 | 144 ± 20.6 | 0.001 b,c |
| DBP, mm Hg | 83 ± 11.7 | 83 ± 10.3 | 83 ± 10.1 | 84 ± 12.7 | 0.284 |
| Biochemical analysis | |||||
| Hemoglobin. mg/dL | 14 ± 1.6 | 14 ± 1.6 | 14 ± 1.4 | 14 ± 1.6 | 0.608 |
| HbA1c. % | 7.9 ± 1.9 | 7.5 ± 1.8 | 8.1 ± 1.7 | 8.1 ± 1.9 | 0.001 |
| Total cholesterol. mg/dL | 182 ± 47.6 | 185 ± 51 | 181 ± 44 | 181 ± 47 | 0.610 |
| LDL-C., mg/dL | 107 ± 37.6 | 110 ± 37 | 109 ± 39 | 105 ± 38 | 0.344 |
| HDL-C, mg/dL | 44 ± 14.6 | 47 ± 19.5 | 46 ± 12.2 | 42 ± 11.9 | 0.004 |
| VLDL-C, mg/dL | 27 (15) | 25 (14) | 25 (14) | 27 (16) | 0.030 |
| Triglycerides, mg/dL | 159 (128) | 145 (110) | 148 (142) | 164 (116) | 0.001 |
| Gfr, ml/min/1.73 m2 | 86 ± 18.1 | 91 ± 16 | 89 ± 15 | 82 ± 18 | 0.010 a,c |
| Proteinuria. % | 19.7 | 0 | 0 | 15.3 | 0.001 |
| Medications | |||||
| Antihypertensive, % | 64.4 | 42 | 52.3 | 80.2 | 0.001 |
| Lipid-Lowering Therapy | 45 | 35 | 41.9 | 51.3 | 0.004 |
| MiS | 36.3 | 29 | 34.8 | 40.8 | |
| HiS | 5.9 | 4.1 | 5.2 | 7.1 | |
| MiS-Ez | 1.3 | 0.6 | 1.3 | 1.6 | |
| HiS-Ez | 1.0 | 0.6 | 0 | 1.4 | |
| Ezetimibe | 0.5 | 0.6 | 0.6 | 0.4 | |
| Antidiabetic therapy | 98.5 | 97.1 | 99.4 | 99.1 | 0.043 |
| Control rate | <0.001 | ||||
| HbA1c. % | 41.2 | 51.1 | 33.6 | 38.4 | 0.003 |
| SBP, % | 30.6 | 23 | 26.4 | 16.1 | 0.007 |
| LDL-C, % | 18.8 | 42.8 | 14.4 | 7.8 | 0.001 |
| Any two | 16.2 | 27.4 | 7.0 | 13.1 | 0.040 |
| All three | 3.2 | 8.5 | 3.5 | 0.6 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, J.; Luchiari, B.; Wolf, V.L.W.; Bonilha, I.; Bovi, T.G.; Assato, B.S.; Breder, I.; Kimura-Medorima, S.T.; Munhoz, D.B.; Quinaglia, T.; et al. Compliance with Cardiovascular Prevention Guidelines in Type 2 Diabetes Individuals in a Middle-Income Region: A Cross-Sectional Analysis. Diagnostics 2022, 12, 814. https://doi.org/10.3390/diagnostics12040814
Barreto J, Luchiari B, Wolf VLW, Bonilha I, Bovi TG, Assato BS, Breder I, Kimura-Medorima ST, Munhoz DB, Quinaglia T, et al. Compliance with Cardiovascular Prevention Guidelines in Type 2 Diabetes Individuals in a Middle-Income Region: A Cross-Sectional Analysis. Diagnostics. 2022; 12(4):814. https://doi.org/10.3390/diagnostics12040814
Chicago/Turabian StyleBarreto, Joaquim, Beatriz Luchiari, Vaneza L. W. Wolf, Isabella Bonilha, Ticiane G. Bovi, Barbara S. Assato, Ikaro Breder, Sheila T. Kimura-Medorima, Daniel B. Munhoz, Thiago Quinaglia, and et al. 2022. "Compliance with Cardiovascular Prevention Guidelines in Type 2 Diabetes Individuals in a Middle-Income Region: A Cross-Sectional Analysis" Diagnostics 12, no. 4: 814. https://doi.org/10.3390/diagnostics12040814
APA StyleBarreto, J., Luchiari, B., Wolf, V. L. W., Bonilha, I., Bovi, T. G., Assato, B. S., Breder, I., Kimura-Medorima, S. T., Munhoz, D. B., Quinaglia, T., Coelho-Filho, O. R., Carvalho, L. S. F., Nadruz, W., & Sposito, A. C. (2022). Compliance with Cardiovascular Prevention Guidelines in Type 2 Diabetes Individuals in a Middle-Income Region: A Cross-Sectional Analysis. Diagnostics, 12(4), 814. https://doi.org/10.3390/diagnostics12040814






